《1. Introduction》
1. Introduction
In our increasingly aging society, the prevention of cardiovascular diseases, Alzheimer's disease and other chronic diseases has become a significant issue for physicians worldwide. Voluminous research indicates that lipid nutrition has a significant impact on the development of cardiovascular diseases. Excessive fat intake is considered an important risk factor for cardiovascular diseases. In contrast, the dietary intake of edible oils with suitable structure and function could delay the onset of cardiovascular and other chronic diseases. Fatty acids from different sources have different carbon chain lengths, numbers of double bonds, and double-bond positions, and therefore exhibit different chemical and physical properties. Due to the high content of fatty acids (about 95%) in oils, the composition, saturation, chain length, and double-bond location of the fatty acids in triacylglycerides (TAGs) have been thought to be associated with the nutrition of edible oils for some time [1]. Moreover, because of variable stereospecific distributions, fatty acids sharing the same structure and composition may exhibit different effects on the absorption and metabolism of the TAG [2,3].
With the development of techniques to measure the acylated position of different fatty acids (i.e., the stereospecific number, SN), the position of fatty acids on the glycerol backbone can now be effectively determined [4,5]. Measuring the distribution of acylated fatty acids in a TAG is important for improving the TAG structure, optimizing its physicochemical properties, and enhancing the nutritional value of an edible oil in order to develop more healthy oils for human consumption. Modifying the fatty-acid composition of rapeseed oil is an important goal of rapeseed breeding [6]. With previous success in breeding low-erucic-acid and low-glucosinolate rapeseed cultivars, current research focuses on breeding high-oleic cultivars because high-oleic oil can reduce low-density lipoprotein (LDL) and lower cholesterol levels in the blood-effectively reducing the risk of developing cardiovascular diseases, and in some cases, cancer. In addition, vegetable oils with high-oleic-acid content are highly desirable with respect to thermal stability, easy oxidation, and longer shelf life. Our group has successfully developed a rapeseed strain by means of γ-60 ionizing radiation that can be used to produce high-oleic oil (more than 80% oleic acid content) [7-10]. After continuous self-breeding and the selection of agronomic traits, several inbred cultivars with stable characteristics were established. However, the molecular structures of the TAGs and the nutritional quality of these strains have not been studied until now.
《2. Materials and methods》
2. Materials and methods
《2.1. Materials》
2.1. Materials
A total of six types of inbred seeds were included in the study. Samples 1 and 2 contained high oleic acid content (more than 80% oleic acid), samples 3 and 4 had medium oleic acid content (about 60% oleic acid) and low erucic acid content, and samples 5 and 6 had low oleic acid content (about 30% oleic acid) and high erucic acid content. All the seeds were provided by Hunan Agricultural University, China.
《2.2. Methods》
2.2. Methods
2.2.1. Extraction of oil from rapeseed
Oil was extracted according to Ref. [4]. In brief, a small quantity of dry rapeseed was homogenized using a microglass pestle to a particle size less than 0.5 mm. Next, the homogenized samples were extracted in a microcentrifuge tube by 2 mL petroleum ether at 45 °C in a water-bath applied with ultrasound for 1.5 h. After centrifugal separation, the supernatants were dried by evaporating the petroleum ether with a gentle stream of nitrogen.
2.2.2. Analysis of composition of fatty acids and total acyl carbon number of triacylglycerides (TAGs)
The methyl esterification method used was the same as that given in Ref. [4], and the analysis of the composition of the fatty acids was done according to ISO standards [4,5], under the category of animal and vegetable fats and oils-that is, analysis by gas chromatography (GC) of the methyl esters of fatty acids. We analyzed the composition of the fatty acids using an Agilent 7890A gas chromatograph (American Agilent Technologies, Columbia, MD, USA), an Agilent HP-FFAP capillary column (30 m × 0.25 mm, 0.25 µm, for the separation and analysis of fatty-acid methyl esters), and a flame ionization detector (FID). The total number of acyl carbons in the fatty acids of the TAGs was analyzed using an Agilent 7890A gas chromatograph, a Varian VF-5ht column (30 m × 0.32 mm, 0.1 µm, Crawford Scientific™ Ltd., Scotland, UK), and an FID.
2.2.3. Analysis of TAGs in rapeseed oil
Qualitative analysis of the TAGs was performed according to Ref. [11]. In brief, the rapeseed oil was dissolved in hexane at about 65 mg·mL−1, and this solution was then diluted with acetonitrile (ACN)/isopropanol containing 0.5% ammonia (7:3 v/v) to 0.65 mg·mL−1. The analysis of the composition of the TAGs in rapeseed oil was performed using electrospray ionization (ESI) tandem mass spectrometry, neutral loss (NL) scan mode, and enhanced product ion (EPI) scan mode with auxiliary judgment. High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) was used for the quantitative analysis of the TAGs. The column was ZORBAX Eclipse Plus C18 (150 mm × 4.6 mm (inner diameter), 5 µm particle size). For the separation of TAGs in the rapeseed oil, the following gradient mode was used: 0-20 min, 70%-30% A; 20-23 min, 30% A; 23-23.1 min, 30%-70% A; and 23.1-28 min, 70% A, where solvent A (100% ACN) and solvent B (0.5% aqueous ammonia in isopropanol) were used as the mobile phase. The flow rate was 1 mL·min−1, the injection volume was 20 µL, and the column temperature was 35°C. An atmospheric-pressure chemical ionization (APCI), multiple reaction-monitoring (MRM) mode, and C18 column were adopted. The quadrupole linear ion-trap mass spectrometer (API 4000 Q-Trap) was purchased from American AB Sciex (Framingham, MA, USA). An Agilent 1200 series HPLC system was purchased from American Agilent Technologies.
《3. Results》
3. Results
《3.1. Fatty-acid composition of rapeseed oil》
3.1. Fatty-acid composition of rapeseed oil
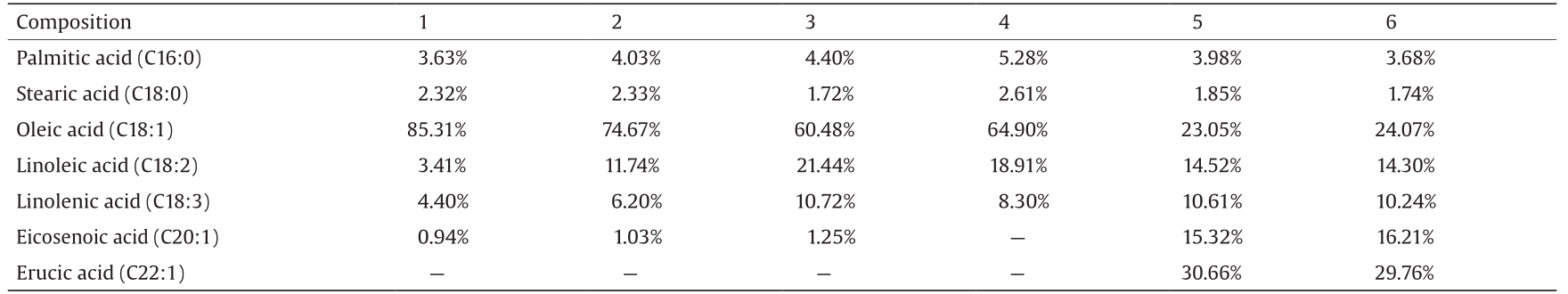
Table 1 presents the composition of fatty acids and their relative percentages in the six tested samples. The results showed the oleic acid content to be 85.31% and 74.67% in samples 1 and 2, respectively, while in samples 3 and 4, the oleic acid content was 60.48% and 64.90%, respectively. The oleic acid content was 23.05% and 24.07% in samples 5 and 6, respectively. The content of erucic acids and eicosenoic acids was rich in samples 5 and 6.
《3.2. The number of acyl carbon atoms in the fatty acids of TAGs in rapeseed oil》
3.2. The number of acyl carbon atoms in the fatty acids of TAGs in rapeseed oil
The total carbon number (CN value) of the acyl chains in the fatty acids of TAGs in the six samples of rapeseed was analyzed using a VF-5ht capillary column with GC-FID detection. The results showed that the CN values of the TAGs in samples 1-4 were 52 and 54, while samples 5 and 6 had a wide range of CN values (from 52 to 62), as shown in Table 2.
《Table 1》
Table 1
Composition and percentage of fatty acids in six rapeseed oils after acylation treatment.
《3.3. Composition of TAGs and their relative percentages in rapeseed oil》
3.3. Composition of TAGs and their relative percentages in rapeseed oil
A qualitative and quantitative analysis of TAGs in the six samples identified a total of 41 types of TAG molecules (Table 3, Fig. 1). Sixteen types of TAG molecules were identified in high-oleic-acid rapeseed (samples 1 and 2) and in low-erucic-acid rapeseed (samples 3 and 4). Twenty-five types of TAG molecules were identified in the high-erucic-acid rapeseed (samples 5 and 6).
《3.4. Basic structure of TAGs in rapeseed oil》
3.4. Basic structure of TAGs in rapeseed oil
The relative proportions of various TAG structures in rapeseed oil were calculated (Table 4). The percentages of 16-carbon saturated fatty acyl, 18-carbon saturated fatty acyl, 18-carbon unsaturated fatty acyl, 20-carbon unsaturated fatty acyl, and 22-carbon unsaturated fatty acyl were measured.
《Table 2》
Table 2
Composition and percentages of TGA in six rapeseed oils.
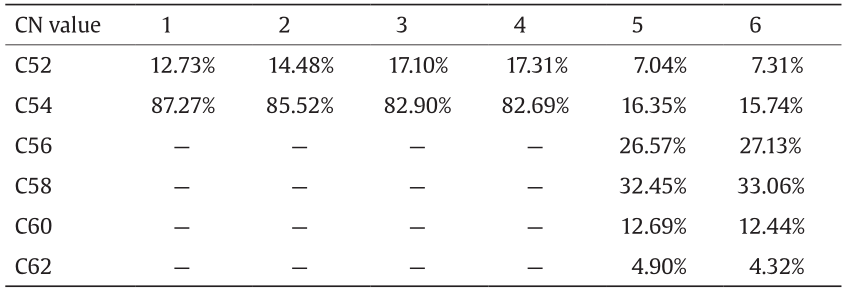
CN value: total number of acyl carbon atoms in fatty acid of TAG molecules. For example, the CN value for both the TAG 18:1/18:1/18:1 and TAG 18:0/18:2/18:2 are 54.
《Table 3》
Table 3
The composition and percentage of total number of acyl carbon atoms in six rapeseed oils.
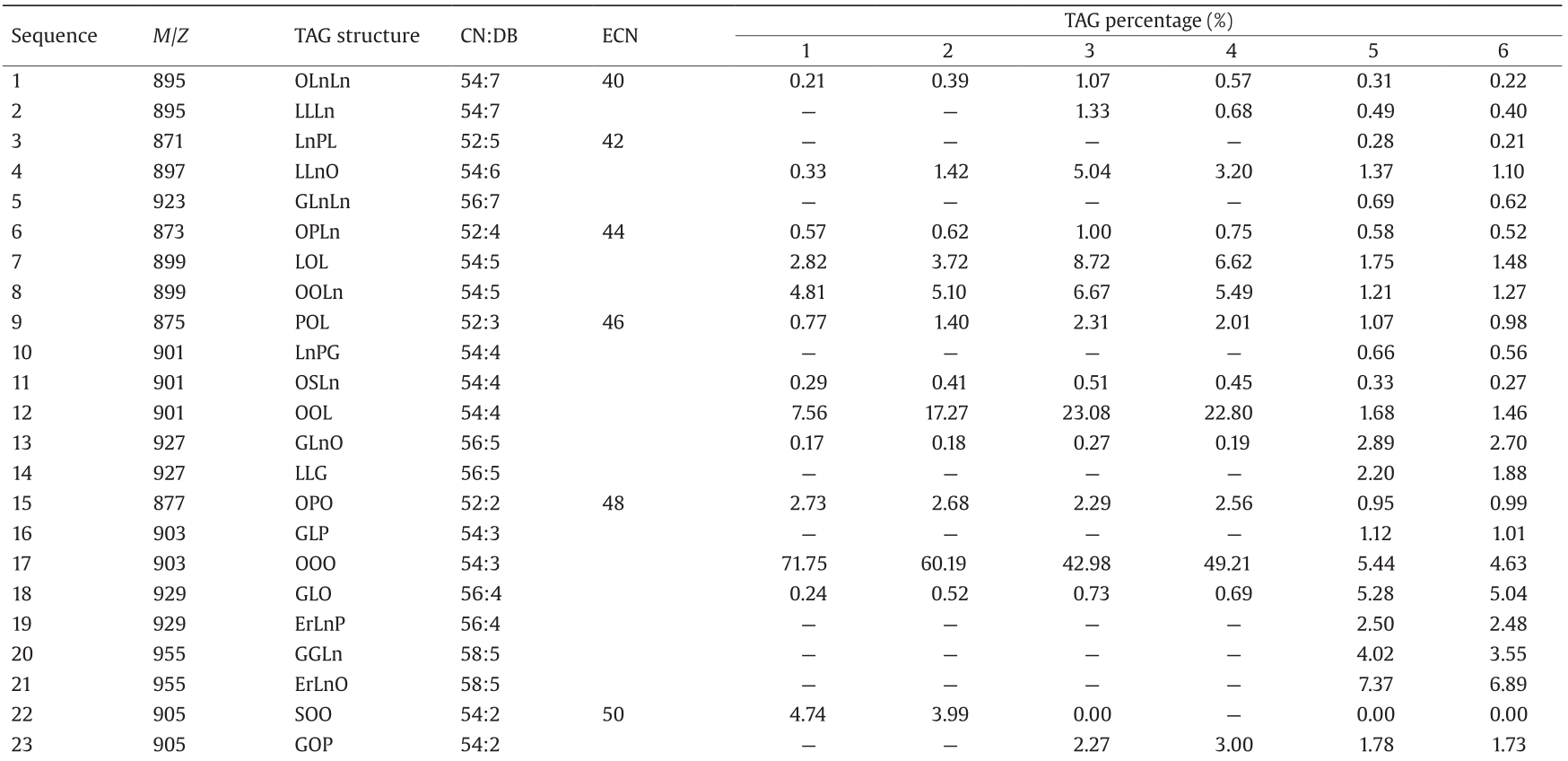

P: palmitic acid; S: stearic acid; O: oleic acid; L: linoleic acid; Ln: linolenic acid; G: eicosenoic acid; Er: erucic acid.
ECN value = CN value – 2 × DB (double bonds) value. For example, for TAG 18:1/18:1/18:1, ECN = 54 – 2 × 3 = 48.
《Fig. 1》
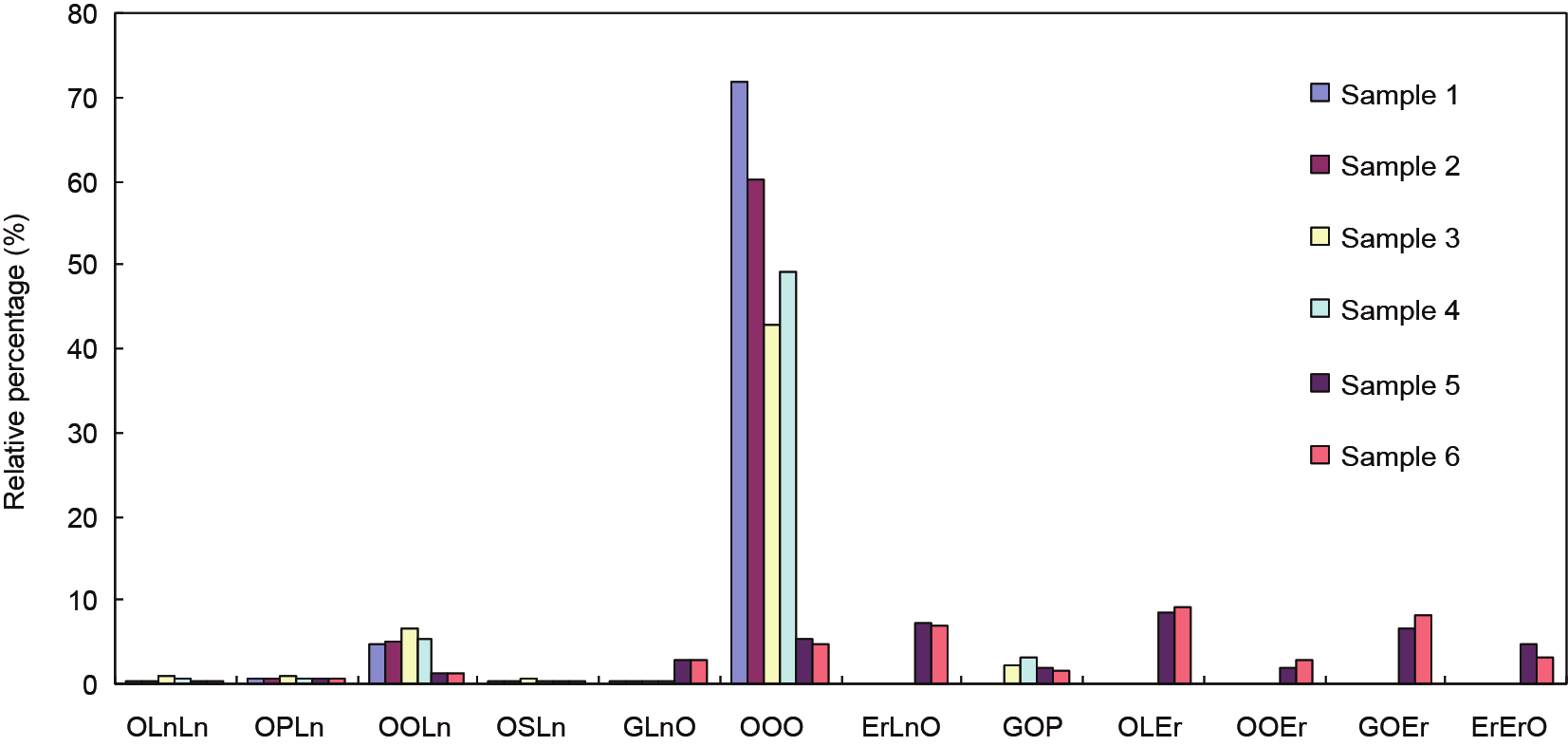
Fig.1 The distribution of TAG content of high-oleic-acid (samples 1 and 2), low-erucic-acid (samples 3 and 4), and high-erucic-acid (samples 5 and 6) rapeseed oils.
《 Table 4》
Table 4
Percentage of several basic structures in six rapeseed oils.

S16: 16-carbon saturated fatty acid acyl; S18: 18-carbon saturated fatty acid acyl; U18: 18-carbon unsaturated fatty acid acyl; U20: 20-carbon unsaturated fatty acid acyl; U22: 22-carbon unsaturated fatty acid acyl.
《4. Discussion》
4. Discussion
Our study demonstrated that the high-oleic-acid rapeseed (samples 1 and 2) not only contained 85.31% and 74.67% oleic acid, respectively, but also contained linoleic acid (3.41%, 11.74%), linolenic acid (4.40%, 6.20%), palmitic acid (3.63%, 4.03%), stearic acid (2.32%, 2.33%), and small amounts of eicosenoic acid. The high-erucic-acid rapeseed contained a high content of long-chain fatty acids, eicosenoic acid, and erucic acid.
TAGs in high-oleic-acid vegetable oil can be classified into four main types. The main TAG composition in the two tested high-oleic-acid rapeseeds was U18/U18/U18, accounting for 87.48% and 88.09% of TAGs in samples 1 and 2, respectively; followed by S18/U18/U18 (4.74% and 3.99%, respectively), U18/S16/U18 (3.31% and 3.30%, respectively), and U20/U18/U18 (3.41% and 2.81%, respectively). Therefore, in oils of the two high-oleic-acid rapeseeds, at least two of the three fatty-acid chains attached to the glycerol skeleton are oleic acid.
The distribution of the total number of acyl carbon atoms in a TAG is relatively simple in high-oleic-acid rapeseed (samples 1 and 2) and low-erucic-acid rapeseed (samples 3 and 4), which mainly included TAGs with 52 and 54 acyl carbon atoms. The relative content of TAGs having 54 acyl carbon atoms was greater than 80% (Table 2). Further structural analysis demonstrated that TAGs having mostly 54 acyl carbon atoms were oleic-oleic-oleic (OOO) (Table 3). The distribution of the total number of acyl carbon atoms is relatively complex in high-erucic-acid rapeseed (samples 5 and 6). The main TAGs contained 56 and 58 acyl carbon atoms, likely because the high-erucic-acid rapeseed contains a high level of eicosenoic acid (C20:1) and erucic acid (C22:1). Further structural analysis revealed that in the high-erucic-acid rapeseed, OOO decreased significantly, while TAGs containing erucic acid increased significantly. This may be the reason why the amount of TAGs with 54 acyl carbon atoms is lower than the amount with 56 and 58 acyl carbon atoms.
In plant seeds, TAGs are formed by combining glycerol with three fatty-acid molecules through ester bonds [12,13]. The composition of the fatty acids determines the types of TAGs in oils [14]. This study showed that the content of OOO TAGs in the high-oleic-acid rapeseed (samples 1 and 2) is 71.75% and 60.19%, respectively, while it is only 42.98% and 49.21% in low-erucic-acid rapeseed (samples 3 and 4). The OOO content is only about 5% in the high-erucic-acid rapeseed (samples 5 and 6), but the relative content of GOEr and ErErO is significantly increased. This result suggests that the oleic acid level has a major impact on the OOO content (Fig. 1). The OOO content is higher in high-oleic-acid rapeseed oil than in low-erucic-acid rapeseed oil. In addition, low-erucic-acid rapeseed oil contains high linoleic acid content (samples 3 and 4), which may be associated with the high content of OOL in the high-oleic-acid rapeseed oil. These findings suggest a positive correlation between high oleic acid content and high OOO content in high-oleic-acid rapeseed oil.
Compared with high-oleic-acid rapeseed, high-erucic-acid rapeseed contains large quantities of linoleic acid (C18:2), linolenic acid (C18:3), eicosenoic acid (C20:1), and erucic acid (C22:1), while the compositions of TAGs in high-erucic-acid rapeseed are more complex. It contains ErLnP, GGLn, ErLnO, OOG, ErLP, GGL, OLEr, ErLnG, ErLnL, SLEr, OOEr, ErLG, ErErLn, SOEr, GOEr, ErErL, and ErErO types of TAGs. We therefore hypothesize that the high content of OOO leads to high levels of oleic acid in high-oleic-acid rapeseed. In contrast, in high-erucic-acid rapeseed, the OOO level is low and the presence of GOEr, ErErO, and so forth, may affect the level of oleic acid.
Fatty acids in TAGs are not randomly distributed in vegetable oils. A study by Vichi et al. [1] showed that unsaturated fatty acids, particular polyunsaturated fatty acids, in TAGs showed a preference of sn-2 > sn-1 > sn-3. Our results were consistent with those of Vichi et al.: For our high-oleic-acid rapeseed oil, the contents of oleic acid in TAGs were 95%, 91%, and 80% (sample 1). In high-oleic-acid materials, lysophosphatidic acid acyltransferase (LPAT)
has an affinity to oleic acid that is greater than those of glycerol-3-phosphate acyltransferase (GPAT) and diacylgycerol acyltransferase (DGAT), according to the Kennedy pathway. No saturated fatty acid was detected at the sn-3 position in our high-oleic-acid cultivar (Table 3), suggesting that in the high-oleic-acid material, DGAT has a low affinity to saturated fatty acid.
Long carbon-chain erucic acids are mainly located in the sn-1 and sn-3 positions in Crambe abyssinica and in the high-erucic-acid Brassica napus, while the erucic acid content at the sn-2 position is low. Therefore, breeding a high-erucic-acid cultivar with erucic acid content as high as> 66% from Brassica napus may be difficult [9]. In cabbage, DGAT can utilize 1,2-diacylglycerol to synthesize three mustard TAGs, but no erucic acid was observed at the sn-3 of TAGs [10]. This finding indicated that DGAT has the ability to transform into erucic acid in rapeseed. High-oleic-acid material does not have this problem. Oleic acid molecules can be inserted into the sn-1/3 and sn-2 positions. Therefore, it is possible to further improve the oleic acid content of rapeseed via breeding.
《Acknowledgements》
Acknowledgements
This study was supported by the Introduction of International Advanced Agricultural Science and Technology program (948 Project) (2006-G04) and the Open Fund of Hubei Key Laboratory of Oil Chemistry and Nutrition.
Mei Guan, Hong Chen, Xinghua Xiong, Xin Lu, Xun Li, Fenghong Huang, and Chunyun Guan declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




