《1.Introduction》
1.Introduction
Due to higher price levels of crude oil, Fischer-Tropsch synthesis (FTS), which converts coal, natural gas, and biomass to chemicals and liquid fuels, has attracted increasing attention in recent years as a way to diversify both the feedstock base for obtaining liquid transportation fuels and the monetization of natural gas and coal resources [1,2]. The principal reaction was discovered by Fischer and Tropsch [3] about 90 years ago. FTS reactions involve the strongly exothermic hydrogenation of carbon monoxide (CO) to paraffins and olefins according to the following chemical equations:

Aside from alkanes and alkenes, oxygenated hydrocarbons and carbon dioxide (CO2) are obtained as by-products in industrial FTS. The mechanism underlying the FTS reaction is complex and involves many steps such as CO dissociation, carbon (C) hydrogenation, CHx coupling reactions, and hydrogenation and dehydrogenation reactions that lead to hydrocarbon product desorption as well as oxygen (O) removal reactions [4]. There continues to be considerable debate about many of these steps, most notably the nature of the active site and the way CO is dissociated, which is closely linked to the question of which is the growth monomer in FTS. From a practical point of view, the main drivers for the improvement of FTS catalysts are higher activity, improved selectivity to targeted product classes such as long-chain hydrocarbons or light olefins, and improved lifetime.
In heterogeneous catalysis, it is well accepted that catalytic performance is governed by the electronic structure of the catalyst [5]. Tunable parameters are active phase composition, particle size, crystal structure, crystal morphology, and the formation of the interface between the transition metal nanoparticles and the support of the catalysts [6–11]. Typical transition metals for catalyzing the FTS reaction are ruthenium (Ru), cobalt (Co), and iron (Fe). All of these present high activity and selectivity toward liquid hydrocarbons in the lowtemperature FTS reaction [12]. Although Ru is usually deemed to be too expensive for this purpose despite its high activity, Co and Fe have been the main components of industrial FTS catalysts. Nickel (Ni)-based catalysts generally produce too much methane (CH4) under practical reaction conditions [13]. Here, we review recent developments in the field of Co-, Ru-, and Fe-based catalysts for the production of alkanes, alkenes, and alcohols via the FTS process. The focus of this review is on the influence of particle size and crystal phase, in which catalyst synthesis, modern characterization techniques, and density functional theory calculations are playing increasingly important roles. This review consists of three main parts: first, a discussion of the particle size effects of Co-, Ru-, and Febased catalysts in FTS; second, a description of the crystal structure effect of Co, Ru, Fe, and Ni metals in FTS; and finally, conclusions and a brief perspective.
《2.Particle size effect of FTS over Co-, Ru-, and Fe-based FTS catalysts》
2.Particle size effect of FTS over Co-, Ru-, and Fe-based FTS catalysts
A general approach to increase catalytic activity is to improve the active phase surface area by decreasing the size of the particles that make up the active phase [7,11,14]. Increasing the number of exposed surface sites works well for structure-insensitive reactions [11]. However, many catalytic reactions are structure sensitive, implying that the specific activity depends on the dispersion in a more complex manner [7,11,15–17]. This becomes particularly evident when metal nanoparticles become smaller than 10 nm, as specific surface sites such as corners, edges, and step edges then become dominant over terrace sites. As the FTS reaction is a well-known structuresensitive reaction, it is no wonder that many researchers have focused on resolving optimum particle size for high activity and selectivity. Given their importance in commercial operation, we limit our discussion of particle size effects to Co, Ru, and Fe nanoparticles.
《2.1. Co particle size effect in FTS》
2.1. Co particle size effect in FTS
Iglesia [18] showed that Co site-time yields are independent of Co particle size in the 10–210 nm range using aluminium oxide (Al2O3), silicon dioxide (SiO2), and titanium dioxide (TiO2) as supports. Bezemer et al. [15] contended that CO hydrogenation activity is insensitive when the (carbon-nanofiber-supported) Co nanoparticles are larger than 6 nm, but demonstrated that smaller Co nanoparticles (< 6 nm) have lower CO hydrogenation activity and higher CH4 selectivity (Fig. 1). Steady-state isotopic transient kinetic analysis (SSITKA) measurements suggested that the lower CO consumption rates of small Co nanoparticles can be attributed to blocking of the active edge/corner sites by CO [7]. Higher hydrogen coverage on smaller particles explains the higher CH4 selectivity that is observed for smaller Co nanoparticles. These trends have been confirmed by other groups for silica-supported Co catalysts [19,20]. An alternative interpretation offered is that smaller Co nanoparticles (< 2.5 nm) can be easily oxidized by water [19]. Different points of view on the effects of particle size may originate from different reaction conditions.
《Fig. 1》
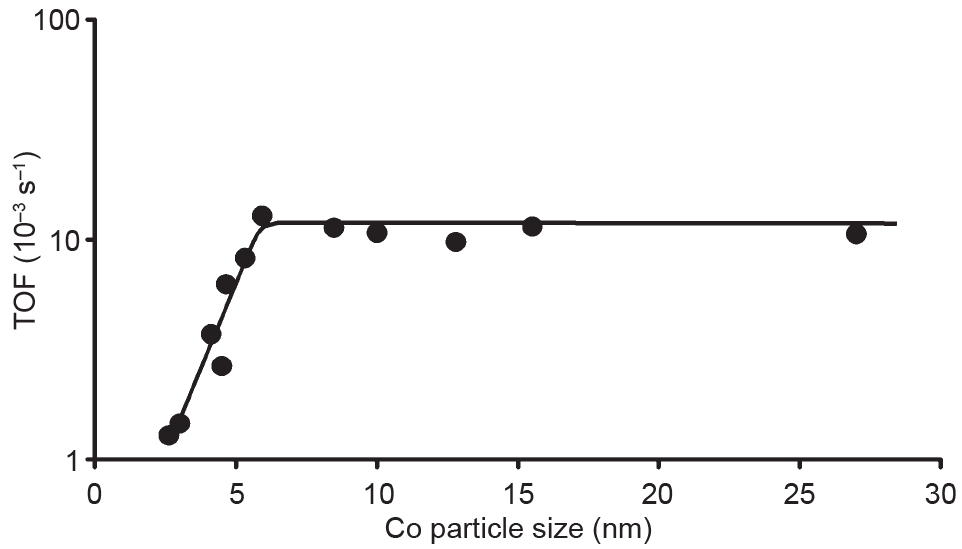
Fig. 1. Co particle size effect in the turnover frequency (TOF) of FTS (220 °C, H2/CO = 2, 1 bar (1 bar = 105 Pa)) [15].
Herranz et al. [21] showed that Co nanoparticles remain metallic under methanation conditions. While CO methanation activity (i.e., the turnover frequency, TOF) of Co/SiO2 catalysts decreases when particles become smaller than about 10 nm, the apparent activation energy for CO methanation is insensitive to Co particle size (Fig. 2). Hydrogen-deuterium exchange experiments indicate that the dissociation of H2 is difficult on small Co nanoparticles, a reason that is used to explain lower rates for smaller particles. Further research provided evidence that CO activation is facilitated by the presence of hydrogen on Co nanoparticles, and that the ability to dissociate hydrogen is the key parameter determining FTS activity [22]. However, Holmen et al. [23] provided an alternative view on the Co particle size effect in FTS. Their SSITKA experiments showed that the number of surface CHx intermediates increased with particle size, while the intrinsic activity remained constant when the Co particle size varied from 4 nm to 15 nm. They suggested that the site-based activity does not change in the 4–15 nm range, but that smaller particles have comparatively less active sites than larger ones.
《Fig. 2》

Fig. 2. CO methanation activity as a function of particle size. (a) TOF of CO hydrogenation at 240 °C; (b) activation energy (Ea) of CO hydrogenation varying with particle size [21]. 1 kcal = 4184 J.
Regarding FTS selectivity, quite small Co nanoparticles generally display increased CH4 selectivity. There is a volcano-like curve for C5+ selectivity that varies with the particle size of Co/γ-Al2O3 catalysts, and the maximum selectivity was found for 7–8 nm Co nanoparticles [24]. This finding was confirmed by Holmen’s [25] lateral work showing that the C5+ selectivity does indeed increase with Co particle size below 8–9 nm, and approaches a constant value for even larger Co particles. It is interesting that the lowest CH4 selectivity as well as the highest olefin-to-paraffin ratio were obtained at maximum C5+ selectivity. Recently, Melaet et al. [26] found that the FTS reaction exhibited obvious changes with Co particle size (3.2–11 nm) in the product distribution at 250 °C. Fig. 3 shows that CH4 selectivity increases with decreasing Co particle size, and is accompanied by a decrease of the C5+ selectivity. This finding indicates that larger Co nanoparticles can produce longer hydrocarbon chains. However, the question of why larger Co nanoparticles produce longer hydrocarbon chains remains an open one.
《Fig. 3》
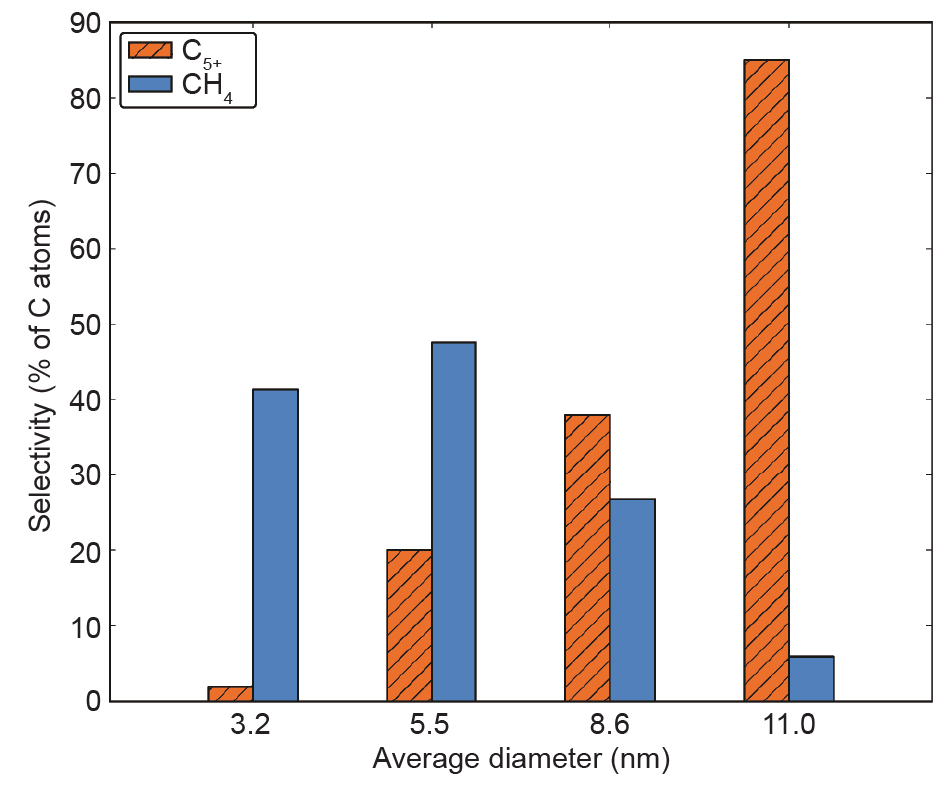
Fig. 3. Selectivities toward hydrocarbons (C5+ and CH4) as a function of Co particle sizes for the CO hydrogenation reaction (250 °C, H2/CO = 2, 5 bar). Both selectivities are calculated with respect to the total number of converted C atoms [26].
《2.2. Ru particle size effect in FTS》
2.2. Ru particle size effect in FTS
In the past, Dalla Betta et al. [27] and Iglesia et al. [28] reported that the intrinsic catalytic activity of a Ru-based catalyst for FTS reaction is not strongly dependent on the particle size. They proposed that the FTS reaction is structure insensitive. However, another two works reached the opposite conclusion—namely, that the specific activity is sensitive to Ru crystal size and metal dispersion [17,29]. The latter finding is supported by Kang’s work [30], which showed that the TOF and product selectivities of the FTS reaction are strongly dependent on the particle size of the Ru-based catalyst. Carbon nanotube-supported Ru-based catalysts with a mean Ru particle size of ~7 nm display the highest activity and selectivity toward longchain hydrocarbons. Xiao et al. [31] have developed Ru nanocluster catalysts that are used in aqueous-phase FTS. These Ru-based catalysts, which have a mean diameter of ~2 nm, presented extremely high activity under these anomalous conditions. Specifically, the FTS activity showed a dramatic increase when the particle size was reduced to 2 nm, with a smaller cluster exhibiting lower performance. Nevertheless, no clear explanation for Ru particle size effect has been advanced by these authors. However, our work showed that the small unsupported Ru nanoparticles have much lower FTS activity and exhibit an unprecedented oxygenate selectivity of 70% for aqueous-phase FTS under low-temperature conditions [32,33].
Carballo et al. [16] studied Ru/Al2O3 catalysts with different metal particle sizes and found that the TOF of the FTS reaction increases with Ru particle size, reaching a constant value for Ru nanoparticles larger than 10 nm; this is similar to what has been observed for Cobased catalysts, as shown in Fig. 4. Furthermore, the values of the rate constant of the FTS reaction do not vary with Ru particle size, which suggests that the particle size does not influence the intrinsic activity of the active sites on Ru-based catalysts. The authors proposed that the lower TOF value for small Ru nanoparticles (< 10 nm) might be related to strong adsorption of CO. A higher coverage of CO will therefore lead to a more difficult CO activation on smaller Ru nanoparticles. This Ru particle size effect is different than that found in Xiao’s work [31], in which the FTS reaction occurred in the aqueous phase.
《Fig. 4》

Fig. 4. CO consumption and CH4 formation rates as a function of Ru particle size (523 K; 5.5 kPa CO, 55 kPa H2, 124.5 kPa inert) [16].
The different conclusions in these works dealing with the effect of Ru particle size may originate from the use of different supports. Our recent work [33] on an aqueous-phase FTS reaction over Rubased catalysts shows that the TOF of FTS increases with particle size (1–5 nm), with a plateau between 2.3 nm and 3.7 nm. Small clusters will not have a step-edge site to dissociate CO, which reduces FTS activity. The chain growth parameter for hydrocarbon formation is not influenced when particles are larger than 2.5 nm.
《2.3.Fe particle size effect in FTS》
2.3.Fe particle size effect in FTS
Previous experimental works showed that FTS activity and selectivity are closely related to the particle size of Fe-based catalysts. For example, Mabaso et al. [34] mentioned that catalysts containing Fe nanoparticles smaller than 7–9 nm present lower TOF and higher CH4 selectivity than those containing larger particles. Olefin selectivity is not affected by particle size, whereas small Fe nanoparticles exhibit lower chain growth probability and higher CH4 selectivity. These findings were confirmed by Liu et al. [35], who showed that smaller Fe nanoparticles generate more short-chain hydrocarbons including CH4. Further investigation showed that the TOF increases when the Fe particle size increases from 2.4 nm to 6.2 nm, and then remains almost constant up to a particle size of 11.5 nm, as shown in Fig. 5 [36]. This set of Fe-based catalysts also presented an increase of CH4 selectivity with decreasing particle size.
《Fig. 5》

Fig. 5. The TOF of FTS as a function of Fe particle size with reaction temperatures of T = 280 °C and 300 °C [36].
Torres Galvis et al. [12] studied the influence of iron carbide particle size using carbon nanofibers as a support. These catalysts showed deceasing activity with increasing particle size from 2 nm to 7 nm. However, the particle size of the Fe-based catalyst had no effect on olefin or CH4 selectivity. In contrast to the unpromoted iron carbide catalysts, the Naand S-promoted catalysts showed quite different catalytic behavior. The TOF of CH4 and C2+ formation as a function of particle size is shown in Fig. 6 [12]. It was found that CH4 selectivity increases when the iron carbide particle size decreases from 7 nm to 2 nm. However, the selectivity of olefins is almost independent of iron carbide particle size. Smaller iron carbide particles display higher activity, mainly due to higher CH4 production. The authors proposed that the corners and edges sites are quite important for CH4 formation, while the terrace sites are feasible sites for olefins generation. A decrease of particle size results in more abundant step and edges sites, and ultimately in higher CH4 production [12].
《Fig. 6》

Fig. 6. Apparent TOF of CH4 and C2+ hydrocarbons varying with iron carbide size (TOS = 1 h). The reaction was performed at 340 °C, 20 bar with H2/CO = 1 on promoted catalysts [12]. TOS: time on-stream.
The results discussed above indicate that the catalytic activity of the FTS reaction is closely related to the particle size of Co-, Ru-, and Fe-based catalysts. Although the origin of the particle size effect is still controversial, the insights revealed are quite helpful for the rational design of highly efficient and stable catalysts with maximum mass-specific reactivity.
《3.Effect of metal particle crystal phase in Fischer-Tropsch synthesis》
3.Effect of metal particle crystal phase in Fischer-Tropsch synthesis
Aside from the particle size effect, it has been found that FTS activity and selectivity depend on the crystal phase of the catalysts [37–56]. Catalysts with different crystal structures generally have distinct morphologies and surface topologies, which expose different concentrations of active sites that can result in very different catalytic performances. Here, we focus on recent works for Co-, Ru-, Fe-, and Ni-based catalysts.
《3.1. Co crystal phase effect in FTS》
3.1. Co crystal phase effect in FTS
For Co, it is mostly assumed that metallic Co nanoparticles comprise the active phase in the FTS reaction. Bulk Co adopts a hexagonal close-packed (HCP) structure under ambient conditions. A temperature-induced phase transformation of HCP Co to the metastable face-centered cubic (FCC) Co occurs at 400 °C [57]. Such an HCP-to-FCC transformation has also been observed under much milder conditions when the Co particle size becomes very small. Specifically, HCP Co is the dominant phase when the particle size is above 40 nm, whereas the FCC lattice is adopted when Co nanoparticles are smaller than 20 nm [58]. Under FTS conditions, the actual crystal structure of metallic Co nanoparticles depends on the support type, the presence of promoters, and the particle size [58]. The pretreatment/activation of the catalyst will also affect this structure [59–61]. Such changes in crystal structure may play an important role in FTS activity and selectivity.
It has been reported by many groups that HCP Co exhibits higher FTS activity than FCC Co [37,44,45,50–52,62]. In particular, Ducreux et al. [45] reported that Co-based catalysts mainly consisting of HCP Co nanoparticles had higher FTS activity than catalysts containing predominantly FCC Co nanoparticles (Fig. 7(a)). Another work demonstrated that the FCC Co nanoparticle is less active than the HCP Co nanoparticle; the cobalt carbide (Co2C) nanoparticle is inactive for FTS (Fig. 7(b)) [37]. Recently, Davis et al. [52] found that Cobased catalysts containing the HCP phase display higher CO conversion rates than FCC Co nanoparticles in FTS. Compared with FCC Co nanoparticles, HCP Co nanoparticles exhibit a slightly higher olefin selectivity in the C2–C4 hydrocarbon range and a lower rate of CH4 formation. It is proposed that the higher conversion of CO on HCP Co nanoparticles is probably due to a higher density of surface defects and stacking faults [45,51].
《Fig. 7》

Fig. 7. (a) CO conversion as a function of time for a Co-Ru/SiO2 catalyst: (i) mixture of FCC-HCP structures (diamonds), (ii) FCC structure (squares), and (iii) HCP structure (triangles) [45]; (b) Co-site yields in FTS on FCC Co-, HCP Co-, and Co2C-based catalysts [37].
 ), (
), ( ), (
), ( ), and (
), and ( )—exhibit higher CO dissociation rates than the most active FCC (100) surface on the FCC Co nanoparticle. The HCP Co nanoparticle shows higher CO dissociation activity than the FCC Co nanoparticle because of the presence of more numerous and more active B5 sites. It was further established that the CO activation reaction pathways are different for HCP and FCC Co phases; that is, CO prefers a direct dissociation pathway on HCP Co phases, whereas an H-assisted route is important on FCC Co phases. Even considering the H-assisted CO activation pathway, the HCP Co phase displays higher activity than the FCC Co phase.
)—exhibit higher CO dissociation rates than the most active FCC (100) surface on the FCC Co nanoparticle. The HCP Co nanoparticle shows higher CO dissociation activity than the FCC Co nanoparticle because of the presence of more numerous and more active B5 sites. It was further established that the CO activation reaction pathways are different for HCP and FCC Co phases; that is, CO prefers a direct dissociation pathway on HCP Co phases, whereas an H-assisted route is important on FCC Co phases. Even considering the H-assisted CO activation pathway, the HCP Co phase displays higher activity than the FCC Co phase.
《Fig. 8》
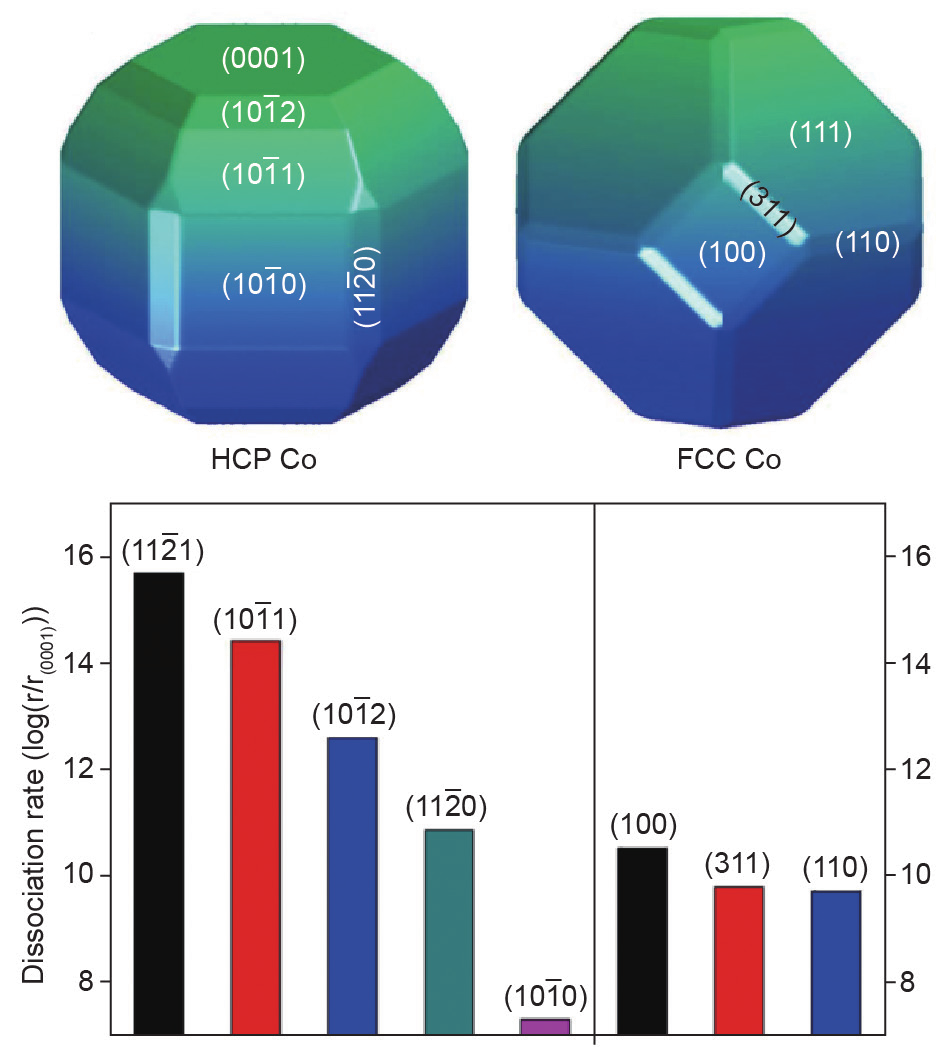
Fig. 8. Wulff shapes for HCP and FCC Co nanoparticles, and CO dissociation rates on the exposed HCP and FCC Co nanoparticle facets. All rates are normalized to that of HCP (0001) [39].
It is widely accepted that Co2C is inert in FTS [37]. However, Ding et al. [64,65] observed that aliphatic C1–C18 α-alcohols could be generated from syngas (CO + H2) by using Co-based catalysts supported on activated carbon. The formation of Co2C during the FTS reaction may be the key to obtaining higher alcohols [4,56,66,67]. The generation of higher alcohols appears to require the coexistence of Co and Co2C, possibly indicating that the interface between Co and Co2C is the active site for the FTS reaction. To test this hypothesis, researchers performed DFT calculations to clarify the active site and reaction mechanism for higher alcohol formation on Co-based catalysts (Fig. 9) [55]. The calculations suggested that CO can adsorb on Co2C, but that it does not dissociate at this location. The researchers therefore argued that this CO can result in a higher rate of CO insertion at the Co/Co2C interface, resulting in more aldehydes that are subsequently hydrogenated to alcohols. The reaction barrier for CO insertion into a CH2 intermediate at the Co/Co2C interface is relatively low at 0.77 eV. This insight opens the possibility of fine-tuning product composition.
《Fig. 9》
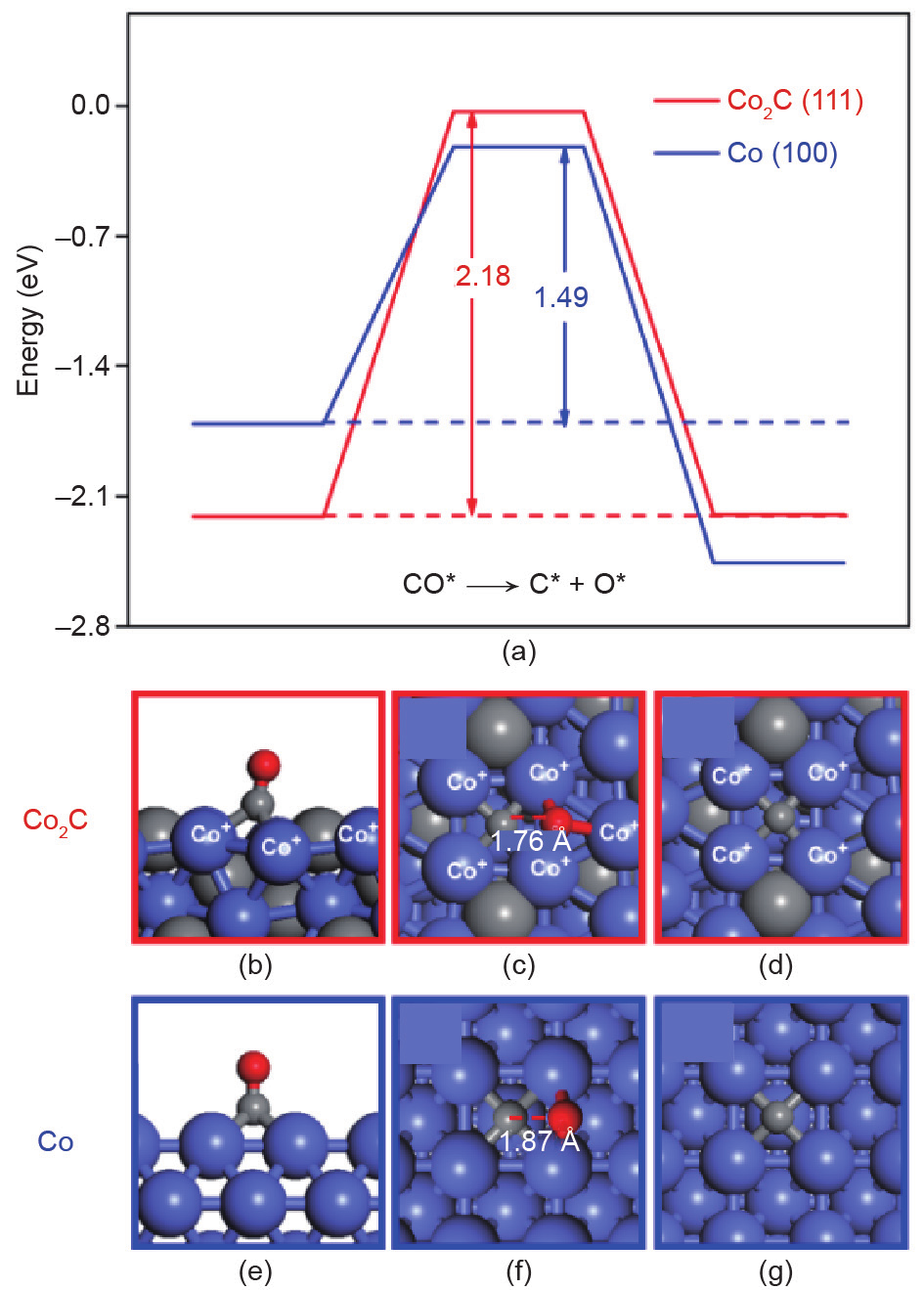
Fig. 9. The (a) potential energy surface and (b–g) geometric information of CO activation on Co2C (111) (red) and Co (100) (blue) surfaces [55].
Recently, Zhong et al. [53] synthesized manganese (Mn)-promoted Co2C nanoprisms exhibiting high FTS activity. Such Co2C catalysts generated much more light olefins (C2–C4 hydrocarbons) at relatively low CH4 selectivity under mild FTS reaction conditions (Fig. 10). Moreover, the Co2C nanoprisms showed excellent stability in the first 15 h. This result is particularly surprising, because conventional spherical Co2C nanocrystals exhibit extremely low activity for FTS. Transmission electron micrograph characterization and DFT calculations showed that the preferentially exposed (101) and (020) facets might present particular active sites for syngas conversion into olefins. This work provides a new way to design the next generation of highly efficient Fischer-Tropsch-to-olefins (FTO) catalysts.
《Fig. 10》

Fig. 10. Catalytic performance of the CoMn catalyst in the initial stages of the reaction. (a) CO conversion and product selectivity as a function of time on-stream; (b) ratio of olefin to paraffin as a function of time on-stream [53].
《3.2. Ru crystal phase effect in FTS》
3.2. Ru crystal phase effect in FTS
Ru nanoparticles are widely used in heterogeneous catalysis. There is a growing interest in the relationship between the crystal structure of Ru-based catalysts and their performance. Kusada et al. [40] have synthesized pure FCC Ru nanoparticle catalysts. It was found that CO oxidation activity was strongly dependent on the crystal structure and particle size of Ru-based catalysts. Above 3 nm, the traditional HCP Ru nanoparticles were less active than the FCC Ru nanoparticles. It was proposed that the higher activity of FCC Ru nanoparticles stems from the presence of abundant (111) facets that can easily be oxidized to RuO2 (110), which is more active than metallic Ru in CO oxidation. Later, Gu et al. [42] synthesized Ru@Pt core-shell catalysts that had an FCC structure and were largely enclosed by (111) facets. This Ru@Pt core-shell catalyst is more active for the hydrogen evolution reaction than conventional HCP Ru catalysts [42]. Higher activity of FCC Ru nanoparticles than HCP Ru nanoparticles was also observed in other reactions, such as the conversion of ammonia-borane [48,68], the oxygen evolution reaction [69], hydrogenation reactions [70], and dinitrogen activation [71]. Thus, the question of how the FCC and HCP crystal structures of Rubased catalysts impact the activity and selectivity of FTS remains an interesting and timely topic.
Li et al. [72] recently studied FTS on Ru-based catalysts with FCC and HCP structures by a combination of DFT calculations, modern materials synthesis, and scanning transmission electron microscope (STEM). DFT calculations showed that there are many open facets with modest CO dissociation barriers available on FCC Ru nanoparticle catalysts (Fig. 11), but only a few step edges with a lower barrier on HCP Ru nanoparticle catalysts. Based on these theoretical results, FCC Pt@Ru core-shell nanocrystal catalysts with high density of the active sites (open facets) were synthesized. The synthesized FCC Ru nanoparticle catalysts showed extraordinarily high specific activity in an aqueous-phase FTS process in the low temperature range of 393–433 K. The activity of FTS was as high as 37.8 molCO∙molru-1 ru ∙h-1 at 433 K, making this by far the most active FTS catalyst working at low temperatures (< 473 K). The origin of the high activity of FCC Ru nanoparticles stems from the higher active site density in comparison with HCP Ru nanoparticles. It is worth noting that the catalytic behavior trend between the HCP and FCC phase is dependent on the transition metals. For example, the FCC Co nanoparticle is less active than the HCP Co nanoparticle, whereas the Ru nanoparticle shows the opposite trend. The kinetic differences between Ru and Co nanoparticles in FTS mainly stem from these structural and electronic differences, which arise from the slightly larger lattice constant of the Ru nanoparticle.
《Fig. 11》

Fig. 11. Scheme for CO activation barriers on the facets of FCC Ru nanoparticle: Wulff construction and STEM image and modeled shapes of the synthesized Pt@Ru nanoparticles [72].
《3.3. Iron and iron carbides crystal phase effect in FTS》
3.3. Iron and iron carbides crystal phase effect in FTS
It is very difficult to synthesize pure iron carbide phases. The evolution of the active phase of Fe-based catalysts is very complicated, resulting in considerable debate on the nature of the active phase and on the FTS reaction mechanism for Fe-based catalysts. Understanding the phase distribution, phase evolution, and activity of different phases will accelerate the design of optimal Fe-based FTS catalysts. Different iron carbides, including ε-Fe2C, ε′-Fe2.2C, Fe7C3, χ-Fe5C2, and θ-Fe3C, have been observed under FTS reaction conditions. Iron carbide phase transformation (ε–χ–θ phase transformation) will occur depending on the temperature and the H2/CO ratio, as shown in Fig. 12 [73]. Specifically, the high temperature and low chemical po-tential of carbon (µC) (high H2/CO ratio) generally result in preferential formation of θ-Fe3C. In contrast, high µC (low H2/CO ratio) and moderate temperature (~250 °C) lead to the formation of χ-Fe5C2. ε-carbides are preferentially formed at lower temperatures and even higher µC.
《Fig. 12》
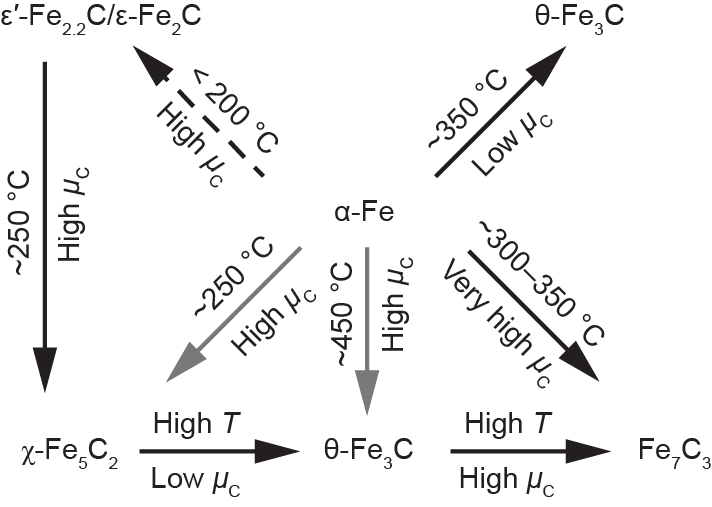
Fig. 12. Iron carbide phases varying with the temperature (T) and chemical potential of carbon [73].
Identification of the active phase of iron carbides for FTS remains a great challenge in heterogeneous catalysis. In fact, metallic iron [74,75] and various iron carbide phases have been claimed to be active for the FTS reaction [76–80]. De Smit et al. [73] reported that Febased catalysts mainly consist of χ-Fe5C2, which is active but prone to oxidation. On the other hand, θ-Fe3C catalysts showed a lower activity and selectivity, which might be due to the buildup of carbonaceous deposits on the catalyst surface. This finding was confirmed by Fierro’s experimental work showing that χ-Fe5C2 is more active than θ-Fe3C catalysts for FTS [81]. Later, Yang et al. [82] first reported a facile wet chemical route for the synthesis of pure χ-Fe5C2 nanoparticles. These χ-Fe5C2 nanoparticle catalysts possess higher activity and selectivity than the conventional reduced-hematite catalysts.
Considering the temperature, pressure, and H2/CO ratio effects, Zhao et al. [83] studied the surface structure and stability of various iron carbide phases (ε-Fe2C, χ-Fe5C2, θ-Fe3C, and Fe4C) by DFT calculations (Fig. 13). Similar to De Smit’s results [73], it was found that higher carbon-content gas always results in a higher carburization degree, which will be suppressed at higher temperature and lower pressure, and under higher H2/CO ratio conditions. ε-Fe2C, χ-Fe5C2, and θ-Fe3C carbides result in different Wulff shapes. In general, the morphologies of iron carbides vary dramatically with the environment (H2/CO ratio and temperature). By increasing the chemical potential of carbon, the most stable termination of the iron carbide surface changes to a carbon-rich surface with a high surface Fe/C ratio. Extensive CO activation has been studied on the obtained stable surfaces. CO adsorption and CH4 formation is most feasible on χ-Fe5C2-(100)-2.25 termination, followed by θ-Fe3C-(010)-2.33, ε-Fe2C-(121)-2.00, and Fe4C-(100)-3.00 termination, the latter of which has the lowest activity. The authors proposed that the different catalytic activities among different iron carbide phases may originate from the surface work function and charge state of the surface atoms [83]. Therefore, χ-Fe5C2 might be the most active phase for CO hydrogenation to CH4.
《Fig. 13》
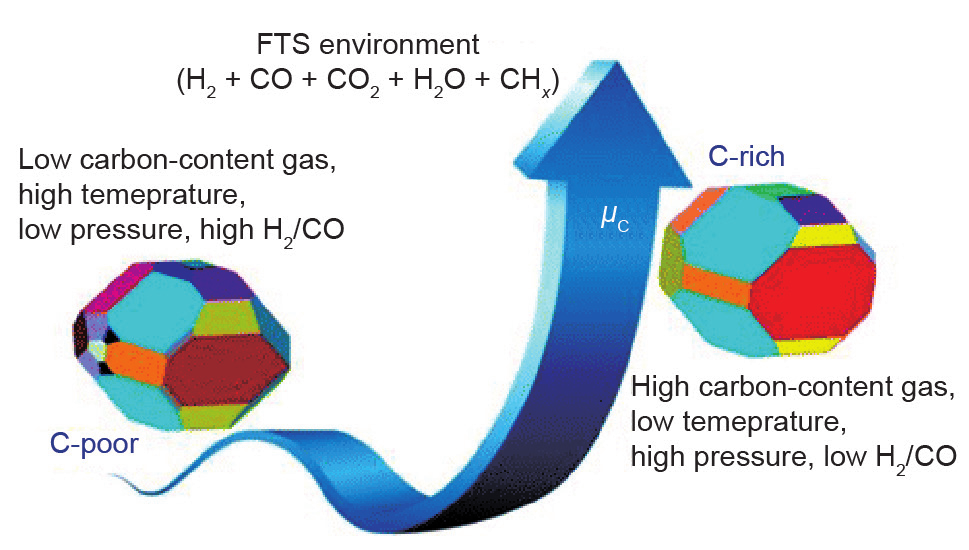
Fig. 13. The morphology of an Fe-based FTS catalyst varying under different reaction conditions [83].
Recently, Yang et al. [84] reported a ceria-supported subnanometer iron oxide cluster catalyst displaying high FTS activity. Using STEM and X-ray absorption fine structure (XAFS) characterizations, it was confirmed that the iron oxide clusters (Fe-Ox-Fey) containing partially reduced Fed+ (d = 2.6-2.9) species in ceria nanorods are the active phase for FTS. Interestingly, these researchers did not observe any iron carbides for this catalyst during the FTS reaction. Their work indicates that the FTS activity is strongly related to the local coordination of the active site, and that iron carbides are not always the demanding phase for FTS. Although many studies have been performed, more experimental and theoretical work needs to be done to clarify the true active phase of Fe-based catalysts for FTS under realistic conditions.
《3.4. Ni crystal phase effect in FTS》
3.4. Ni crystal phase effect in FTS
Nickel is not a good FTS catalyst because of its poor ability to dissociate CO, such that its use results predominantly in CH4, and because of its instability, which is due to the formation of volatile carbonyls under practical FTS reaction conditions [85]. However, Ni can be used as an efficient catalyst for CH4 production and as a promoter for FTS [86]. Bulk Ni adopts an FCC structure under ambient conditions. However, FCC Ni can transform into HCP Ni when the Ni particle size decreases to 4 nm [87]. In fact, HCP Ni nanoparticles have already been synthesized via a one-pot chemical route [87] and via other chemical methods [88–95]. It was found that HCP Ni nanoparticles have higher activity than FCC Ni nanoparticles in the aqueous-phase reforming of glycerol. Furthermore, HCP Ni nanoparticles can present higher H2 selectivity and hinder CH4 formation, compared with FCC Ni nanoparticles [96].
CO activation is the first crucial step in the CO methanation reaction. It is suggested that CO activation is closely related to the surface structures of Ni nanoparticles, and that under-coordinated step/ edge sites are the active sites for CO dissociation [97,98]. CO activation via a COH intermediate is proposed to be the rate-determining step during CO methanation [99,100]. Liu et al. [54] performed DFT calculations to study the influence of the Ni crystal phase on CO activation (Fig. 14). Their computational analysis indicated that CO dissociation is strongly dependent on the crystal structure and morphology of Ni catalysts. It is important to note that CO dissociation assisted by hydrogen is kinetically favored over a direct dissociation pathway, irrespective of the crystal phases. As seen in Fig. 14, (311) and ( ) are the most active facets for FCC Ni and HCP Ni, respectively. CO dissociation prefers the COH intermediate on the most active surface of FCC and HCP Ni catalysts. FCC Ni is more active than HCP Ni because FCC Ni can expose abundant facets with low activation barriers. The distinct behaviors of different crystal phases (FCC versus HCP) revealed here are valuable for catalyst optimization to expose abundant active sites for activating diatomic molecules.
) are the most active facets for FCC Ni and HCP Ni, respectively. CO dissociation prefers the COH intermediate on the most active surface of FCC and HCP Ni catalysts. FCC Ni is more active than HCP Ni because FCC Ni can expose abundant facets with low activation barriers. The distinct behaviors of different crystal phases (FCC versus HCP) revealed here are valuable for catalyst optimization to expose abundant active sites for activating diatomic molecules.
《Fig. 14》

Fig. 14. CO activation on FCC and HCP Ni morphologies, obtained by Wulff construction [54].
《4.Conclusions and perspective》
4.Conclusions and perspective
The identification of the relationship between structures and the catalytic activity of the FTS reaction will accelerate optimal catalyst design. In this paper, we summarized recent progress on the dependence of the FTS performance on particle size and crystal phase effects. FTS activity and selectivity strongly depend on the particle size of the catalysts. However, the origin of the particle size in FTS remains controversial due to the high complexity of the FTS reaction mechanism and to limitations of in situ characterizations. Catalysts with different crystal phases and structures have different morphologies; the resulting different electronic states result in substantial activity and selectivity variations. It is more necessary than ever to develop modern characterization techniques and advanced material synthesis methodologies, and to combine these with DFT calculations applied to model and technical catalyst systems, in order to identify active sites and explore novel concepts that can dramatically improve the performance of metallic catalysts in the hydrogenation of CO to chemicals and fuels.
《Acknowledgements》
Acknowledgements
We acknowledge financial support by NWO-VICI and NWO-TOP grants awarded to Emiel J. M. Hensen.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jin-Xun Liu, Peng Wang, Wayne Xu, and Emiel J. M. Hensen declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




