《1.Introduction》
1.Introduction
Cyclic redox reactions of metal oxides are the key to many energy-conversion systems such as photocatalysis and chemical looping [1]. Consequently,improvements in the redox reactivity of metal oxides, made possible by a deep understanding of the associated reaction mechanisms,are critical in energy-related fields. Chemical looping technology has been demonstrated to be one of the most promising and versatile technologies in the clean energy industry. In a chemical looping oxygen uncoupling (CLOU) system, oxygen gas molecules are released through the creation of an oxygen vacancy(VO):

where 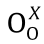 denotes the lattice oxygen of metal oxide X.
denotes the lattice oxygen of metal oxide X.
During a regeneration step, the oxygen vacancy is annihilated through the adsorption, dissociation,and diffusion of molecular oxygen(O2):

Active oxygen carriers for CLOU applications include transition metal oxides such as copper/ manganese-based oxides [2] and modified perovskite materials [3,4]. In a chemical looping combus-tion(CLC), chemical looping gasification (CLG), or chemical looping reforming (CLR) system, oxygen carriers react with solid or gaseous fuels by providing lattice oxygen in a reducer and are regenerated by air in an oxidizer where heat is released, thus allowing for auto-thermal operation.Unlike in the CLOU system,lattice oxygen directly reacts with hydrocarbon intermediates in the CLC, CLG, or CLR system without gaseous oxygen being involved in the reducer. According to the carbonaceous feedstock reaction conditions in plete/ full oxidation and partial/ selective oxidation. Complete/full oxidation represents typical CLC applications,whereas partial/ selective oxidation represents CLG or CLR applications. Chemical looping reactions can also be applied to systems without fossil feedstock, such as solar or nuclear systems. Fig. 1 shows four typical chemical looping systems, in which Fig.1 (a) and( b) show the systems with iron (III) oxide (Fe2O3) as the oxygen carrier and methane (CH4) as the carbonaceous feedstock.
《Fig.1》

Fig.1.Classification of chemical looping systems. (a)Complete/full oxidation system; (b)partial oxidation system;(c)selective oxidation system; (d)solar/nuclear system.
Major efforts have been made toward the conversion of various carbonaceous fuels including coal, methane,and biomass to carbon monoxide and hydrogen with improved utilization efficiency based on the chemical looping concept [5]. Nevertheless, the design and improvement of cost-effective, environmentally friendly, highly reactive,and recyclable oxygen carrier materials remain a challenging topic due to the complex reaction mechanism in chemical looping redox reactions. Extensive research has been conducted into the development of optimum oxygen carrier materials. During the early stages of development, these studies focused on single-component materials.More recently, the development of oxygen carriers has been directed toward using multiple metal-based composite materials for performance enhancement, such as binary metals or bimetallic oxygen carriers [6]. Different with the conversion reactions of carbonaceous fuels on solid catalysts which have stable reaction structures, the cyclic chemical looping reactions are accompanied by a tremendous change in the morphology of solid oxygen carriers due to the ionic diffusion and oxygen vacancy formation both in the bulk and on the surface of the oxygen carriers. With the development of advanced experimental techniques, computation,and simulation, the field of chemical looping has attracted increasing attention in the past decade. In this article, we focus on new insight into the development of oxygen carriers for chemical looping systems based on the latest theoretical and experimental studies.
《2.Ionic diffusion with morphological evolution》
2.Ionic diffusion with morphological evolution
The morphological evolution of oxygen carriers plays a significant role in chemical looping processes because it determines the reaction surface area and structural stability. During redox reac-tions,the morphology evolves with the ionic diffusion in the solid phase. Therefore, an understanding of ionic diffusion is crucial in the development of oxygen carriers.
To determine the ionic diffusion mechanism macroscopically,inert marker experiments have been performed on many oxygen carrier materials.Li et al.[7] studied the effect of titanium dioxide (TiO2) support in Fe2O3 during oxidation, and found that the dominating ionic-transfer mechanism changes from the outward diffusion of iron(Fe)ions to the inward diffusion of oxygen(O)ions. Naturally occurring ilmenite (FeTiO3) shows a similar inward diffusion pattern for lattice oxygen in the bulk. Day and Frisch [8] used chromium chromate as an inert marker to study the copper(Cu) oxidation process and found that the diffusion mechanism is the outward diffusion of Cuions. In general, the dominating diffusing species during the oxidation of oxygen carriers are metal cations. Once the ionic diffusion mode of a volume-expansion or volume-constant reaction is determined, the morphological evolution induced by oxidation can be deduced. The molar volumes for typical oxygen carrier materials are listed in Table 1. The oxidation reaction involving outward ionic diffusion often smoothens concave structures on the particle surfaces [9]. On the other hand, the convex surface is smoothened via a lower outward diffusion flux in the vertical direction.Ionic diffusion in the solid phase with molar volume changes can affect the available surface area during the oxidation reaction. Similar phenomena have been observed during reduction reactions. Hence, these factors should be considered when investigating the morphological evolution of oxygen carriers during redox reactions.
《Table 1》
Table 1 Molar volume information for typical oxygen carrier materials at different oxidation states.

Molar volume reported in the literature varies from study to study, based on different experimental and theoretical methods.
The morphological evolution of oxygen carriers,induced by solid-state ionic diffusion, was also studied on the microscopic level. Qin et al.[10] investigated the oxidation of Fe particles in randomly distributed pores with no visible grain boundaries and found that oxidation at 700°C results in a mean volume expansion of about 25% and the formation of a porous center arising from the outward diffusion of the Fe ions and inward diffusion of the O ions. Because the outward diffusivity of Fe ions is higher than the inward diffusivity of O ions,there is net Fe transport from the core of the Fe particle to the particle surface, where Fe2O3 forms.This outward diffusion of Fe ions is further improved because the volume xpansion of Fe particle during oxidation creates more physical space for Fe ion transport. The diffusion also is accompanied by the formations of nanowires and nanopores due to the curvature-driven grain growth (Fig. 2)[10].
《Fig.2》
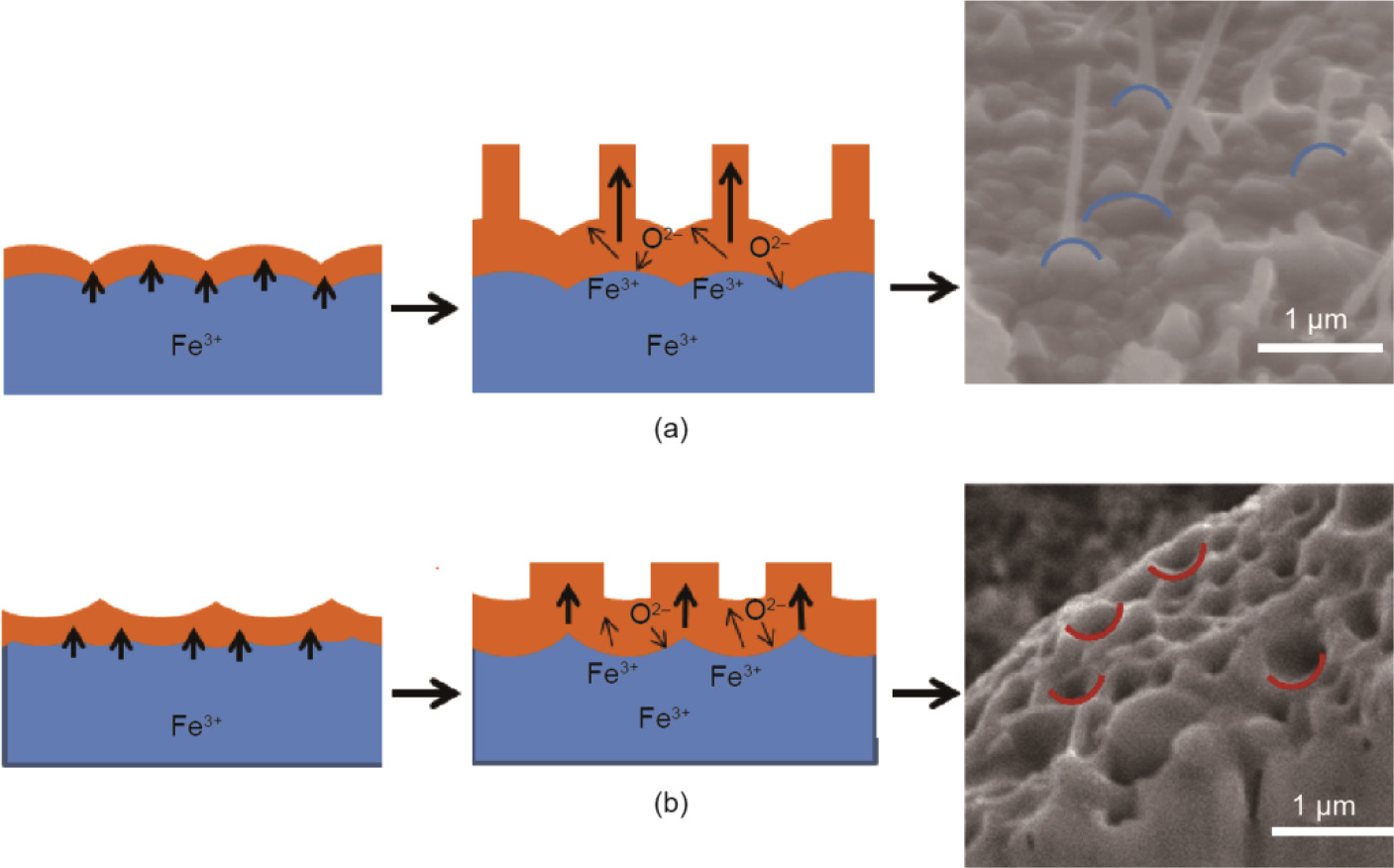
Fig.2.Formations of (a)Fe2O3 nanowires and (b)nanopores. (Reproduced from Ref.[10] with permission from the Royal Society of Chemistry)
However, for the bimetallic oxygen carrier Fe–Ti binary system, the morphological evolution is different than that of the Fe microparticle due to the different diffusion behavior of the tita-nium (Ti)ion and the Fe ion. At an oxidation temperature of 700°C, Fe and Ti are oxidized to FeTiO3 with the formation of nanobelts[11]. These nanobelts, which have widths of 150–200 nm and width-to-thickness ratios of 5–10, protrude from the surface of the FeTiO3 oxygen carrier and mainly contain Fe and O [12–14]. Compared with Fe2O3 microparticles, FeTiO3 microparticles not only exhibit higher porosity after multiple redox cycles, but also show higher stability which has been confirmed through the reactivity study of over 1000 redox cycles[15]. It indicates that the addition of Ti support can enhance the reactivity and recyclability of iron based oxygen carriers.
The application of density functional theory(DFT)calculations to study the ionic diffusion of oxygen carriers during chemical looping processes began in the late 2000s[13,16]. The methodology is to create possible interstitial sites for the ions of interest to diffuse through based on ground state energy searching,then to identify the most favorable diffusion path-ways by energy barrier calculations.Recently, the diffusion paths of the Fe and Ti ions in oxygen carrier FeTiO3 were investigated by combining first-principles microkinetics and ab initio thermodynamics. It was found that the diffusion barrier of Fe ions was much lower than that of Ti ions. Therefore, the out-ward diffusion of Fe ions is more favorable than that of Ti ions, which explains the formation of Fe2O3 nanobelts during the oxi-dation process[11].
《3.Oxygen vacancy of oxygen carriers》
3.Oxygen vacancy of oxygen carriers
During reduction in chemical looping processes,the oxygen carrier donates lattice O atoms for the full oxidation, partial oxidation, or selective oxidation of carbonaceous fuels,leading to the formation of oxygen vacancies.Oxygen vacancies in the bulk or on the surface of metal oxide oxygen carriers may greatly affect their morphology,electronic structure, and chemical properties. An efficient oxygen carrier should allow for sufficient transportation of lattice O atoms from the bulk toward the surface. Thus, an effective oxygen carrier needs to have appropriate oxygen vacancy formation energy so that the required amount of lattice O atoms can release for the oxidation of carbonaceous fuels. Due to the limit of spectroscopic techniques ,many computational studies on oxy-gen vacancies of metal oxides have been performed.
Ceria (CeO2) has a fluorite structure, which is favorable for the diffusion of lattice O atoms and oxygen vacancy formation.Yang et al. [17] investigated the structural modifications induced by the oxygen vacancy formation on CeO2 using the spinpolarized PW91 functional, and found that the structural modifications followed a pattern in which cerium (Ce) atoms near to vacancy sites migrated away from the defect sites, while the next-nearest vacancy sites of the lattice O atoms contracted in the region. Vanadium oxide (V2O5), which was considered to be most effective oxygen carrier for the selective oxidation of CH4 to formaldehyde (HCHO), was widely studied recently [18]. Sauer and Dobler [19] investigated the oxygen vacancies on V2O5 surface using the 2L cluster model and the B3LYP functional,and reported an oxygen vacancy formation energy of 1.17 eV. This indicated that the lattice O atoms on V2O5 surface are easy to release due to the low oxygen vacancy formation energy. In addition to ceria and vanadium oxide, iron oxide is an important oxygen carrier for CH4 CLR, in which it converts CH4 to syngas via partial oxidation. In this process,the adsorbed CHx radicals on the surface bind to lattice O atoms transferred from the bulk of the iron oxide,resulting in the formation of oxygen vacancies. The CH4 partial oxidation reaction on the iron oxide oxygen carrier is a complex process due to the stepwise reduction: Fe2O3→Fe3O4→FeO→Fe. Fan et al. [20,21] investigated the characteristics of the multiple oxidation states of iron, and developed a countercurrent moving bed reactor system for their application in chemical looping system. Fe2O3, Fe3O4, and FeO have similar close-packed oxygen structures, thus the redox transitions between each phase are faster than that between FeO and Fe [22,23]. Cheng et al.[24] studied oxygen vacancies in these oxidation phases using the atomistic thermody -namics appraoch and DFT calculations,and found that the formation energies of oxygen vacancies on the surface are lower than that of oxygen vacancies in the subsurface (Table 2 and Fig.3), which significantly affects the phase transition of iron oxide oxygen carriers.
《Fig.3》
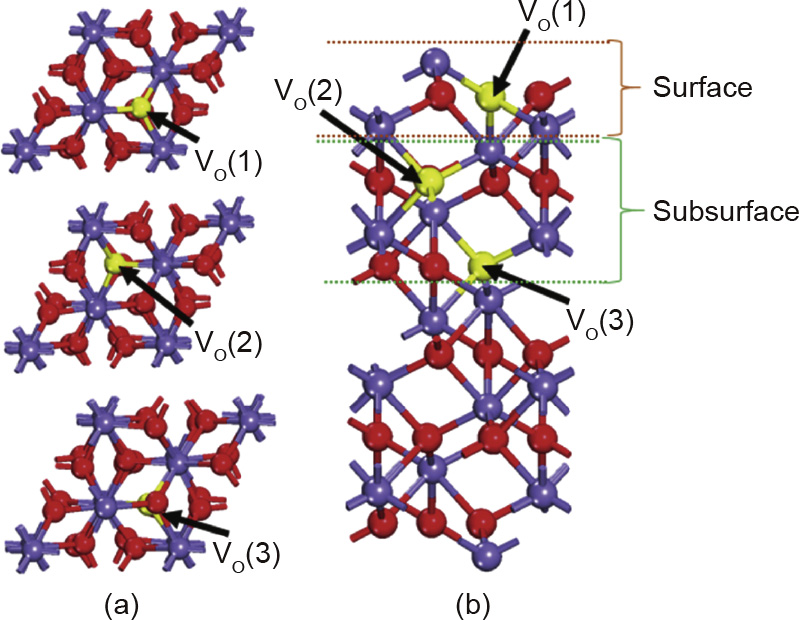
Fig.3.(a)Top view and(b)sider view of a-Fe2O3(001) surface model with oxygen vacancies on the surface and in the subsurface. Purple balls denote Fe atoms,red balls denote lattice O atoms, and yellow balls denote oxygen vacancies. (Reproduced from Ref. [24] with permission from the PCCP Owner Societies)
《Table 2》
Table 2 Formation energy of oxygen vacancies VO(1), VO(2), and VO(3)in a-Fe2O3(001)surface[24].

Ef:formation energy; dmin: the minimal length of Fe–O bonds; dmax:the maximal length of Fe–O bonds.
Without oxygen vacancies,the oxidation process will result in the formation of an iron oxide product layer outside of the original surface, since the diffusion energy barrier for the inward diffusion of O ions is larger than the barrier for the outward diffusion of Fe ions. However, with oxygen vacancies, the diffusion barrier for the inward diffusion of O ions is lower than the barrier for the out-ward diffusion of Fe ions; thus, the formation of the iron oxide product layer occurs at the Fe2O3Fe interface (Fig. 4).
《Fig.4》

Fig.4.Oxygen vacancy effect on the ionic diffusion mechanism with diffusion activation energies obtained from DFT calculations. VO and Vm represent the oxygen vacancy and metal cation vacancy, respectively; In and Im represent the initial state and the intermediate state, respectively.
Cheng et al.[24] also found that TiO2 support can decrease the formation energy of oxygen vacancies in the iron oxide, thus facilitating oxygen vacancy formation and the transportation of lattice O atoms [11]. Therefore, oxygen vacancy formation in the FeTiO3 phase during reduction is substantially easier than in Fe2O3, which explains the superior recyclability of Fe–Ti binary oxygen carrier particles over iron oxide oxygen carrier particles[25].
《4.Surface reaction on oxygen carriers》
4.Surface reaction on oxygen carriers
As discussed in the previous sections,the oxygen carriers donate lattice O atoms for the conversion of carbonaceous fuels in chemical looping processes. However, the conversion may proceed via full oxidation, partial oxidation, or selective oxidation. Therefore, a comprehensive understanding on chemical looping redox reaction mechanism is extremely important.
Syngas is a crucial intermediate resource for production of ammonia,methanol,and synthetic hydrocarbon fuels and commonly produced from the partial oxidation of carbonaceous fuels such as methane[26]. A great number of oxygen carriers have been studied for chemical looping partial oxidation applications, including the transition metal oxides of manganese(Mn), Fe, cobalt(Co), nickel(Ni), and Cu[25,27–29]. Iron oxide has been widely studied and applied as chemical looping oxygen carrier because it possesses high oxygen capacity meanwhile it is a low-cost environmentally-friendly material. In addition, using iron oxide oxygen carrier, the syngas selectivity can reach over 90% by chemical looping partial oxidation of methane[30]. Jin et al.[31] studied the reduction of iron oxide with methane under different reaction conditions using thermogravimetric analysis/ mass spectrometry(TGA-MS), and found that the ventilation air is unfavorable for the release of lattice O atoms. Recently, Cheng et al. [32] investigated the role of oxygen vacancies of iron oxide on chemical looping partial oxidation of methane using DFT+U calculations and TGA. They found that O ions in the subsurface prefer to diffuse out-ward into the vacancy sites on the surface. More importantly, they found that oxygen vacancies can facilitate methane partial oxidation by reducing the activation barrier of the C–H bond in CHx radicals. The proposed mechanism for the complete and partial oxidation of methane is shown in Fig.5 [33]. As the figure shows, carbon dioxide (CO2) and water (H2O) molecules form during the initial stage of methane oxidation because CH4 molecules prefer to adsorb on surface Fe sites and then proceed via a complete oxidation pathway when the oxygen vacancy concentration is very low. Monazam et al.[33] applied TGA to investigate methane oxidation on Fe2O3 over the range of 700–825°C, and demonstrated that most of the CH4 molecules were completely oxidized to CO2 and H2O in the early stage of the reduction period; CO and H2 molecules were then generated via the partial oxidization path way. This finding was in good agreement with the proposed mechanism depicted in Fig. 5.
《Fig.5》
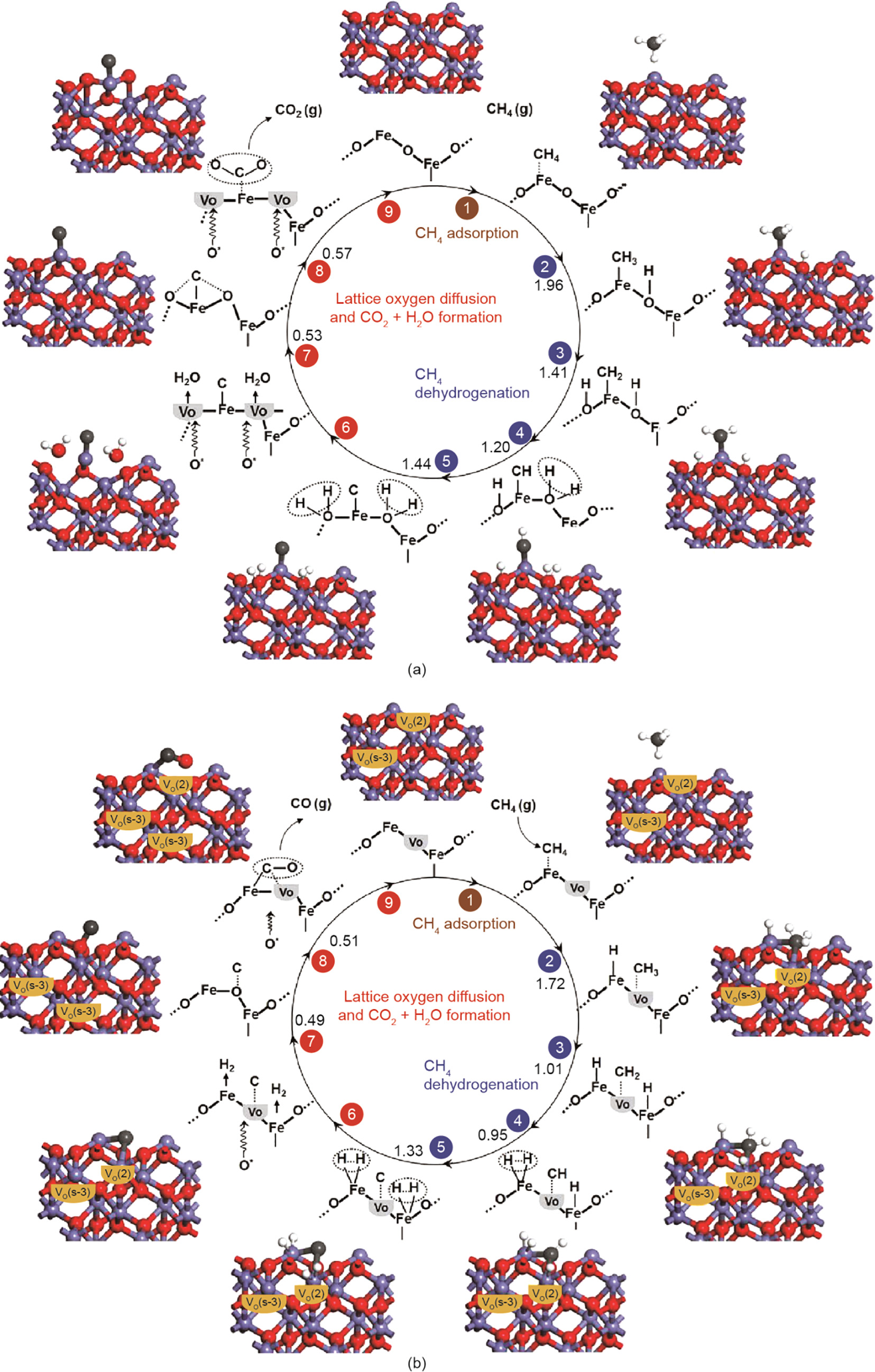
Fig.5.(a)Complete oxidation and (b)partial oxidation mechanism of methane on iron oxide oxygen carriers. The activation barrier for each elementary step is given in eV units. VO(s-3)represents the oxygen vacancy in the subsurface, VO(2)represents the oxygen vacancies on the surface, and O*represents the lattice oxygen which migrates into the oxygen vacancy. (Reproduced from Ref.[33] with permission from the PCCP Owner Societies)
Oxygen carrier materials for the oxidative coupling of methane (OCM) have also been extensively studied for their physiochemical properties, especially in terms of structural defects such as oxygen vacancies. The oxygen vacancies of OCM oxygen carrier materials play a vital role in facilitating methane activation from charged oxygen species[34]. Studies have shown that there is a correlation between electrical conductivity and C2 yields. OCM oxygen carriers with p-type and O ion conductivity within the band gap of 5–6 eV are reported to be the best-performing OCM materials[35]. In addition, methane conversion and C2 selectivity are important factors for the selection of OCM materials. Many types of oxides have been tested as OCM oxygen carriers,including but not limited to lead(II) oxide(PbO), tin dioxide (SnO2), gallium(III)oxide (Ga2O3), germanium dioxide (GeO2), indium(III) oxide (In2O3), zinc oxide (ZnO), cadmium oxide (CdO), calcium oxide (CaO), magnesium oxide (MgO), and aluminum oxide (Al2O3) (Table 3) [36]. A general conclusion obtained from the material screening is that rare earth metal oxides, particularly the lanthanide oxides, tend to have high C2 selectivity (>70%), whereas the acidic oxides, including Al2O3 and ZSM-5, have low C2 selectivity[37,38].
《Table 3》
Table 3 Performance of OCM oxygen carriers[36].


a Based on methane converted into carbon-containing product.
The general reaction mechanism for the chemical looping OCM is shown below.
Oxygen carrier reduction:

Oxygen carrier oxidation:

Although a great deal of ambiguity remains regarding the OCM mechanism that determines the OCM yield and selectivity due to the complicated OCM reaction network[39,40], researchers keep trying to develop efficient approaches for understanding the OCM process and improving the reactivity. Huang et al.[41] proposed a hybrid genetic algorithm to design the OCM materials. Based on the multi-turn design strategy with global optimization,they designed a multi-component OCM catalyst which can achieve C2 hydrocarbon yield of greater than 25%. Chua et al .[42] developed a sodium-tungsten-manganese-supported-silica catalyst (Na-W-Mn/SiO2) and found both Na2WO4 and Mn2O3 crystalline phases contributed to achieving high selectivity of C2 products by exploring the interaction effects of the component–component and component–support on the methane conversion and C2+ yield. In general, current OCM materials can achieve a C2 selectivity of 50%–70% and methane conversion of 35%–55%[43]. The continuing studies on the chemical looping technology will facilitate the development of OCM oxygen carriers for achieving higher selectivity and yield
《5.Reactivity improvement of oxygen carriers》
5.Reactivity improvement of oxygen carriers
Since surface chemistry plays a key role in chemical looping redox processes, extensive efforts have been devoted to catalytically modifying the surface of oxygen carriers for reactivity enhancement [44–46]. Liu and Zachariah [47] synthesized alkali-metal-doped Fe2O3/Al2O3 oxygen carriers and found alkali metal doping (~5 mol%) with Na, K,and Cs can stabilize the reactivity of the Fe2O3 more than 50 redox cycles.Wang et al.[48] investigated a series of Zr-doped Cu-based oxygen carriers,and demonstrated that the Zr-doping amount can improve the oxygen-release capacity. Mohamed et al.[49] studied the effect of Ce dopant on a NiO/Ce-Al2O3 oxygen carrier and reported that NiO with 2%–10% Ce can greatly enhance CO conversion.Chen et al. [50] recently demonstrated that tungsten-based oxygen carriers with nickel modification were effective for the chemical looping partial oxidation of methane. Their results indicated that the oxygen availability, methane conversion,and syngas yield can be significantly increased over Ni0.5WOx/Al2O3 compared with WO3/Al2O3. Qin et al. [51] recently initiated a work on low-concentration dopant modification over oxygen carrier for reactivity improvement. They used isovalent lanthana (La) dopants at a very low concentration relative to the total concentration of iron oxide oxygen carriers (e.g.,<1%) so that the oxygen carrier can keep high oxygen capacity without phase change. They found 1% of La dopant leads to a 178% increase in reduction reactivity and a 156% increase in reoxidation reactivity for CH4 gasification reactions, compared with updoped iron oxide. It is because La dopant significantly lowers the barrier of C–H bond (CH4 and CHx radicals) activation during oxygen carrier reduction. Recently, Chung et al.[52] reported an iron-based composite oxygen carrier with an Al-based skeleton which exhibits high reactivity and recyclability over 3000 TGA redox cycles at 1000°C. This oxygen carrier also was tested extensively in both pilot and sub-pilot scale reactors exhibiting low attrition rates as shown in Fig. 6[52]. These findings provide a strategy for designing highly active oxygen carrier using a relatively simple fabrication process.
《Fig.6》
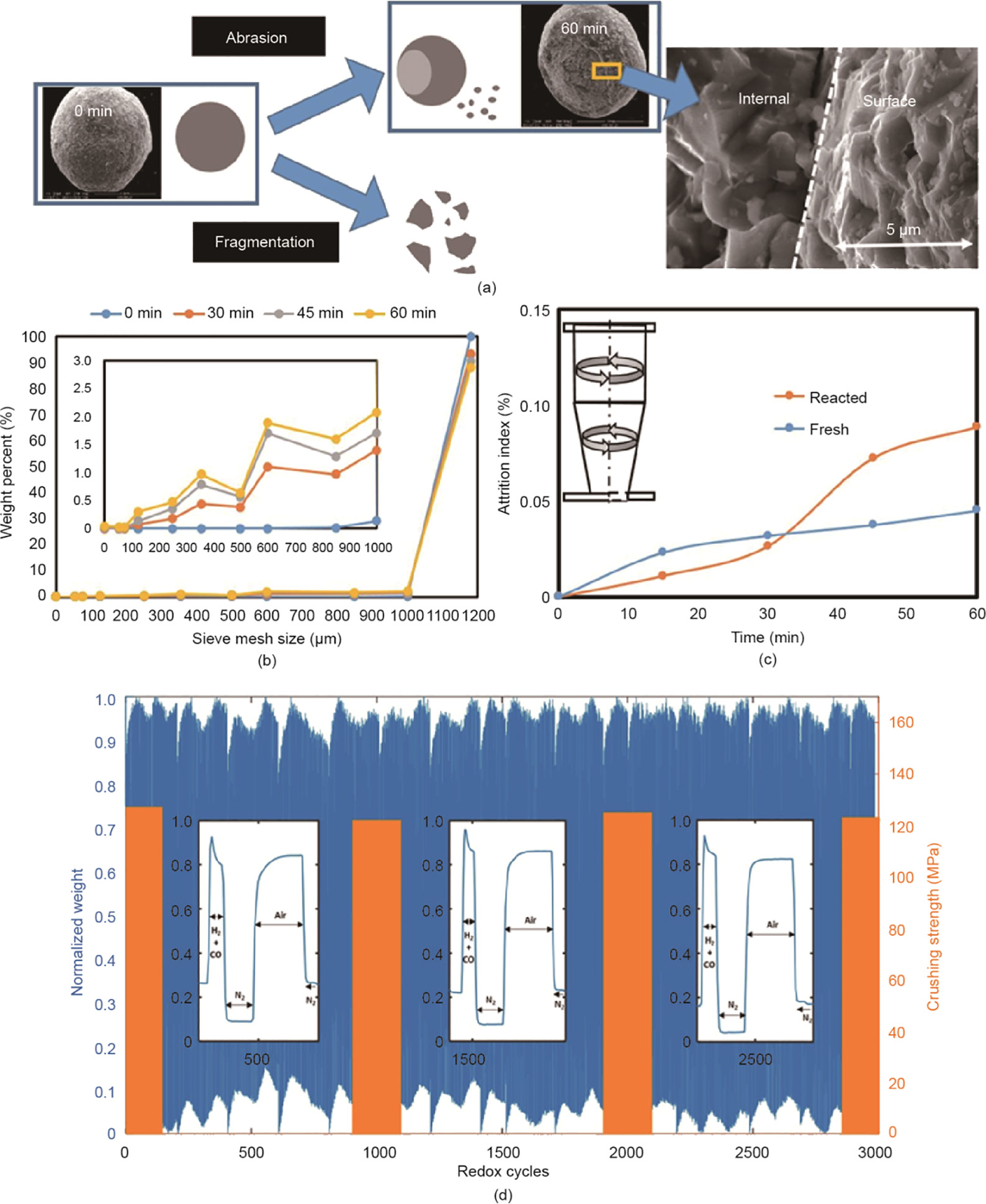
Fig.6.(a)Mechanism of attrition with SEM images of fresh (0 min)and eroded oxygen carrier (60 min) after jet-cup; (b)evolution in particle size distribution in a jet-cup experiment; (c)comparison of attrition index between fresh and reacted composite oxygen carrier from pilot operation; (d)normalized real-time TGA data over 3000 redox cycles with crushing strength measurements. (Reproduced from Ref.[52] with permission from the EES Owner Societies)
In chemical looping partial oxidation processes,methane oxidation occurs in the reducer , which is typically either a fluidized bed or a moving bed. The fluidized bed has excellent heat-transfer characteristics, but it is difficult to maintain a uniformly desired oxidation state of the metal oxide oxygen carrier for methane oxidation due to a wide distribution of the residence time in a fluidized bed. The moving bed can achieve high CH4 conversion (>99.9%) with a syngas purity of 91% at the sub-pilot scale[26]. In addition, it can maintain a high oxygen carrier conversion and hence a low oxygen carrier circulation rate in the reactor. However, there are still challenges to be overcome in industrial applications, such as stabilizing the continuous long operation of the chemical looping process,controlling the proper heat balance between the oxidizer and reducer, and maintaining the recyclability properties of the oxygen carriers. Therefore, an appropriate design for an oxidizer and reducer to treat solid feed materials during the sustained redox reactions of reactive oxygen carriers under high temperature and pressure will also be a focus of studies on chemical looping technology development.
《6.Summary》
6.Summary
The oxygen carrier materials in chemical looping play a crucial role in achieving high efficiency compared with conventional fuel-conversion processes. Metal oxide oxygen carriers are required to have high reactivity and recyclability while undergoing cyclic redox reactions with lattice oxygen transport at high chemical looping temperatures. Therefore, it is essential to fully understand the effects of the various chemical and physical characteristics of the crystal phases on the ionic diffusion process, with particular focus on the diffusion energy barrier, oxygen vacancy formation energy, and surface reaction mechanism. Understanding the mechanism can both provide theoretical guidance on fabricating active oxygen carrier materials and predict the performance of particles with minimum trial-and-error screening. It is noted that earlier experimental marker and isotope studies that confirmed the ionic diffusion mechanism for the sulfation reaction[53,54] of CaO and SO2 provide a fundamental framework for the current analyses of the redox reactions of the metal oxides involved in chemical looping systems. The recyclability of metal oxide oxygen carriers can be promoted by adding supportive oxides that lower the oxygen vacancy energy and increase the lattice oxygen diffusivity. On the other hand, dopant modification can significantly improve the surface reactivity of metal oxide oxygen carriers, such as iron-oxide-based oxygen carriers. These findings provide new insight into the factors affecting the properties of oxygen carriers during chemical looping redox processes. It should be noted that a combined experimental and computational approach will facilitate the development of metal oxide oxygen carriers with improved ionic diffusion, reactivity, selectivity, and structural stability.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Zhuo Cheng, Lang Qin, Jonathan A. Fan, and Liang Shih Fan declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




