《1.Introduction》
1.Introduction
Industrial goethite,hereafter called simply goethite, is a residue generated during the electrolytic production of zinc. Goethite is mostly composed of hydrated ferric oxide, with a 5%–10% content of zinc(Zn), as well as traces of other valuable metals. This concentration of zinc is too high to allow goethite recovery in iron production and, at the same time, too low to make zinc recovery economically profitable with the currently available technologies.
Consequently, nowadays goethite is not recycled and is mostly stockpiled in impoundments located close to the electrolytic plant[1]. Goethite landfilling presents, however, several economic and environmental disadvantages. Economic disadvantages derive from the high costs of landfilling and the losses of the zinc and other metals contained in goethite. Environmental threats are posed by the potential leaching of hazardous compounds, occurring when untreated goethite is disposed of in landfills[2,3].
In the past, scholars have investigated the technical and economic viability of different alternatives for goethite management. Piga et al.[4] obtained a higher zinc concentrate product by treating the goethite through a sequence of thermal processes. However,the obtained product was still not suitable for industrial applications, due to the poor separation between iron (Fe) and zinc. Pelino et al.[5] evaluated the possibility of recovering valuable zinc and lead (Pb) from goethite, and using the metal-poor residue to produce glass-ceramic products. This study showed that the recovery of zinc and lead by washing with distilled water is technically feasible, but not economically viable on an industrial scale.Nevertheless, the conversion of the Zn/ Pb-free residues into glass ceramic is a promising possibility.
In conclusion, sustainable goethite management remains an ongoing challenge. At present,industrial research strategies aim to find technical and economic viable solutions to ①recover valuable metals and ②process goethite to obtain a byproduct that can be subsequently valorized.
In this context, the Flemish governmental "Strategic Initiative Materials”(SIM) program MaRes (Materials from Solid and Liquid Industrial Process Residues) aims to stimulate new technologies for metal extraction from solid industrial residues and the subsequent valorization of the residual mineral matrix. The program helps to build collaboration between universities and metal producers in Flanders, the northeast region of Belgium. As a result of this collaboration, a near-zero waste strategy has been developed aiming at sustainable management of goethite . This near-zero waste strategy is based on the combination of two existing processes: ①plasma fuming and ②inorganic polymerization.
In the plasma-fuming process, the slag is heated up by electric plasma torches, which supply the slag with both heat and mixing. Several metals contained in the slag are vaporized and leave the system through the fumes. The vaporized metals are later precipitated in the form of metal oxide powder. This powder can then be recycled in metal smelters, replacing metal concentrates coming from natural zinc/ lead ore mining. The leftover of the fuming process is a nearly metals-free fumed slag that can be recycled in several applications[6–8].
Inorganic polymerization can be applied to industrial residues with a low calcium content; it consists of mixing the residue with alkaline-silicate activators to produce a high-strength polymer material with a non-carbon-containing skeletal structure[9,10]. This inorganic polymer presents a significant reduction to the carbon footprint when compared with traditional concrete based on ordinary Portland cement (OPC) [11–14].
The application of plasma fuming and inorganic polymerization to goethite brings up many technical and environmental questions. While the state of the art indicates plasma fuming as a promising technology for slag cleaning,fundamental understanding of the interaction between plasma and solid, liquid,and gaseous phases is still missing and is necessary to achieve a high degree of slag purity. In addition, most of the available work on inorganic polymerization is relevant for traditional aluminum (Al)-silicon(Si)-based residues, while goethite is an Fe-Si-based residue. The required mix design for the novel inorganic polymer, the potential leaching of hazardous compounds, and the physical properties of the inorganic polymer are fundamental issues that require further research.
In addition to the technical challenges, the success of near-zero waste strategies for goethite also depends on its environmental acceptability. Although goethite recycling permits landfilling avoidance, the recovery of valuable metals and the production of a low-carbon binder for construction material,plasma fuming and inorganic polymerization require high amounts of energy , the use of chemicals, and additional transportation. Therefore, a better understanding of the environmental implications of the near-zero waste strategy for goethite is needed.
Along with the ongoing investigation to solve the technical issues described above, a part of the MaRes program focuses specifically on the final environmental balance of the whole goethite valorization process. Life-cycle assessment (LCA) is used as the environmental impacts evaluation methodology. LCA is a framework for estimating the environmental impacts attributable to the life cycle of a product; it is widely used today to evaluate alternative products coming from waste recycling[15,16].
The environmental performance of the goethite-based inorganic polymer (GIP) will be compared with the environmental performance of a traditional OPC concrete block that is currently available on the market.
The analysis of the LCA results will help to increase our understanding of the tradeoff between the environmental costs of the fuming process plus inorganic polymerization versus the environmental benefits of avoided landfilling, metal recovery, and OPC substitution. Finally,the LCA will also be useful for identifying the environmental hotspot of the proposed processes, thus helping to increase the sustainability of the whole system.
《2.Methodology》
2.Methodology
LCA is a methodology that is used to evaluate the environmental impacts and benefits of a product,process, or system, by considering the whole life cycle. As described in the ISO 14040:2006[17], LCA consists of four main steps: ①defining the goal, scope, and system boundaries of the study; ②creating a life-cycle inventory; ③assessing the impacts; and④interpreting and analyzing the results.
《2.1.Goal,scope,and system boundaries》
2.1.Goal,scope,and system boundaries
The goal of the LCA study is to compare the environmental impacts and benefits of two equivalent construction blocks used for insulation: ①a newly developed GIP, made through the fuming and subsequent inorganic polymerization of goethite slag from zinc production; and②an autoclaved aerated concrete block made with OPC (OPCaer). The system boundaries and functional unit (FU) are introduced below.
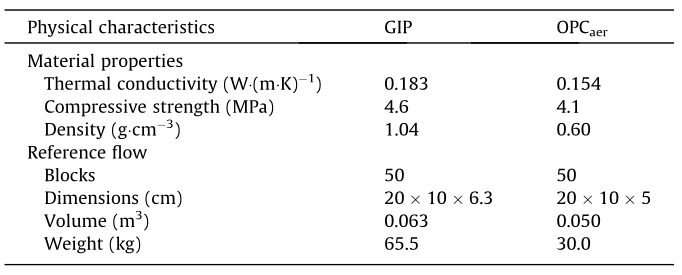
In the goal and scope definition phase, it is fundamental to define the system boundaries and FU of the study. The FU represents the product’s ability to perform a given function, and provides a reference to which all the inputs and outputs must be referred. When using LCA to compare different products, a common FU must ensure comparability among the analyzed alternatives. In the case presented in this paper, the compared products are two insulating blocks. Therefore, the FU can be defined as the capacity of the materials to insulate a surface of 1 m2. The required mass of each material to provide that FU is referred to as the reference flow. Table 1 depicts the physical properties of the GIP and the OPCaer, which indicates that GIP presents higher density and higher thermal conductivity.
One square meter surface is assumed for both materials to be made by 50 blocks measuring 20 cm (length) 10 cm(width). Table 1 shows that the thermal conductivity of GIP is higher than that of OPCaer (0.183 W·(m·K)-1 vs.0.154 W·(m·K)-1). Since both GIP and OPCaer must ensure the same thermal conductivity, the thickness of a GIP block must be higher than that of the OPCaer. Therefore, assuming a 5 cm thickness for the OPCaer, the required thickness for the GIP blocks to meet the required thermal insulation is 6.3 cm. The reference flows for each scenario can be finally defined as follows: ①the production of 50 OPCaer with the dimensions 20 cm×10 cm×5 cm and②the production of 50 GIP blocks with the dimensions 20 cm×10 cm×6.3 cm.
《Table 1》
Table 1 Physical characteristics of the GIP and OPCaer.
The system boundaries define the processes to be included in the analysis. As with the FU, the system boundaries need to be con-sistent and ensure comparability among the alternative scenarios. Fig. 1 shows the considered system boundaries for the two products. For the GIP, the goethite landfill and the natural Zn/ Pb ore mining and transport are considered to be avoided impacts. Therefore, they are accounted as a credit (negative value) to the recycling process. The approach used in this study is a cradle-to-gate analysis, meaning that only the production phase is considered.
《Fig. 1》

Fig.1.Considered system boundaries for GIP and OPCaer.AA:alkali activator.
《2.2.Life-cycle inventory》
2.2.Life-cycle inventory
The life-cycle inventory (LCI) phase estimates the consumption of resources and the quantities of waste flows and emissions caused by or attributable to a product’s life cycle[16]. Therefore, the LCI phase creates a list of inputs and outputs related to the FU chosen, and represents the basis for the calculation of the environmental impacts. The data used in this study were collected from real industrial applications and laboratory works within the MaRes program, integrated with data from the previous literature. More specifically, data were collected from the real industrial processes of an international zinc and metals producer operating a plasmafuming plant in Norway and a metal smelter in Belgium.
2.2.1.Goethite-based inorganic polymer
The goethite considered in this study is produced as the residue of a zinc production plant located in Norway. Goethite is fumed in a fuming plant located in the same area. The fuming process has four outputs: the off-gas, the metal oxide powder, the fumed slag, and an impure fused material called matte. In this study,the matte is not considered in the LCI because of the low quantity that is produced during the goethite-fuming process. Metal oxide powder and fumed slag are shipped to Belgium, where the metal oxide powder is recovered as a metal concentrate in a metal smelter,and the fumed slag is processed to produce new inorganic polymers. Table 2 summarizes the inventory for the production of 65.5kg of GIP. All the background processes (referring to the inputs of materials and energy) are modeled using the ecoinvent database v3. The next paragraphs further detail the main assump-tions adopted in the development of the GIP production process.
《Table 2》
Table 2 The inventory for the production of 65.5 kg of GIP.

(1)Goethite. Among various samples, the goethite chosen for this study is a sludge residue of an electro-metallurgic zinc production process in Norway, which produces 80 000 t of goethite every year. The chemical composition of the goethite is reported in Table 3.
《Table 3》
Table 3 Goethite composition from zinc production;goethite is an Fe-Si-based residue with a low amount of calcium(Ca).

The production of GIP avoids the landfilling of the goethite. The avoided goethite landfilling, hence, can be considered as a credit to GIP production, and is accounted as a negative impact. The inventory for the goethite landfilling refers to the ecoinvent module described by Doka[18].
(2)Plasma-fuming process. The plasma-fuming plant is equipped with two electric plasma torches that are able to fume up to 2000 kg of slag in total. A schematic of the submerged plasma process,as described in Verscheure et al.[7], is depicted in Fig.2. The furnace is fed with premixed goethite, which forms the liquid slag bath. The torches supply heat as well as a good mixing to the slag bath, thus transforming the external blast primary and secondary air into a plasma gas. Operating temperatures range from 1150 to 1300°C[19]. Methane (CH4) is subsequently mixed with the plasma gas to reduce the partial pressure of oxygen (O2). Inside the furnace, the slag bath is injected with the plasma gas/CH4 mix and with petroleum coal,which acts as a heat source through combustion with primary and secondary air,and as a reductant through the production of carbon monoxide(CO)[8].Thanks to the low O2 partial pressure and the high temperature,the oxides of zinc are removed from the slags bath by reduction and the generation of volatile metal species. Due to the wide range of chemical species involved, the reactions occurring during the fuming process are numerous and complex[20]. However, the chemical reactions that lead to the fuming of zinc can be summarized as follows[19]:
《Fig. 2》

Fig.2.Submerged plasma fuming[7].
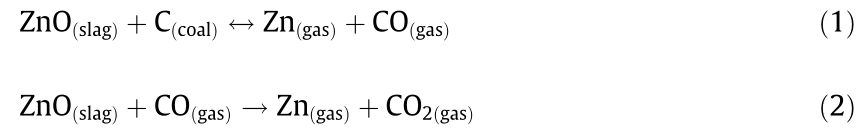
Both reactions are strongly endothermic. Thus ,the partial combustion of coal below the slag bath also provides the heat for the reactions. As a consequence of the reducing reactions described above, volatile zinc leaves the furnace with the off-gas. Equivalent reducing reactions also occur for other volatile metals present in the goethite,such as lead ,indium,and germanium.
The reactions described above indicate that the availability of coal is among the factors that determine the efficiency of zinc fuming. Therefore, the coal is usually supplied in surplus compared to the stoichiometric needs for Zn and other metal reduction.Consequently,the exceeding coal may react with the ferric oxide and other metal oxides present in the slag, creating a metal matte containing sulfur, which is collected separately at the end of the fuming process. According to the data collected directly at the fuming plant, the quantity of matte produced can reach 4% of the initial quantity of the goethite. The matte can be further processed to recover some metals (copper(Cu) and nickel (Ni), among others). However, the recovery of matte is outside the scope of the MaRes program, and literature data in this field are missing. Therefore, due to the complexity of the reactions and the missing information, the presented environmental analysis focuses only on the fumed slag and does not consider the production of matte. Although this can be considered as a limitation of the study, the production of matte is not expected to change the results significantly.
The vaporized metals in the fumes enter into contact with secondary air, and volatile metals re-oxidize and precipitate as oxidized powder. This powder contains various metal oxides,and its composition is equivalent to that of a natural zinc concentrate ore, which is commonly used today in metal smelters. After metal vaporization, the metal concentrations in the leftover slag are significantly lowered[7].
The inputs of the process are the goethite,the electric energy to fume the slag and run the off-gas treatment, the CH4 and coal that are used as reductants. For the electricity, the Norwegian mix used is composed of hydropower (96.8%), natural gas (2%), and wind(1.2%)[21]. The outputs of the process are the off-gas, the nearly metal-free fumed slag, and the metal oxide powder. The off-gas is mostly composed of carbon dioxide (CO2), which is produced during the reduction of zinc oxide (ZnO) and other metal oxides, and sulfur dioxide (SO2), which comes from the sulfur content in goethite and coal.
The quantity of CO2 released into the atmosphere with the off-gas is calculated stoichiometrically, under the hypothesis that the coal contains 90% carbon and that all carbon is finally released as CO2. The quantity of SO2 released with the off-gas refers to quantities measured directly at the fuming plant.
The fumed slag is collected and further processed in an inorganic polymerization process. Both the fumed slag and the metal oxide powder are transported by ferry to Belgium to be processed. The average transportation distance for the fumed slag and the metal oxide powder is assumed to be 1500 km. Metal oxide powder contains mostly ZnO, with a lower amount of lead oxide (PbO), and can be recovered in a metal smelter for the production of zinc, lead, and other metals, thus replacing natural zinc concentrate ores produced through ore mining. Therefore,the avoided production of an equivalent amount of zinc concentrate ore from mining is given as a credit (negative value) for the fuming process. The inventory for the avoided mining of natural zinc ores is modeled using the ecoinvent module described by Classen et al.[22]. The metal smelter considered in this paper belongs to the same zinc and metals international producer, and is located in Belgium.Today, most of the natural zinc ores used in the metal smelter are mined in Australia and in South and North America, and are then shipped to Belgium. The average avoided transportation for the natural zinc ores is assumed to be 5000 km.
(3)Inorganic polymerization. The fumed slag goes into the inorganic polymerization process, where it is mixed with an alkali activating solution with a ratio of 3.04 (slag to activating solution). The activating solution contains two commonly used alkaline activators, sodium hydroxide and sodium silicate,mixed in a solution with a ratio of 14.11 wt% sodium oxide (Na2O), 22.49 wt% silicon dioxide (SiO2), and 63.4 wt% water(H2O). Aluminum powder at 0.05 wt% is added to the mix as a foaming agent. Since the fumed slag does not dissolve completely in the process, it also acts as an aggregate; therefore, no additional external aggregate is added to the process. Steam curing was carried out at 60°C for 24 h. Electricity consumption refers to the electricity consumed by the mixing process and used to produce the steam for the final curing. All the electricity consumed refers to the Belgian electricity mix for 2017, which was composed of nuclear(46.4%), natural gas (26.5%), coal(6.1%), biofuels(4.9%), wind(4%), solar(2.1%), and other energy sources (10%)[23].
As described in the introduction, research is currently being carried out to optimize the use of alkali activators in the process and to minimize the risk of hazardous metal leaching in the inorganic polymerization of Fe-Si-based residues [24,25]. The data for the inventory of the inorganic polymerization process were determined empirically after several experiments at the lab scale that were conducted to find the optimal process conditions. Plasma fuming theoretically removes a large number of metals from the fumed slag. For the present study,it is assumed that no significant leaching occurs from the produced inorganic polymers.
2.2.2.Autoclaved aerated OPC concrete
The production process starts from silica sand, which is mixed with binding agents (quicklime and OPC) and water. Before the suspension is poured into the casting molds, the expanding agent(aluminum powder) is added. The aluminum reacts with the alkaline water,producing hydrogen. The hydrogen creates pores that decrease the thermal conductivity of the material. A more thorough description of the OPCaer production process can be found in previous literature[26,27]. Table 4 [27] provides the inventory for the OPCaer production used in this study. The quantities assumed for each input refer to the work by Kellenberger et al.[27]. The ecoinvent database was used to model the inventory of the background processes, namely the inputs of materials and energy. The electricity used refers to the Belgian electricity mix in 2017[23].
《Table 4》
Table 4 Inputs/outputs for autoclaved aerated OPC concrete(reference flow=30 kg)[27].

a Belgian electricity mix[23].
《2.3.Life-cycle impact assessment》
2.3.Life-cycle impact assessment
The life-cycle impact assessment (LCIA) phase translates each piece of LCI data into the corresponding environmental impacts. LCIA results commonly refer to two different approaches: ①the problem-oriented (midpoint) approach and ② the damageoriented (endpoint) approach. A midpoint analysis assesses how each single process contributes to several different environmental impact categories, while an endpoint analysis translates the mid-point impacts into three endpoint damages: human health, ecosystem, and resources. As explained by Benetto et al.[28], the midpoint analysis provides a reliable evaluation of the environmental performances of a product. However, when comparing two products, midpoint results are not easily comparable, as the environmental impact categories are calculated in different units, and it is not possible to sum them up. Endpoint analysis aggregates the mid-point results to only three endpoint indicators. While this can facilitate the comparison between different products, it increases the uncertainty of the results, since it involves an aggregation process. Therefore, to have a reliable and comparable set of results, findings from both midpoint and endpoint analyses should be presented. For the current study, the LCA model was implemented using the software GaBi. For the LCIA phase, the ReCiPe 1.08 calculation methodology was used.As one of the most-used calculation methodologies in LCIA, ReCiPe 1.08 includes both midpoint and endpoint approaches, and provides high reliability regarding characterization factors and reduced uncertainty[29]. A more detailed description of ReCiPe 1.08 can be found in Ref.[30].
《3.Results》
3.Results
《3.1.Result description》
3.1.Result description
The estimated midpoint environmental impacts for GIP and OPCaer are reported in Fig. 3 and Table 5.As categories are measured in different units, in order to facilitate the comparison between the environmental performances of GIP and OPCaer, Fig. 3 shows the relative impacts of the two products. Positive values indicate an induced impact, while negative values indicate the net impacts savings.
《Fig. 3》
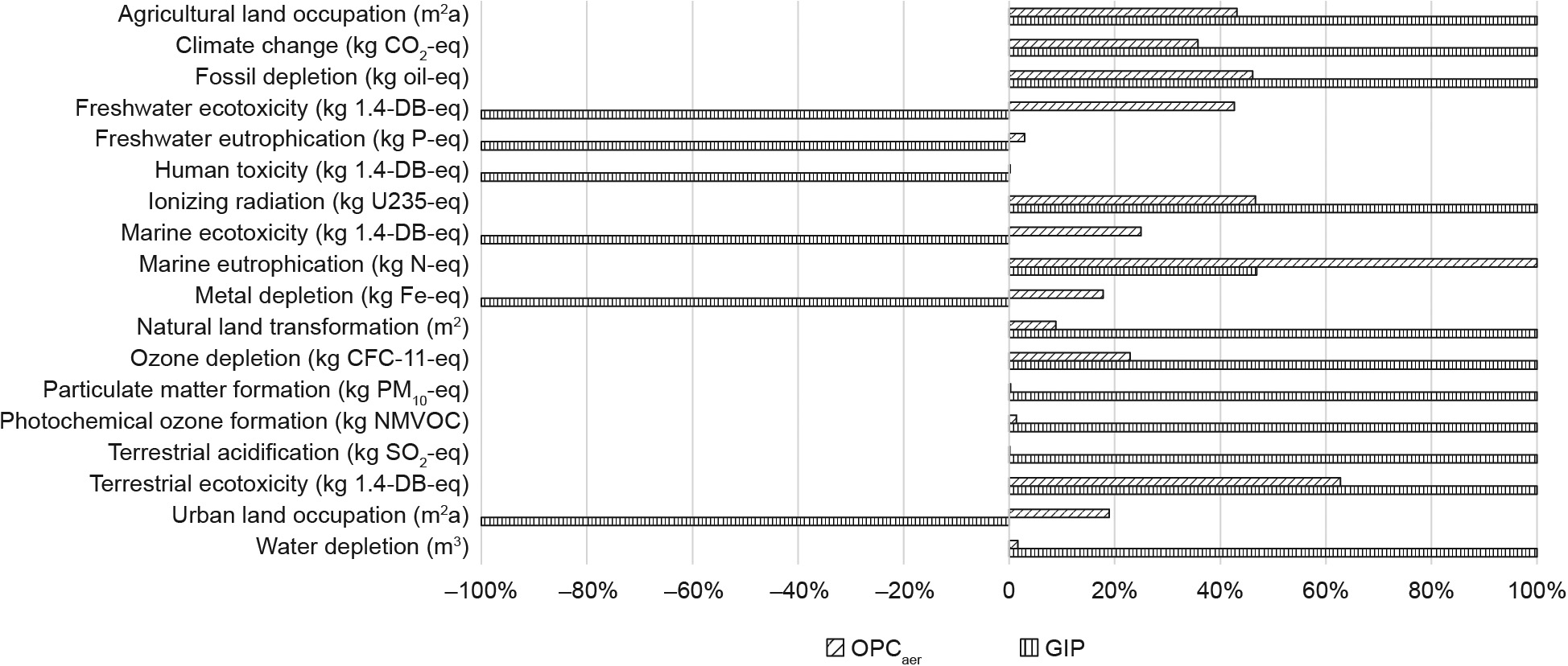
Fig.3.LCIA midpoint results for GIP and OPCaer. CFC-11: fluoro trichloro methane; NMVOC:non-methane volatile organic compound; 1.4-DB:1.4 kg dichlorobenzene.
《Table 5》
Table 5 LCIA midpoint results.
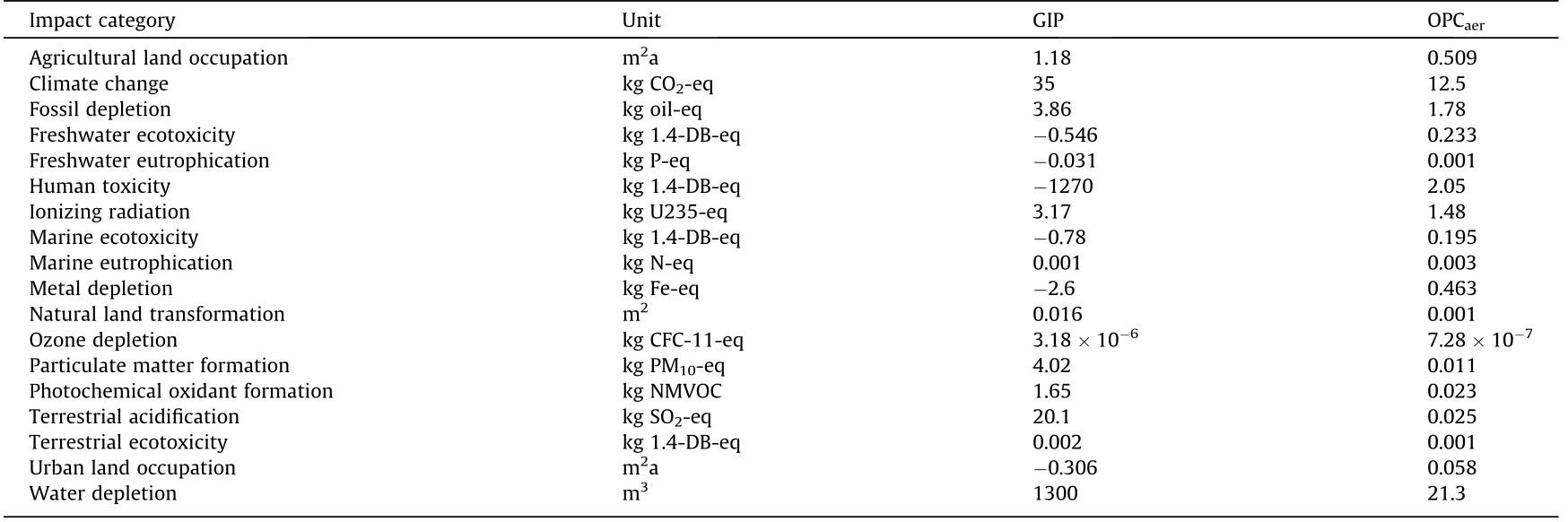
Table 5 shows that 35 kg CO2-eq are emitted to produce the GIP, which is a value 2.8 times higher than the emissions for the OPCaer. At the same time,in some other categories such as freshwater ecotoxicity and eutrophication, the final value for the GIP is negative, meaning that the avoided impacts (i.e.,goethite landfilling and zinc ore production) are higher than the caused impacts. GIP shows negative impacts in many other categories, such as human toxicity, marine ecotoxicity, metal depletion, and urban land occupation.Moreover, compared with OPCaer, GIP shows an impact reduction in marine eutrophication, whereas GIP performs worse than OPCaer in agricultural land occupation, fossil depletion, ionizing radiation, natural land transformation, ozone depletion,particulate matter formation, photochemical oxidant formation, terrestrial acidification, terrestrial ecotoxicity, and water depletion.
The midpoint results provide a detailed description of the direct impacts generated by the production of GIP and OPCaer. However, the results in Fig.3 and Table 5 do not help to identify the environmental tradeoff between the two construction materials. Therefore, an aggregation to the endpoint can help to identify which of the two options presents the highest environmental advantages.
Following the ReCiPe endpoint methods,three endpoint indicators for damage assessment are identified: human health,ecosystem, and resources, which are calculated from the aggregation of all the midpoint indicators. The comparative damage assessment between GIP and OPCaer is shown in Fig. 4. Compared with GIP, OPCaer has lower impacts in all categories, presenting 91% in human health, 75% in ecosystem,and 27% in resources.
《Fig. 4》
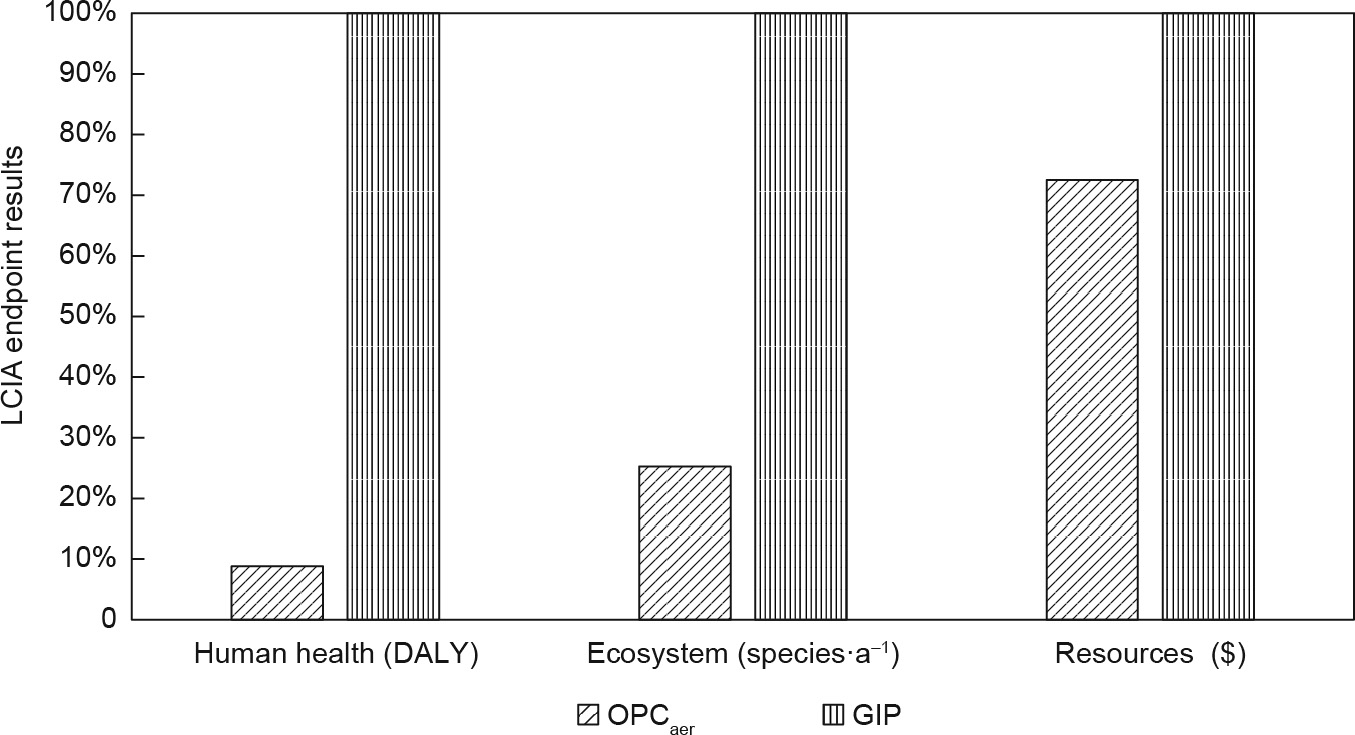
Fig.4.LCIA endpoint results for GIP and OPCaer.DALY:disability adjusted of life years.
Finally,it is useful to assess the contribution of each single process to the final results, in order to identify possible environmental hotspots. Fig.5 and Table 6 show the contribution of each process to the final endpoint results. In Fig.5, the first column on the left,represents the total for the OPCaer, while the rest of the columns on the right represent the contribution of each single process to the final impact for GIP.
《Fig. 5》

Fig.5.LCIA endpoint results and processes contributions to (a)human health(DALY), (b)ecosystem (species·a-1) ,and(c)resources($). The final impact for GIP is the sum of the contributions from each single process.
《Table 6》
Table 6 Process contribution analysis of endpoint results.

In all three endpoint damages to human health, ecosystems, and resources, the impact coming from the plasma fuming has the highest value (1.09×10-3 disability adjusted of life years (DALY), 3.89 ×10-7 species a-1, and$0.502). Considering the damages to human health and ecosystems, most of the impacts come from the electricity production and the direct emissions (CO2 and SO2) released during the fuming. Regarding the damage to resources, the main contributions for the plasma fuming come from electricity and coal production.
For alkali activation, the main contributor to damage to human health is sodium hydroxide (8.69 ×10-6 DALY), while sodium silicate is the main contributor to damage to ecosystems (2.82×10-8 species a-1) and resources ($0.1648).
Regarding the damage to human health,the avoided landfilling of goethite has a high avoided avoidance impact (8.51× 10-4 DALY), while the avoided mining and transportation of natural zinc ores have an important negative contribution to damage to resources ($0.3404). In both cases, however,the avoided impacts cannot offset the impacts caused by the plasma fuming and the inorganic polymerization, and the final value of each endpoint damage for the GIP result is positive (see Fig. 5). The transportation of fumed slag and metal oxide powder has a relatively low impact on human health (1%), ecosystems (2%) ,and resources (11%), despite the long transportation distances that were assumed (1500 km from Norway to Belgium). Finally, the avoided mining of natural zinc ore and its transportation from Australia to Belgium (5000 km), has a low effect on human health (—5%) and ecosystems(—3%), but significantly lowers the final results for damage to resources (—75%).
《3.2.Result interpretation》
3.2.Result interpretation
The results of the LCA analysis presented above highlight the advantages and environmental hotspots of goethite valorization when producing GIP through plasma fuming and inorganic poly-merization. The midpoint results showed that in some midpoint categories, the GIP production process has a lower impact than the equivalent OPCaer production, due to the avoided impacts of goethite landfilling and natural zinc ores mining and transport. However ,in some other midpoint categories, the goethite valorization presented higher impacts than the production of the equivalent OPCaer. The endpoint results identified electricity and direct emissions (CO2 and SO2)as the main environmental hotspots of the GIP production process.
The electricity consumption in the plasma-fuming process is one of the major contributors to the final impact of GIP, although the electric mix used for the plasma fuming refers to the Norwegian electric mix (which is nearly fossil fuel-free, with 96.8% based on hydropower). Therefore, electricity represents a preeminent environmental hotspot of the whole valorization process,especially if less environmentally clean electric sources are used.In addition, a second major environmental concern is represented by the direct emissions of CO2 and SO2 that, even if they are below the legal thresholds, contribute significantly to the final impact of GIP production. The avoided goethite landfilling and mining and transportation of natural zinc ores lower the overall impact of the GIP production process, but they cannot offset the impacts caused by the plasma fuming and the inorganic polymerization processes.
Considering that the thermal energy requirement and CO2 emissions are two unavoidable components of the plasma-fuming process, some reduction of the environmental impact can already be achieved with higher energy efficiency and a more effective exhausted fumes cleaning system (i.e.,the use of scrubbers for SO2). However, the most effective potential means to reduce the environmental impact is identified in the better use of slag in the inorganic polymerization process. The proposed recipe to make GIP requires a high quantity of fumed slag to reach specific cemen-titious properties. As a consequence,the high use of a fumed slag attributes high environmental impacts to the GIP.As a side note, while OPCaer is a well-developed historic material that contains only 24 wt% of binder (OPC), the analyzed GIP represents a new product at its early development stage, containing 75 wt% of fumed slag as the activated binder. Considering that OPC and fumed slag are the main contributors to the final environmental impacts of OPCaer and GIP, decreasing the content of fumed slag to a percentage that is closer to that of OPC in the OPCaer can level down the differences in the environmental impacts of the two materials.
To conclude: ①the use of clean electricity, ②a higher energy efficiency, ③a more effective control of the fumed gas emissions, and④a reduced use of fumed slag through an increased efficiency of the inorganic polymerization process are the key elements toward the sustainability of goethite valorization.
《4.Conclusions and future perspectives》
4.Conclusions and future perspectives
Industrial goethite, a residue that occurs during the electrolytic production of zinc, is today mostly landfilled in controlled tailing ponds. However, goethite landfilling can pose several economic and environmental disadvantages, due to the high cost of its impounding and the risk of heavy metal leaching. Although goethite contains a substantial amount of valuable metals , the currently available technology does not permit an economically convenient extraction of these metals. Therefore, industries are currently looking for new technologies for goethite recycling and metal recovery. The present study analyzes the environmental performances of a newly developed strategy for sustainable industrial goethite management that aims at the production of GIP that can be used in construction applications. More specifically, this study analyzes the environmental performances of insulating blocks made of GIP compared with those of equivalent OPCaer that are currently available on the market. LCA methodology is used to evaluate the environmental performances of the two products, using ReCiPe 1.08 midpoint and endpoint as impact calculation methods. Comparison of the environmental results for ReCiPe midpoint shows that the production of GIP, compared with the equivalent OPCaer, can increase the environmental impacts in some of the considered categories, such as 2.8 times higher impact on global warming. At the same time, GIP production presents negative values in many other midpoint categories, meaning that the avoided impacts of goethite landfilling and natural zinc ore mining are higher than the caused impacts of the GIP production. The three endpoint damage indicators confirm the criticalities in the environmental profile of GIP. OPCaer presented a lower value for all endpoint indicators: human health (-91%), ecosystems(-75%), and resources (-27%).
LCA is also useful for identifying the environmental hotspots in the analyzed production system. Looking at the results for each single process in GIP production, the analysis shows that plasma fuming provides the main contribution for all three endpoint indicators, especially due to its electricity consumption and the direct emissions of the plasma-fuming plant. The production of the alkali activators used during the inorganic polymerization also provides an important contribution to all three endpoint indicators. Transportation of the fumed slag and the metal oxide powder does not have a significant negative effect on the final environmental performance of GIP, whereas the avoided transportation of natural zinc ore yields significant environmental credits to the goethite recycling, especially regarding the endpoint indicator resources.
The LCA of GIP compared with OPCaer shows that the potential environmental benefits of the proposed goethite recycling strategy lie especially in the avoided impacts of goethite landfilling and natural zinc ore production and transportation. However ,these do not offset the environmental costs of goethite fuming and inorganic polymerization, resulting in an overall impact that is significantly higher in some categories than that of traditional OPCaer. To conclude, this study represents a first exercise in analyzing the environmental hotspots in goethite recycling. Rather than providing a definitive answer regarding the sustainability of the process,the scope was to provide useful information to help the involved parties take the correct actions toward the sustainability of the process.Future strategies to lower the environmental impact of GIP are identified as follows: ①increasing energy efficiency during plasma fuming and②making more efficient use of the fumed slag during the inorganic polymerization;these strategies can reduce the impact that is assigned to GIP by the fuming process.
Optimizing the quantity of slag that is needed to reach the required cementitious property appears to be the priority development in order to lower the impacts from the plasma-fuming process. It appears clear that the future development of GIP technology must aim at increasing the cementitious properties of the fumed slag. This goal can be achieved through the optimization of the inorganic polymerization recipe used to activate the slag. Reduction in the use of the fumed slag in GIP could also be achieved by substituting the inert part of GIP (i.e.,the fumed slag that is not activated during the process) with materials that have lower environmental impact (i.e.,natural aggregates or slag from other metallurgical processes).
These results can help the zinc-producing industries to strive for goethite recycling while limiting the factors that hinder the sustainability of the whole process.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Andrea Di Maria and Karel Van Acker declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




