《1.Introduction》
1.Introduction
Osteoarthritis (OA) is a chronic, debilitating, and painful disease, and is the most common form of arthritis in the world. It is a complex disease that involves the entire synovial joint, including the articular cartilage, synovium, and subchondral bone [1,2]. The disease involves the release of pro-inflammatory factors in the joint, leading to structural derangements (Fig. 1). Importantly, OA is the leading cause of disability due to pain; it accounts for approximately 70% of arthritis-related hospital admissions and 23% of clinic visits for arthritis [3]. Knee OA is the most common form of OA, given the anatomical position, since the knee bears most of the weight of the body. Older adults above the age of 50 are at an increased risk for knee OA, possibly due to hormonal changes or senescence of chondrocytes [3,4]. With an estimated one-third of US adults living with obesity, two-thirds of these individuals are at risk of developing knee OA in their lifetime [1,5,6]. This risk could be due to increased weight bearing on the joint or to the release of adipokines, which are cytokines released from adipose tissue. In the US, 27 million adults were estimated to have OA in 2008, with the number of adult arthritis patients projected to rise from 47.8 million in 2005 to more than 67 million by the year 2030 [7,8]. However, the number of individuals seeking medical help is predicted to increase even further through care programs such as the Affordable Care Act [9]. The total cost of the treatment of knee OA for each individual diagnosed with the disease exceeds $6000 USD per year, with a lifetime cost of over $100 000 USD [10,11]. This number is still modest, however, as the true cost of OA has not been taken into account. These additional costs include the loss of hours from work and the effect on the patient’s quality of life due to disability from pain [12]. Thus, OA is a very significant health problem that poses a physical burden to patients and an economic burden to both patients and the healthcare system.
《Fig. 1》

Fig. 1. Structural changes between (a) a healthy joint and (b) an OA joint. Expression of matrix proteinase plays an important role in inducing OA. (Adapted with permission from Ref. [2])
The end-stage surgical solution for knee OA, which accounts for most of the cost of OA treatment, is total knee arthroplasty (TKA) [3,10,11]. TKA involves replacement of the knee joint with prostheses made of metallic alloys and polymers. This procedure gives relief from pain, improves physical functions, and improves quality of life for most patients. However, it is not uncommon for revision surgeries to be necessary for failed joint replacements. It is projected that more than 3 million TKA procedures will be demanded by the year 2030, along with a nearly concomitant increase in revision surgeries [13]. This is an increase from 450 000 TKA procedures in 2005. With this increasing prevalence and cost of the management of knee OA, it is important to develop newer, less-invasive, and more cost-efficient therapeutic intervention for the disease. These treatments should also postpone or eliminate the need for a TKA.
Due to the increasing efforts of researchers, surgical procedures with varying success rates have already been developed. One notable example of such innovations is the less-invasive procedure of subchondroplasty. This involves the use of calcium phosphate bone substitutes to treat bone marrow lesions (BML), which are associated with the development of end-stage OA; thus, the procedure prevents severe OA and the need for TKA [14,15]. Despite the usefulness of this procedure, some patients do not fare well due to variability in patients [14]. In addition, the use of subchondroplasty is limited to BML. Hence, novel regenerative engineering strategies utilizing the latest advancements in biomaterial science, stem cell science, aspects from developmental biology, and physical forces will enable the development of translational therapies for knee OA treatment. “Regenerative engineering” has been defined as the convergence of advanced material science, stem cell science, physics, developmental biology, and clinical translation for the regeneration of complex tissue and organ systems [16,17].
In this review, we discuss the pathogenesis of the OA disease and current biomaterial and cell-based technologies to treat knee OA.
《2.Osteoarthritis pain and treatment modalities》
2.Osteoarthritis pain and treatment modalities
Pain is the number one reason for a patient’s visit to the clinic when symptoms of knee OA arise [3]. This pain can occur without simultaneous radiographic evidence of knee OA [3,18]. The pain may be dull and constant with intermittent intense exacerbations. The etiology of OA pain is complex, with several modifiable and non-modifiable factors involved. Modifiable risk factors include weight, structural derangements, biological processes such as inflammation, and sociocultural factors [3,19]. Non-modifiable factors include patient genetics. We discuss the effects of inflammation, structural pathology such as articular cartilage damage, and other factors on pain in subsequent sections.
《2.1. Osteoarthritis pain》
2.1. Osteoarthritis pain
The biological mechanisms that contribute to knee OA pain are patient dependent and not fully understood. Chondrocytes and other cell types present in the knee joint produce inflammatory mediators and degradative enzymes, leading to cellular apoptosis. The inflammatory process is induced by cytokines such as proinflammatory interleukins and tumor necrosis factor-α (TNF-α) [20]. These mediators may be recognized by receptors of nerve terminals present in the synovial joint. Such noxious stimuli can activate high-threshold ion channels, resulting in the propagation of pain signals from peripheral tissues to the central nervous system [21]. The cytokines produced in the osteoarthritic joint may act on the innervating joint nociceptors (myelinated or unmyelinated sensory neurons that carry pain signals from peripheral tissues to the central nervous system) [20] and generate pain. Although OA is associated with the frequent occurrence of pain, the real mechanism is not clearly elucidated. Further understanding of the pain mechanisms may help in developing novel therapeutic strategies for managing OA pain.
《2.2. Treatment modalities for osteoarthritis pain》
2.2. Treatment modalities for osteoarthritis pain
Since knee pain is the most debilitating symptom of knee OA, several non-pharmacological and pharmacological therapies have been investigated. Non-pharmacological treatments are particularly important in the elderly due to increased risk of comorbidities and medication toxicity [22], which may complement or prevent medication use. Non-pharmacological treatments include mechanical approaches such as physical therapy, rest, canes, ice bracing, and weight loss. The American College of Rheumatology (ACR) and the Osteoarthritis Research Society International (OARSI) guidelines for knee OA management strongly suggest that the best way to relieve OA pain is to lose weight by exercising regularly [22,23]. Landand water-based therapeutic exercises are the most dominant non-pharmacologic therapies. Land-based exercises provide a temporary reduction in pain and physical disability [24]. A Cochrane (independent and non-governmental organization) review of 44 randomized controlled trials (RCTs) with 3537 participants presented high-quality evidence that exercise reduced pain immediately after treatment and for at least two to six months. In addition, moderate-quality evidence from these studies (44 RCTs, 3913 participants) showed that physical functions improved immediately after exercises [25]. Water-based exercises are also beneficial for people with serious mobility and functional limitations. A clinical trial with 64 participants showed that water-based exercise was better than land-based exercise for pain relief before and after a 50-foot walk test during a period of 18 weeks [26]. However, a recent meta-analysis of other RCTs shows only moderate beneficial effects on pain, physical function, and quality of life in patients with musculoskeletal conditions, similar to those achieved with land-based exercise [27,28]. Lowerimpact physical exercises such as tai chi and yoga are also becoming popular. A systematic review of six RCTs investigating the effect of tai chi suggested that this exercise controlled pain and improved physical function for knee OA patients [29]. A pilot study of an eight-week yoga course was done at the University of Pennsylvania Medical Center that showed reduction in pain and disability in obese patients over 50 years of age [30]. Although limited studies have been reported to date, the results for yoga and tai chi have been encouraging for OA knee patients.
In regard to pharmacological treatments, the most commonly used drugs are nonsteroidal anti-inflammatory drugs (NSAIDs) [31–33], opioids [34–36], a combination of NSAIDs and opioids [37], hormones, chondroprotective agents, calcium and vitamins, and intra-articular steroid injections. For example, a phase III clinical trial [38] evaluated the efficacy and safety of a low-dose meloxicam formulation administered orally once daily for three months in patients with OA-related pain. Patients treated with meloxicam reported significant improvements at week 12 compared with a placebo, but serious adverse effects were reported including headache, diarrhea, urinary tract infection, and nausea. Certain opioids such as tapentadol and tramadol have also been reported to be efficacious for the treatment of moderate to severe acute OA knee pain [39,40]. The adverse effects reported regarding tapentadol in phase III trials involve the gastrointestinal and central nervous systems [39]. Regarding tramadol, the most frequent adverse events were dizziness, nausea, and constipation [40].
Recently, trials in a real-life setting [41] demonstrated the efficacy and safety of topical NSAIDs in the management of OA. Topical and oral NSAIDs exhibited an equivalent effect on knee pain over one year of treatment, with fewer adverse effects for topical NSAIDs; in addition, fewer patients change medication due to adverse effects with topical NSAIDs in comparison with oral treatments. Topical NSAIDs may be preferred to oral NSAIDs because their lower peak plasma concentration results in a lower tendency to cause unwanted side effects. Current clinical strategy to treat moderate OA is by intra-articular injections of antiinflammatory drugs. For example, corticosteroid and paracetamol injections are often used for pain relief [42]. Overall, several effective therapeutics have been investigated for inflammation and pain relief in knee OA, albeit with significant side effects [43–45]. These led to the exploration of alternative treatment such as the biomaterial-based approach (viscosupplementation) for knee OA pain relief.
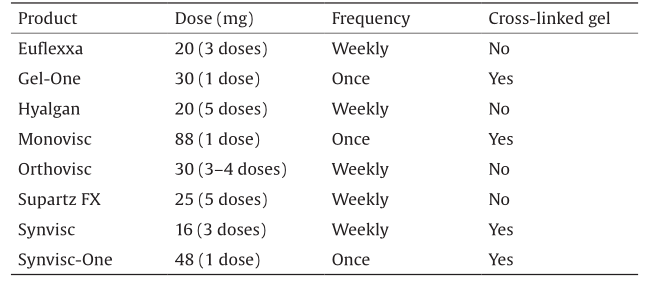
The concept of viscosupplementation is based on intraarticular hyaluronic acid (HA) injection, in order to restore the viscoelasticity in the synovial fluid [46,47]. HA, or hyaluronan, is a natural lubricant that is secreted by synovial fibroblasts and type-B synoviocytes into the joint [48]; thus, it is widely used for OA treatment. HA is also the main component of the extracellular matrix (ECM), principally of connective, epithelial, and neural tissues. HA is an anionic and linear polysaccharide of a repeating non-sulfated disaccharide unit composed of D-glucuronic acid and N-acetyl-D-glucosamide, linked via alternating β-1,4 and β-1,3 glycosidic bonds. The molecular weight (MW) of native HA is around 106 Da and its concentration in the synovial fluid (approximately 4 mg·mL–1) is reduced considerably by OA. Today, there are several types of Food and Drug Administration (FDA)-approved injectable HA viscosupplementation products (Table 1) [49–57] such as Hyalgan, Synvisc, and Supartz, along with others that differ in their biological properties, MW, production procedure, and injection protocols. RCTs have recently been published comparing the efficacy of the intra-articular injection of HA with a placebo or with corticosteroid intra-articular injections, in patients with OA [58–61]. Overall, after eight weeks, the use of HA seems to be more effective than a placebo or corticosteroid after the last injection, in terms of OA knee pain or improved function.
《Table 1》
Table 1 FDA-approved injectable HA viscosupplementation products [50–57].
《3.Cartilage damage and treatment modalities》
3.Cartilage damage and treatment modalities
《3.1. Mechanism of cartilage damage》
3.1. Mechanism of cartilage damage
Knee OA is characterized by the progressive degradation of joints, involving articular cartilage damage, subchondral bone thickening, osteophyte formation, and secondary inflammation of the synovial membrane [62]. Articular cartilage is an avascular tissue rich in type II collagen, glycosaminoglycans (GAGs) such as HA, and proteoglycans such as aggrecan. Articular cartilage is divided into three regions that vary in cell morphology as well as in type II collagen arrangement, which plays an important role in governing the mechanical properties. Articular cartilage degeneration begins with the onset of fibrillation at the articular surface and the disruption of the macromolecular framework of the matrix. The collagen fibrils start to disorient significantly just beneath the surface and the proteoglycan content decreases, not only in the superficial zone but also in the middle zone of the cartilage [63]. With disease progression, fibrillation extends into the deeper layers, reaching the subchondral bone; gradually, the fibrillated cartilage begins to tear at its superficial tip. Aggrecan undergoes rapid and extensive degradation with the truncation of its core protein. The core protein is cleaved into fragments, some of which remain bound to HA and are retained within the tissue, whereas others diffuse into the synovial fluid. This diffusion leads to a reduction of the net anionic charge present in the aggrecan and thus to the loss of its ability to resist compression stress, predisposing the tissue to erosion. Moreover, aggrecan cleavage was found to vary among individuals and was the highest in regions closer to cartilage erosion [64,65]. Along with aggrecan content reduction, the composition of the collagen changes from type II to type I [66]. This change greatly affects the mechanical stability of the tissue, as type II collagen contains a higher content of hydroxylysine, as well as glucosyl and galactosyl residues, facilitating its interaction with proteoglycans [67]. The matrix degeneration is mediated by unbalanced metalloproteases production, including matrix metalloproteases (MMPs) and aggrecanase, which are known to digest cartilage matrix [68]. The major MMPs include stromelysin-1 (MMP3) and collagenase-3 (MMP13), which are activated by the proteolytic removal of their propeptides; they then cleave aggrecan at its short interglobular domain and chondroitin sulfate (CS)-rich domains (CS1 and CS2) [64]. In addition, the aggrecanase family includes a disintegrin and metalloproteinase with thrombospondin motif-4 (ADAMTS4) and ADAMTS5 [69], which are potent degrading enzymes that cleave the aggrecan core protein in a similar way as that of MMPs. Moreover, collagenase has been demonstrated to digest the collagen fiber network, resulting in a decrease of the proteoglycan content. Such alterations in the structure and composition of cartilage matrix lead to fibrillation at the articular surface and hence lower the mechanical strength of the tissue [70]. ECM changes are caused by many factors, one of which is the inflammation triggered by either mechanical damage or wear and tear of the tissue. The chondrocytes present in cartilage tissue respond to inflammation by participating in catabolic activities, ultimately causing ECM degradation [71]. Various factors contributing to the catabolic processes in OA include TNF-α, interleukin 1β (IL-1β), IL-12, IL-15, and various associated chemokines; these significantly increase the expression of matrix-degrading proteins, including both MMPs and ADAMTSs in chondrocytes [72]. The matrix disruption (due to loss of proteoglycans, PG) leads to an increase in surface porosity and permeability, causing increased interstitial fluid flow out of the tissue. This in turn leads to tissue degradation and complete loss of its functional properties. Understanding the cartilage degeneration mechanisms may thus help to develop new potential treatment strategies to repair damaged cartilage tissue.
《3.2. Treatment modalities for cartilage damage》
3.2. Treatment modalities for cartilage damage
Treatment modalities for cartilage repair include both nonsurgical and surgical methods. The non-surgical methods discussed in Section 2.2 can be used to relieve early-stage symptoms of OA; however, there is no proof of restoration of the anatomy of the native tissue and healing of the lesion [73]. Surgical approaches aim to restore the structural and functional properties, thereby repairing the damaged cartilage tissue. These approaches include arthroscopy, subchondral drilling, abrasion arthroplasty, and microfracture, which focus on the use of mesenchymal stem cells (MSCs) from subchondral bone for cartilage regeneration. The major limitations of these methods include the formation of fibrocartilage, which has less ability to absorb shock and which thus compromises the functional properties of the native cartilage tissue. Other approaches include mosaicplasty and autogenous chondrocyte transplantation, in which autologous tissues or cells are harvested and used to repair chondral defects. Autologous chondrocyte implantation (ACI) is a commonly used treatment that may result in a greater proportion of hyaline-like tissue at the repair site [74,75]. In first-generation ACI, chondrocytes are isolated from a biopsy of healthy cartilage obtained from a minimal loading area. The chondrocytes are expanded in vitro and then injected into the patient between six weeks and 18 months after the biopsy [75]. In order to avoid chondrocyte leakage from the defect site, a periosteal patch is either sutured or glued using fibrin glue to cover and seal the area [76]. In order to avoid periosteal patch complications and surgical difficulties, second-generation ACI was developed, and is known as matrix-assisted autologous chondrocyte implantation (MACI). MACI was developed using either collagen or hyaluronan-based matrices seeded with autologous chondrocytes, which are inserted into the defect site or fixed with fibrin glue. The clinical results of the hyaluronan-based MACI treatment of chondral lesions showed stability and a low failure rate in long-term follow-up [77,78]. The major problems associated with ACI are donor site morbidity, nonhomogeneous distribution of chondrocytes, and loss of chondrocytes. Allografts are being used as an alternative, but poor integration between the edge of the chondral defect and the donor graft leads to graft failure [79]. These treatments suffer from major limitations related to the availability of sufficient cells for repair and the quality and quantity of repaired tissue; due to these limitations, they may fail to produce long-lasting repair. Based on favorable clinical outcomes, cell therapy along with biomaterials can be an alternative therapy for the repair of articular cartilage defects.
Repairing articular cartilage is highly challenging because of its avascular and aneural nature, and its sparse cell population. Thus, the selection of a suitable cell source is extremely critical. With this aim, various researchers have employed a wide source of cells to identify the most optimum cell source, such as chondrocytes and MSCs, including bone marrow, adipose-derived, and synovium-derived MSCs [80–83]. These cell sources can be easily isolated, are capable of expansion, and can express and synthesize cartilage-specific molecules (e.g., type II collagen and aggrecan) with or without scaffolds for successful cartilage repair [83].
With advancements in the field of medical science and technology, the focus has shifted toward the use of biomaterials for cartilage regeneration. A wide variety of biomaterials, including both natural and synthetic biomaterials, has been used to date to deliver cells and other signals to efficiently regenerate cartilage tissue. Several different scaffolds have been made out of biomaterials using various techniques such as hydrogels, sponges/foams, fibrous matrices, and layered structures. Fig. 2 represents cellbiomaterial approaches for cartilage repair.
《Fig. 2》
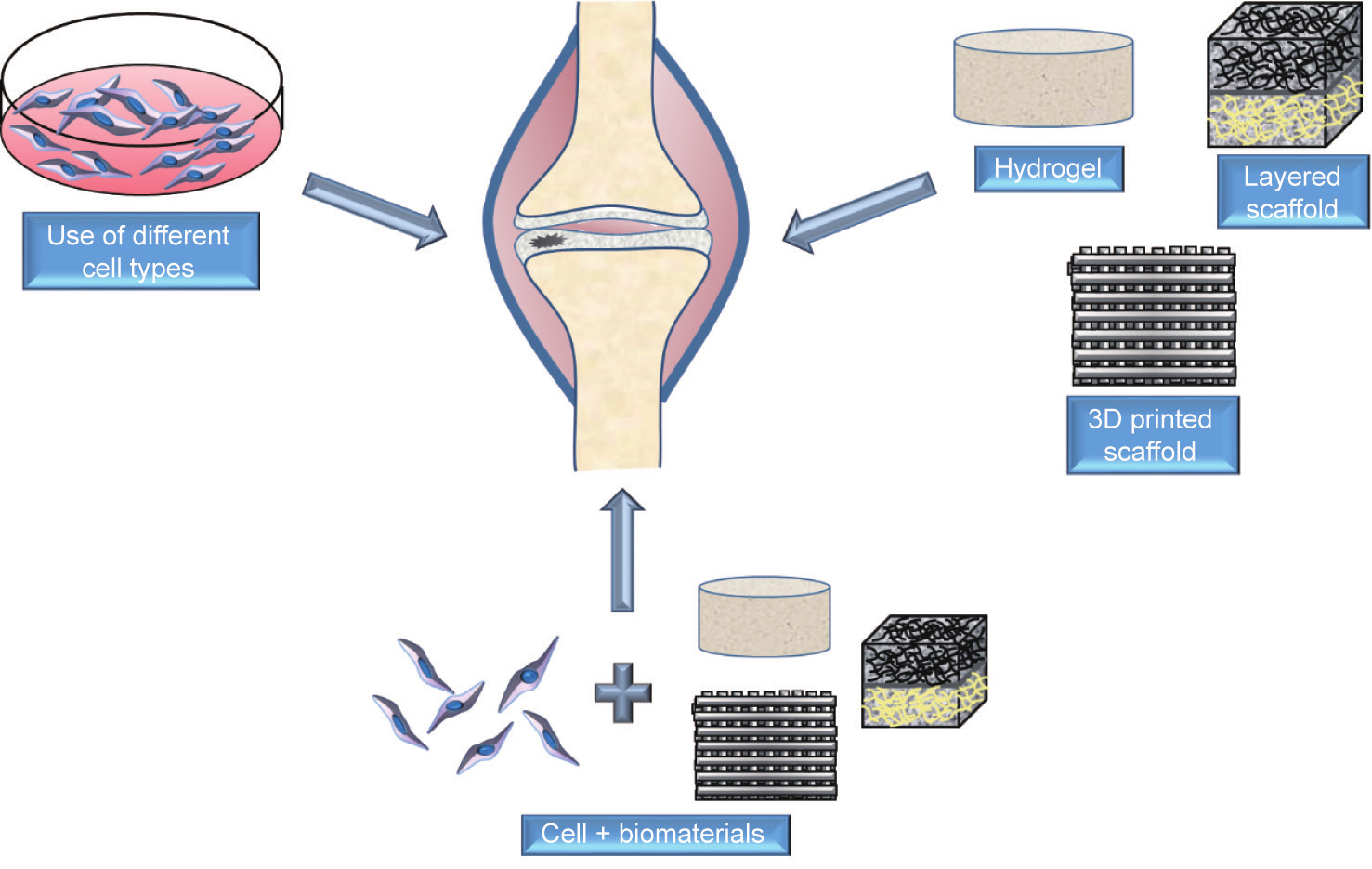
Fig. 2. Cell-biomaterial approaches for cartilage repair.
3.2.1. Hydrogels
Hydrogels are 3D polymer networks with high water content, which mimic the water content of the native ECM [84,85]. Hydrogels are formed either by physical or chemical cross-linking methods and are promising delivery carriers for the controlled release of cells and bioactive molecules into the target site. The type and degree of cross-linking influence important properties of the hydrogels such as swelling properties and the elastic modulus [84,85]. Hydrogels are also used in the form of injectable hydrogels, which can easily fill defects of any size and shape and can be implanted in a minimally invasive manner [86].
Several synthetic and natural biomaterials have been tested for developing hydrogels for cartilage regeneration. One of the most prevalent synthetic biomaterials used for developing hydrogels is polyethylene glycol (PEG), as it is relatively inert and biocompatible. However, PEG does not support cartilage-specific matrix production and the production of chondrogenesis on the same level as observed in other natural materials such as HA [87]. Thus, the incorporation of HA into PEG hydrogels has been shown to improve the bioactivity of the PEG hydrogels [88]. Poly-L-lysine (PLL), a natural polypeptide, has been explored for cartilage regeneration because it is known to be an early chondrogenic stimulant of MSCs. PLL has been shown to up-regulate mesenchymal condensation in vitro, thereby mimicking the stages of cartilage developmental processes. PLL was incorporated into oligo(PEG fumarate) (OPF)-based hydrogels in order to evaluate the efficacy of a developmental-biology-inspired strategy for cartilage regeneration. These hydrogels resulted in early up-regulation of type II collagen and aggrecan genes as well as the expression of a condensation marker, the N-cadherin gene, in the encapsulated MSCs. The study demonstrated that cationic PLL induces the expression of early chondrogenic markers, replicating the condensation stages of cartilage development [89,90].
Alginate hydrogels have also been used for cartilage regeneration and the promotion of cartilage ECM synthesis and chondrogenesis. The negative charges present on the alginate chemical structure induce the retention of newly synthesized aggrecan molecules. However, the limitations of these materials include weak mechanical stability, slow degradation, and poor cell adhesion. In order to overcome poor cell adhesion, the arginineglycine-aspartic acid (RGD) peptide sequence (cell adhesion motif) was immobilized in alginate scaffolds. RGD immobilization substantiated the formation of focal cell adhesions and thus improved cell adhesion [91]. Collagen is an important component of cartilage matrix; therefore, collagen hydrogels have been investigated to support cartilage regeneration. Collagen hydrogels also contract during in vitro culture, which provides an advantage in cell aggregation and the initiation of chondrogenic differentiation that is similar to the events in embryonic chondrogenesis [92]. CS is another important component of cartilage matrix and thus has been used extensively for cartilage regeneration. Due to the weak mechanical properties of CS, attempts were made to develop a composite material by incorporating another material such as PEG. CS has been modified using N-hydroxysuccinimide (NHS) to yield a CS-NHS macromer that can react with six PEGamine arms forming chemically cross-linked hydrogels [93]. Varying the pH of the PEG-amine precursor leads to CS-PEG hydrogels with tunable mechanical, swelling, and gelation properties. Lowering the pH increases the gelation time, stiffness, and the degree of cross-linking, and decreases the degree of swelling. Moreover, the adhesive strength of CS-PEG hydrogels was evaluated and found to be comparable with that of fibrin glue, a commonly used adhesive for clinical application. This is due to its ability to form covalent cross-links with biomolecules containing primary amine groups in the ECM.
One of the most commonly used hydrogels for cartilage regeneration is HA hydrogel, as it is abundant in cartilage matrix. The abundance of functional groups (—OH and —COOH) allows for its chemical modification and covalent cross-linking. As described in Section 2.2, HA is widely used for viscosupplementation therapy for OA. HA with a high MW produces a highly viscous solution, but remains in the damaged area for a short time. The most common method to prolong HA duration in vivo and improve its mechanical properties is the chemical modification of HA.
The mechanical and physical properties of the hydrogels greatly depend on the cross-linking density. The chemical functionalization of HA using different functional groups has been investigated (Fig. 3), allowing the formation of hydrogels without using potentially cytotoxic cross-linkers. Covalent cross-linking of HA can be achieved via ester and ether linkage using —COOH and —OH functional groups (Fig. 3). For example, dihydrazide-modified HA (HA-ADH) has been synthesized using an excess of adipic acid dihydrazide (ADH) and in the presence of carbodiimide/1-hydroxybenzotriazole (HOBt) [94,95]. In addition, aldehyde-modified HA (HA-CHO) has been prepared by sodium periodate-mediated oxidation [96,97]. The physical and mechanical properties (gelation time, viscosity, elastic modulus, and cross-linking density) of HA hydrogels can be modulated by changing the ratios of HAADH and HA-CHO in the feed mixture. The reaction between HAADH and HA-CHO leads to the formation of hydrazone linkages (C1=N1—N2H—(C=O)N3H), which are not quite stable under physiological conditions and which are rapidly excised by lysosomal enzymes [98]. Oommen et al. [99] designed a more stable HA system composed of HA-carbodihydrazide (HA-CDH) and HA-aldehyde. The hydrazone linkage thus formed is stable and non-reversible due to the delocalization of the positive charge in its chemical structure. HA-CDH hydrogels were 15-fold more stable under acidic conditions, with a higher storage modulus (G′ = 1196 Pa) than that of HA-ADH (G′ = 297 Pa). Moreover, periodate-mediated oxidation affects the biological functions of HA (lack of cell recognition), limiting its application. In order to preserve the native structure of HA, Wang et al. [100] designed a synthesis route to graft aldehyde moieties onto the HA under mild conditions. Alternatively, HA hydrogels can be prepared by a thiol-Michael addition reaction using difunctional electrophiles such as PEG diacrylate [101,102]. The reaction can be carried out under physiological conditions; its gelation kinetic is fast and proceeds without a catalyst. Another study of a thiol-based reaction investigated the use of thiolated HA with PEG vinyl sulfone to yield 3D hydrogel networks under physiological conditions with tunable degradation rate and gelation time [103]. Here, gelation time was decreased from 14 min to less than 1 min by increasing the polymer concentration (from 0.02 w/v to 0.06 w/v) and HA MW (from 45 kDa to 185 kDa). The biodegradation and mechanical strength of the hydrogels depended on the MW of HA and PEG, polymer concentration, and functionalization degree of thiolated HA. The modification of the HA chemical structure with acrylate and methacrylate moieties is another synthesis route used to modulate the chemical and mechanical properties of HA. Methacrylate-HA hydrogels can be achieved by photo-cross-linking methacrylate groups linked to HA chains, which then undergo free-radical polymerization upon exposure to ultraviolet (UV) irradiation. Leach et al. [104] described the transesterification reaction between HA and glycidyl methacrylate. A range of glycidyl methacrylate-HA (GMHA) conjugates yielded GMHA hydrogels with different and controllable properties. High-functionalization-degree conjugates lead to hydrogels with increased cross-linking densities and decreased degradation rates.
《Fig. 3》
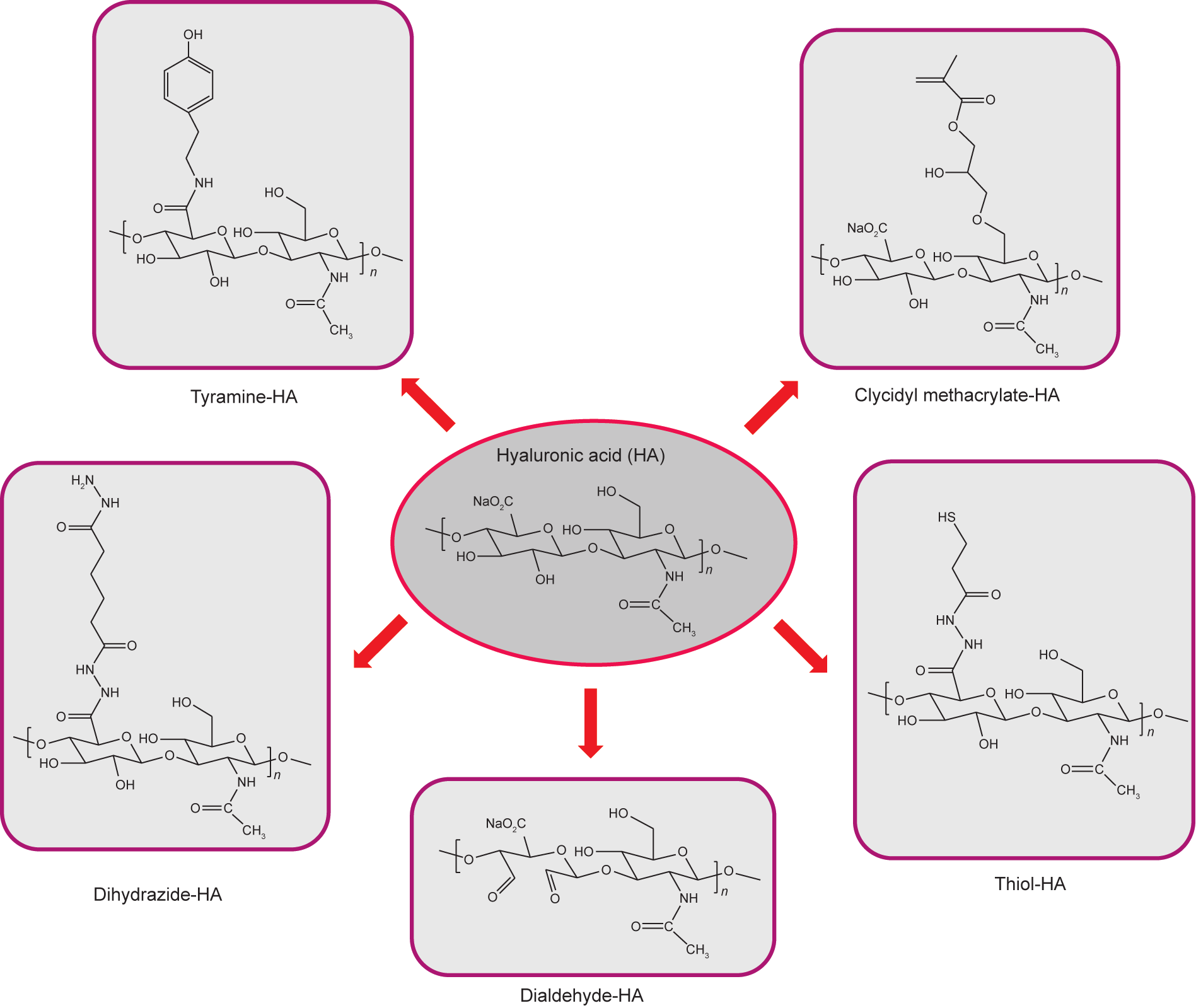
Fig. 3. Chemical modifications of HA.
Composite approaches using reinforced hydrogels such as fillers or fibers have the potential to improve the mechanical properties of the hydrogel. Thus, along with the use of reinforcing fillers such as cellulose or fibers of another nature, such approaches can be used to improve the mechanical properties of the hydrogel [105]. Researchers have attempted to replicate the complex mechanical properties of native cartilage tissue in fiber-reinforced composite using 3D woven polycaprolactone (PCL) scaffolds encapsulated in a fibrin hydrogel and seeded with human adipose-derived stem cells. The scaffold developed functional tissueengineered constructs with similar biomechanical properties as those of native cartilage tissue [106].
The above-mentioned hydrogels lacked a zonal organization similar to that of native cartilage tissue. Thus, new strategies are being developed to replicate zonally organized native articular cartilage-like tissue, as this property plays an important role in governing the mechanical behavior of the native cartilage. A three-layer PEG-based hydrogel was developed with a top layer containing CS and MMP-sensitive peptides (superficial zone, PEG:CS:MMP-pep), a middle layer containing CS (transitional zone, PEG:CS), and a bottom layer containing HA (deep zone, PEG:HA). This layered hydrogel facilitated zone-specific chondrogenesis and developed cartilage-like tissue with spatially varying mechanical and biochemical properties [107].
Although several techniques have been developed to increase the mechanical properties of hydrogels, the hydrogels still lack hierarchical organization and thus lead to isotropic tissues with varied mechanical behaviors compared to those of the host tissue.
3.2.2. Sponges/foams
Sponges are porous scaffolds whose properties are defined by pore size, porosity, and interconnectivity. Pore size is an important factor for tissue regeneration; thus, controlling the pore structure is very important. To date, various methods have been employed to develop sponges, including porogen leaching, freeze drying, and gas foaming for cartilage regeneration. Purified alginate sponges have been prepared in order to minimize the immunological reaction and were found to support chondrogenesis [108]. Porous alginate scaffolds were prepared using a microfluidic device to generate alginate droplets upon gelation, and formed a highly organized porous scaffold. The scaffold maintained the chondrocyte phenotype and facilitated the formation of cartilagelike tissue in the dorsal subcutaneous site of mice [109]. Another study investigated the effect of pore size on cartilage regeneration using a porous collagen scaffold with gradient pores developed using ice particulates (150–250 μm, 250–355 μm, 355–425 μm, and 425–500 μm) as a porogen. The scaffold showed compactly packed spherical pores with good pore interconnectivity and 98% porosity in all the scaffolds with different pore sizes. The constructs were subcutaneously implanted into the dorsa of sixweek-old mice. The study demonstrated that scaffolds with 150– 250 μm diameter promoted the highest expression of type II collagen and aggrecan and increased the formation of cartilage-like tissue with better mechanical properties than scaffolds with different pore sizes [110]. Due to the higher chondrogenic gene activity in collagen scaffolds with a 150–250 μm pore size, this system was used for the delivery of insulin. The porous scaffold loaded with poly(lactic-co-glycolic acid) (PLGA) microparticles containing insulin showed a sustained release of insulin, which supported the survival and proliferation of chondrocytes [111]. Other materials that have been used as porous scaffolds for cartilage regeneration include silk, gelatin, chitosan, and HA [112–116].
However, these scaffolds lack zonal organization with varied composition and properties similar to native cartilage tissue. Thus, the recent focus has been toward developing a multilayered 3D scaffold that is similar to native cartilage, with varied mechanical properties and functions. Multilayered porous scaffolds were developed by stacking chitosan and PCL copolymers, which were blended with different concentrations of type II collagen. To replicate the collagen composition of cartilage tissue layers, type II collagen content (in the scaffold) was reduced from the top to bottom layers. The study showed the feasibility of these scaffolds to be similar to native cartilage in terms of composition, porous architecture, water content, and compressive mechanical properties [117]. Another study developed multilayered 3D constructs using layer-by-layer technology combined with template leaching using chitosan and CS, which can form a polyelectrolyte complex. The constructs showed controlled pore size, a water-uptake capacity of up to 300%, a viscoelastic nature, and the supported chondrogenic differentiation of MSCs [118]. Attempts to mimic the zonal organization of native cartilage were performed using electrospun PCL fibers deposited onto particulate-leached foam. The two-layered 3D scaffold contained an upper aligned fiber region to simulate the morphology of the superficial zone of articular cartilage, whereas the bulk porous particulate template scaffold allowed cellular infiltration and extensive ECM deposition. The incorporated aligned fibers also enhanced the tensile properties, and the porous region facilitated the infiltration of seeded chondrocytes, resulting in high rates of proliferation and GAG production [119]. The two-layered scaffold showed improved mechanical properties due to the use of synthetic polymers, which usually lack the biochemical cues to induce chondrogenesis. However, the zonal organization present in native cartilage was still not achieved in these scaffolds.
3.2.3. Fibrous meshes/scaffolds
Fibrous meshes are networks of non-woven and woven fibers with variation in the fiber diameter and void volume, which dictates cellular behavior. Fibrous scaffolds are used because of their high porosity and interconnected pores, which may provide higher mechanical strength but which fail to fill irregular defects. Microscale and nanoscale fibers are commonly used to mimic the ECM components of native cartilage tissue.
Several attempts have been made to develop 3D fibrous scaffolds with biomimetic mechanical properties and the ability to sustain physiological loading after implantation. 3D woven PCL scaffolds infiltrated with a slurry of homogenized cartilagederived matrix were developed and cultured with human adiposederived stem cells for up to 42 d. These woven scaffolds supported ECM accumulation and maintained the mechanical properties [120,121].
Electrospun scaffolds have also been studied for cartilage regeneration. The nanofibers generated by electrospinning have great potential as a cartilage ECM mimic substrate. Despite their ECM-mimicking properties, nanoscale fibers suffer from limited cellular infiltration because of the closed pore network mesh. Thus, in order to overcome these limitations, scaffolds having fibers of variable sizes (microfibers and nanofibers) were developed. Electrospun scaffolds were developed containing two differently scaled fibers (microfibers and nanofibers) composed of two discreet materials, specifically fibrin and PCL. The nanofibers were incorporated into the microfiber mesh such that they were evenly distributed throughout the entire construct. The presence of both nanofibers and microfibers improved the cellular proliferation and GAG deposition in comparison with scaffolds composed solely of microfibers [122]. This result was due to the larger pore sizes maintained by the combined microfibers and nanofibers and to the close resemblance of the nanofibers to the components of native ECM, thereby improving the cellular responses (differentiation and ECM production) [123]. Newer strategies are also under development to replicate the zone-specific organization and properties of cartilage. A trilaminar PCL-based scaffold was developed by sequential electrospinning in which the fiber size and orientation were varied in a continuous construct. The trilaminar composite scaffolds displayed mechanical properties similar to those of native cartilage tissue and supported in vitro cartilage formation [124]. The major limitations of fibrous meshes for cartilage regeneration are: ① the formation of 2D meshes via electrospinning, which leads to the flattening of chondrocytes and thus to the formation of fibrocartilaginous-like tissue; and② the finding that nanofibrous electrospun scaffolds have high stiffness, resulting in a mechanical mismatch between the engineered construct and the host tissue [125,126].
As collagen fibers are known to control the mechanical behavior of cartilage tissue, collagen fibers were woven in a 3D-aligned pattern to create macropores. MSC cell pellets were seeded within these macropores in order to replicate mesenchymal condensation-driven chondrogenesis. The scaffold framework supported enhanced chondrogenesis and mechanical robustness, and thus holds significant potential for cartilage regeneration [127].
Fibrous scaffolds have shown potential as cartilage ECM mimics; however, further structural refinement is needed to replicate the structure-function properties of native tissue.
《4.Minimally invasive cell therapy approach for osteoarthritis treatment》
4.Minimally invasive cell therapy approach for osteoarthritis treatment
Surgical therapies for cartilage regeneration suffer from major limitations such as significant post-operative infection and longer hospital stay. To overcome these limitations, researchers have started to explore less invasive treatments.
New insights have been gained into stem cell therapy for the treatment of OA, wherein stem cells are isolated from different tissue sources (e.g., bone marrow, adipose tissue, and synovial membrane), expanded in vitro, and transfused back into the patient. MSCs are an attractive cell source to support reparation following cartilage damage due to: the ease of expanding MSCs in vitro; multilineage potential; immunosuppressive properties; and pro-angiogenic, antifibrotic, anti-apoptotic, and wound-healing properties [128]. The immunosuppressive properties of MSCs facilitate down-regulation of the pro-inflammatory response, thereby promoting tissue repair [129].
Among various sources of MSCs, bone-marrow-derived MSCs (BMSCs) have been extensively studied as a cell source for cartilage regeneration. The intra-articular injection of BMSCs into rat joints with multiple tissue injuries, including injuries to the anterior cruciate ligament (ACL), medial meniscus, and cartilage of femoral condyles, showed mobilization of BMSCs into the injured site and tissue regeneration with ECM synthesis around the cells. In addition, the study reported that injecting a higher cell number (1 × 107 cells) resulted in more effective mobilization to the injured tissue compared with the injection of 1 × 106 MSCs. However, the injection of 1 × 107 cells led to the formation of scar tissue, which the authors of the study suggested to be an adverse effect of using a higher cell number and the cause of considerable dysfunction of the joints. Thus, it is important to optimize the cell number. In addition, the use of an appropriate delivery system may further aid the development of better strategies [130].
In another study, a single intra-articular dose of autologous BMSCs at a density of 2 × 106 cells, cultured either in chondrogenic media (CM) composed of a 1:1 mixture of Ham’s F12:Dulbecco’s Modified Eagle Medium (F12/DMEM, or FD) + 1% of fetal bovine serum (FBS) + 5 ng·mL–1 transforming growth factor-β3 (TGF-β3) + 50 ng·mL–1 of insulin-like growth factor-1 (IGF-1), or in basal media (BM) composed of FD + 10% FBS, was injected into an osteoarthritic sheep model. OA was induced in the sheep via total medial meniscectomy and resection of the ACL. Six weeks post-injection, cartilage destruction and OA progression were reduced in the osteoarthritic knee joints treated with CM and BM, as compared with the control group. The control group showed the highest lesion score of 3.44 ± 0.38 points, followed by the BM group, which showed a smaller lesion score of 1.22 ± 0.89 points. The CM group showed the lowest lesion score of 0.8 ± 0.35 points, with a shallow lesion and smooth edges. The authors of the study concluded that good cartilage histoarchitecture, thickness, and quality, along with meniscus repair, were observed in the CM group compared with the BM and control groups. These studies demonstrated the potential of BMSC injection for treating OA [131].
Based on promising preclinical studies, several clinical trials have recently been completed in order to understand the potential of intra-articular injection of BMSCs. Centeno et al. [132,133] performed direct injection of MSCs to knee joints; the MSCs were suspended in PBS and injected into the knee of patients suffering from degenerative OA. The patients were then given a second injection of 1 mL of 10 ng·mL–1 dexamethasone in the second week, as this is known to promote chondrogenesis when given in a small dose. Threeand six-month follow-ups with the patients showed increased volume of cartilage (up to 28.64%), with reduction in pain in OA patients. The limitation of this study is that only one patient was studied and no long-term effects were studied [132,133]. Another study by Centeno et al. [134] used a larger patient population and followed the efficacy of BMSC injections up to 10.6 ± 7.3 months. No sign of tumor formation or neoplastic complication was observed in the knee joint following BMSC injection [134]. The safety of intra-articular injection of BMSCs was further investigated by Davatchi et al. [135], who studied four patients with moderate to severe knee OA for up to six months with 2 × 107–2.4 × 107 BMSCs, and by Emadedin et al. [136], who studied six OA patients for one year with 8 × 106–9 × 106 BMSCs. Significant reduction in pain and improvement in walking ability were reported six months post-injection in patients. However, the pain reduction or improvement in walking ability decreased after six months, suggesting the need for a second injection [135,136]. A more promising outcome of intra-articular injection was observed by Orozco et al. [137], who used a higher cell number than was used in previous studies. In that study, researchers used 4 × 107 cells of autologous BMSCs in 12 patients with chronic knee pain who were unresponsive to conservative treatments. The clinical outcomes were followed for one year. It was found that pain was significantly reduced at all time points, with rapid and progressive increase in cartilage volume [137]. Soler et al. [138] performed a phase I to II clinical trial using a single-dose intra-articular injection of (4.09 ± 0.04) × 107 autologous BMSCs in 15 patients with knee OA. After eight days of infusion, there was a relative decrease in pain intensity, which was maintained even after 12 months. Even after 12 months, there was improvement in bodily function and physical functioning, with signs of cartilage regeneration [138]. These studies showed significant potential in the use of BMSC therapy to treat knee OA. However, the variability in the protocol and cell number used in these studies indicates that further optimization is required [139,140].
Unlike BMSCs, adipose-derived MSCs (ADSCs) obtained from the adipose tissue can be isolated using a less-invasive procedure and can be obtained in large amounts [141]. The intra-articular injection of ADSCs has been studied with encouraging outcomes in animal models of OA. Ter Huurne et al. [142] studied the antiinflammatory and chondroprotective effect of intra-articular injection of ADSCs in mice joints with collagenase-induced OA. ADSCs inhibited synovial lining thickening and cartilage destruction, and protected against joint destruction by both anabolic and catabolic mediators. A similar study was conducted using the intra-articular administration of 1 × 106 ADSCs in a rabbit OA model. The results showed that ADSC injection was highly tolerated by the animals; it produced no signs of immunological reaction and showed a reduction in the clinical signs of OA, possibly due to the anti-inflammatory characteristics of ADSCs and their low immunogenicity. Furthermore, intra-articular ADSC therapy has been shown to improve limb function in dogs with hip OA within a period of less than three months [142–144]. ADSCs have also been used for clinical studies as an alternative cell source. Koh et al. [145] injected 4.04 × 106 ADSCs in the knee of 30 patients. The ADSC injection was effective in healing cartilage, reducing pain, and improving function in elderly patients. The authors also concluded that ADSC injection was a simple and cost-effective method, as the cells can be harvested and re-injected on the same day, with no need for hospitalization. Jo et al. [146] conducted another study in which they administered different doses of ADSCs—a low dose (1 × 107 cells), middle dose (5 × 107), and high dose (1 × 108)—in 18 patients suffering from knee OA. The lowand middle-dose groups showed significant improvement in joint function and pain reduction, whereas the size of the cartilage defect increased in the low-dose group and decreased in the middleand high-dose groups. In addition, the high-dose group showed a thick hyaline-like cartilage covering the defect sites [146]. The promising outcomes of these studies demonstrated that intra-articular ADSC injection may serve as a potent and safe therapy for OA. However, the major limitation of the study was that cells were not characterized, and thus may contain adipocytes.
Synovial-derived MSCs (SDSCs) have also become a suitable choice of cell source because of the ease of harvest and strong potential for chondrogenic differentiation [147]. SDSCs can be extracted from the synovial membrane, which is harvested in a less-invasive manner with few complications at the donor site [148]. Although BMSCs and ADSCs have shown encouraging results for OA treatment, it may be more effective to use developmentally closer cells to the chondrocytes such as progenitor/stem cells. During the developmental process of synovial joints, cartilage and synovium originate from a common pool of cells, and the SDSCs derived from the synovium and synovial fluid possess cartilage-repair potential. The intra-articular injection of SDSCs in a pig model showed improved cartilage resurfacing, as evident from the intense staining of hyaline cartilage markers [149]. SDSCs isolated from two different strains of mice (MRL or C57BL6) have also been used for intra-articular injection in mice; both have been found to preserve cartilage proteoglycan levels, enhance cartilage repair, and protect from joint deterioration [150]. In another study, the effects of single or repetitive intra-articular injections of SDSCs were investigated in a rat OA model. OA was induced by transection of ACL, and 1 × 106 SDSCs were injected in the rat knee joint. The study showed that, although a single injection was ineffective, repetitive intra-articular injections of SDSCs had significant chondroprotective effects for 12 weeks. Periodic injections of SDSCs not only maintained cell viability without causing the loss of MSC properties, but also inhibited OA progression by the secretion of trophic factors. Upon injection, most SDSCs migrated to the synovium and did not survive in the longer term. The remaining few cells maintained their properties and produced proteoglycan 4 (PRG-4) and bone morphogenetic proteins (BMPs) for cartilage homeostasis and TNF-stimulated gene 6 protein (TSG-6) for anti-inflammation, thus contributing to chondroprotection and preventing the progression of OA [151]. These studies showed that exogenous delivery of SDSCs may be a viable treatment option for patients with OA.
Although these studies showed promising outcomes, as in the case of other cell types, several factors still need to be addressed in order to develop an optimal treatment strategy. These factors include: dose size, number of doses (single or multiple), and the use of an appropriate vehicle for cell delivery (if needed), so as to ensure that the cells engraft and populate the target tissues.
《5.Future perspective and challenges》
5.Future perspective and challenges
Knee OA is a chronic disease characterized by the slow degradation of cartilage, resulting in pain and disability in patients. This disease can have an adverse impact on the quality of a patient’s life. New therapeutic strategies using biomaterials and cells have shown significant potential in preclinical and clinical studies. The major limitation of the cell therapy approach is cell retention in the target tissue, which may be due to the rapid recycling of synovial fluid, resulting in movement of the cells to other parts of the body. In order to avoid this issue, advanced delivery systems need to be designed to help with longer cell retention and enhance the healing of the damaged tissue. Furthermore, the use of biomaterials has shown encouraging outcomes in treating OA. However, the structural and functional properties of these biomaterials can be refined further. In addition, understanding the pathophysiology of OA and the mechanism of action of these treatments would help us to improve the current technology. Convergence approaches proposed by regenerative engineering, which bring together the use of advanced materials science, stem cell science, physics, developmental biology, and clinical translation, have the potential to develop novel innovative strategies to address the limitations of current knee OA treatments.
《Acknowledgements》
Acknowledgements
Support from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, NIH DP1 AR068147, and from the Biomedical Trust Fund from the State of Connecticut is gratefully acknowledged.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jorge L. Escobar Ivirico, Maumita Bhattacharjee, Emmanuel Kuyinu, Lakshmi S. Nair, and Cato T. Laurencin declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




