《1 Introduction》
1 Introduction
Hydrogen is an important raw material, and is ideal as a secondary energy source or for use as an energy carrier. Hydrogen (H2) is convenient for both storage and transportation when acting as a secondary energy source. On the other hand, hydrogen can also be applied directly as a fuel. In addition to industrial applications (such as the syntheses of ammonia and methyl alcohol) and refining processes, H2 has great potential applications in other fields, including hydrogen metallurgy, coal liquefaction, and in vehicular fuel cells. Nuclear hydrogen production is a highly efficient method for mass producing H2 without carbon emissions.
Supported by the National 863 Program, a 10 MWt, high-temperature gas-cooled test reactor (HTR-10) was constructed and operated at the Institute of Nuclear and New Energy Technology (INET), Tsinghua University. With support from major national scientific and technological projects, a 200 MWe high-temperature gas-cooled nuclear power demonstration plant (HTR-PM) is currently under construction [1]. Moreover, China is engaged in the research and development of advanced technologies in this field, including nuclear hydrogen production and helium turbine. High temperature gas cooled reactors (HTGRs) are superior to other reactor models when considering nuclear hydrogen production, due to the high outlet temperature and inherent safety of these reactors. Therefore, they are regarded the most suitable reactors for use in producing hydrogen [2]. Nuclear hydrogen production and power generation provided by HTGRs will allow for new applications of this technology in the future. Moreover, research and development of nuclear hydrogen technologies will ensure the leadership of China in terms of HTGR technologies, provide an effective method for the large-scale production of H2, and provide a new application of the heat produced in HTGRs. In addition, this development is important for the strategic energy transformation of China in the future.
《 2 A brief introduction of nuclear hydrogen production technologies》
2 A brief introduction of nuclear hydrogen production technologies
In nuclear hydrogen production, H2 is extracted from a hydrogen-containing substance (such as water or fossil fuels) by using heat produced by the nuclear reactor as the primary energy source.
《2.1 Reactors for nuclear hydrogen production》
2.1 Reactors for nuclear hydrogen production
Existing reactors that are extensively used for power generation, such as pressurized water reactors (PWRs), use high-temperature steam as a heat carrier. Due to their relatively low outlet temperatures, PWRs are mainly used for power generation. The Generation IV International Forum (GIF) has selected six reactor types (including the sodiumcooled fast reactor, gas-cooled fast reactor, lead-cooled fast reactor, molten salt reactor, supercritical water reactor, and very/high-temperature gas-cooled reactor) for future development. These developments are not only expected to be economically viable, safe, and sustainable, but may also expand the applications of nuclear power into fields other than power generation. Among the six reactor types, the very/high-temperature gas-cooled reactor is considered the best reactor for hydrogen production, which is determined by its inherent safety, high outlet temperature, and appropriate power output [3]. It is noteworthy that in GIF, a hydrogen production project management board is responsible for coordinating international communications and cooperation in relation to nuclear hydrogen production.
《2.2 Nuclear hydrogen production technologies》
2.2 Nuclear hydrogen production technologies
As a secondary energy source or energy carrier, H2 must first be extracted from hydrogen-containing substances by using primary energy sources. The typical routes of nuclear hydrogen production are shown in Fig. 1.
《Fig. 1》
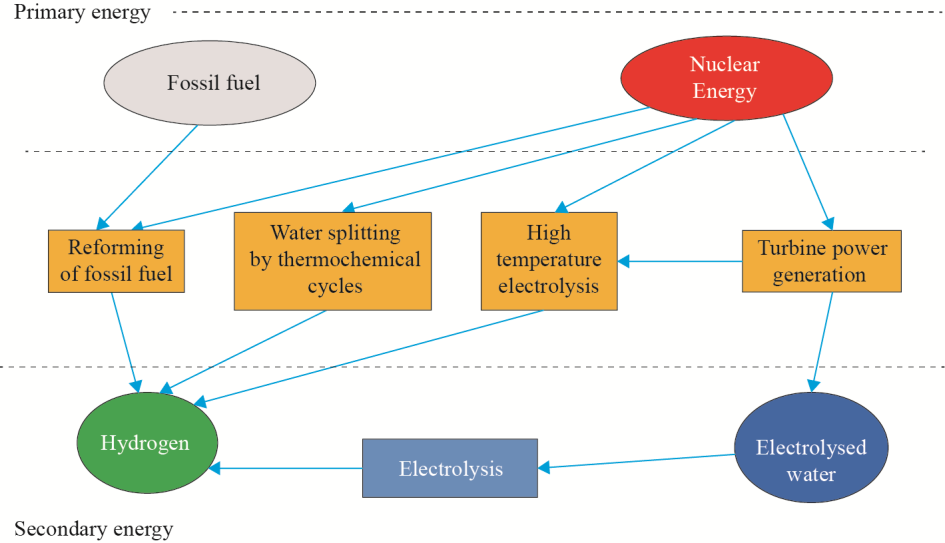
Fig. 1. Routes of nuclear hydrogen production.
It can be seen from Fig. 1 that in the nuclear heat-assisted reforming of hydrocarbons, the heat produced in HTGRs can reduce the use of fossil fuels and thereby decrease CO2 emissions (by some extent) compared with conventional technologies. Hydrogen production may be achieved via a combination of nuclear power generation and conventional electrolysis. However, this process demonstrates low transformation efficiency when converting the primary energy source into hydrogen. Hydrogen production via alkali electrolysis can be used for energy storage, providing H2 when a surplus of power is generated by a PWR, or in other such special cases. To achieve a highefficiency transformation of nuclear energy into hydrogen energy, heat resulting from the reactor processes must be used either partially or completely; hence, decreasing the energy lost in a heat–power transformation. Currently, the dominant nuclear hydrogen production technologies include thermochemical cycles (iodine-sulfur cycle and hybrid sulfur cycle) and high-temperature steam electrolysis.
2.2.1 Iodine-sulfur cycle (IS cycle)
The iodine-sulfur cycle (IS cycle) was proposed by General Atomics (GA) of USA [4], and is viewed as the most promising nuclear hydrogen production technology. The IS cycle is a closed process formed by three chemical reactions in Equation (1)––Equation (3). The net outcome of the IS cycle is water decomposition into H2 and O2 in the presence of high temperatures. In this process, water decomposition that is originally only feasible under very high temperatures (>2500 ℃) can be realized in a range of 800–900 ℃.
Bunsen reaction (producing sulfuric acid and hydroiodic acid):
SO2+I2+2H2O=H2SO4+2HI (20–120℃) (1)
Sulfuric acid decomposition (producing oxygen):
H2SO4= SO2+1/2O2+H2O (830–900℃) (2)
Hydroiodic acid decomposition (producing hydrogen):
2HI=H2+I2 (400–500 ℃) (3)
An IS cycle using an HTGR as the heat source is shown in Fig. 2.
In this IS cycle, the sulfuric acid decomposition is a high-temperature endothermic process that matches well with the outlet temperature of the HTGR. The hydrogen production efficiency of the IS cycle is expected to be higher than 50%. Because this operation occurs under a flow state that is both easy to scale up and continuous, the IS cycle is applicable to the large-scale production of hydrogen. Moreover, greenhouse gas emissions are basically eliminated when using the IS cycle.
《Fig. 2》

Fig. 2. IS cycle in conjunction with a high-temperature gas-cooled reactor.
2.2.2 Hybrid sulfur cycle
The hybrid sulfur cycle (HyS cycle) was proposed by Westinghouse Electric (USA) [5], and is another nuclear hydrogen production technology with promising industrial applications. The principle of the HyS cycle is shown in Fig. 3.
《Fig. 3》

Fig. 3. Principle of the HyS cycle.
The HyS cycle contains two reactions:
SO2 depolarizing electrolysis: SO2+2H2O=H2SO4+H2 (30–120 ℃) (4)
Sulfuric acid decomposition: H2SO4=H2O+SO2+ 1/2O2 (850℃) (5)
SO2 electrolysis produces sulfuric acid and H2, the sulfuric acid is further decomposed into SO2, and the process is repeated. In this way, a closed-cycle is formed. In the HyS cycle, water is decomposed into O2 and H2. The HyS cycle is composed of two steps and uses both high-temperature process heat and electricity. This cycle demonstrates a significantly higher hydrogen production efficiency compared with conventional electrolysis. Moreover, it partially avoids the material and engineering problems encountered when solely using a high-temperature thermal process.
2.2.3 High-temperature steam electrolysis
High-temperature steam electrolysis (HTSE) decomposes high-temperature water vapor by using a solid oxide electrolysis cell (SOEC), as shown in Fig. 4. Compared with conventional electrolysis, high-temperature steam electrolysis requires a heat source, which can significantly increase the hydrogen production efficiency [6].
《Fig. 4》

Fig. 4. Principle of hydrogen production based on SOEC.
Hydrogen production from water electrolysis based on an SOEC involves the inverse operation of a solid oxide fuel cell (SOFC). The operational procedure of an SOEC is shown in Fig. 4. Vapor enters into the hydrogen electrode of the SOEC and undergoes a reduction reaction with electrons provided by the external circuit to produce H2. Meanwhile, oxygen ions are produced and transmitted to the oxygen electrode by the external electric field via oxygen vacancies in the electrolyte layer. Subsequently, oxygen is produced through an oxidation reaction and the produced electrons are returned to the external circuit.
《3 R&D status of nuclear hydrogen production techniques》
3 R&D status of nuclear hydrogen production techniques
《3.1 International R&D status》
3.1 International R&D status
3.1.1 Japan [7]
The Japan Atomic Energy Agency (JAEA) has been devoted to the research of hydrogen production based on HTGR and IS cycle since the 1980s. The outlet temperature of the 30 MW high-temperature gas-cooled test reactor (HTTR) constructed by JAEA was increased to 950 ℃ in 2004. JAEA designed a commercial reactor (GTHTR300C) for hydrogen-electricity co-generation, which was mainly applied towards nuclear hydrogen production and helium turbine. Additionally, JAEA successively constructed a proof-of-concept facility (1 NL/h, 1997) and a bench scale facility (50 NL/h,2004) for the IS cycle, and the continuous operation of these facilities was successfully realized. Recently, an engineering material facility was constructed (200 NL/h) for R&D on the materials, integrity of the equipment, long-term operation, and membrane separation involved in this process. This facility was used to investigate and verify the manufacturability of the equipment, determine the performance in a tough environment, and to develop advanced technologies that may improve the efficiency of the equipment. In addition, many studies on the dynamic simulation of the IS process and safety of nuclear hydrogen have been reported upon. JAEA also plans to demonstrate nuclear hydrogen technologies by using HTTRs. Additionally, JAEA is designing a multifunctional commercial high-temperature reactor demonstration for hydrogen production, power generation, and seawater desalination. The feasibility of applications involving nuclear hydrogen steel-making are also being explored.
3.1.2 America [8,9]
America has placed emphasis on the study of nuclear hydrogen production in the 21st century. A series of hydrogen energy development plans have been released, including the national hydrogen energy roadmap, hydrogen fuel plan, nuclear hydrogen initiative (NHI), and the next generation nuclear plant (NGNP). All of these plans involve content related to nuclear hydrogen production. A large amount of R&D has been performed concerning high-temperature water splitting technologies, which has been motivated by advancements in nuclear systems and basic research on related processes, including the IS cycle, HyS cycle, and high-temperature electrolysis. GA, the Sandia National Laboratory, and the French Atomic Energy Commission have conducted a collaborative development of the IS cycle. They constructed a small facility for engineering material manufacturing and it was tested in 2009. The HyS cycle is currently being developed by the Savannah River National Laboratory (SRNL) in conjunction with several universities. An SO2 depolarized electrolyzer has also been successfully developed. Hightemperature steam electrolysis has been performed by the Idaho National Laboratory, and a 10 kW SOEC stack was developed and tested with HTSE facilities.
3.1.3 South Korea [10]
South Korea is undertaking R&D and demonstrations of nuclear hydrogen, aiming to realize the commercialization of nuclear hydrogen technologies after 2030. Korea implemented the nuclear hydrogen development and demonstration (NHDD) project in 2004. In this plan, a method for achieving economically viable and high-efficiency hydrogen production based on HTGRs was determined, and a preliminary concept for a commercial nuclear hydrogen production plant was designed. The IS cycle was selected for use as the main nuclear hydrogen production technology. The Korea Atomic Energy Research Institute (KAERI) is responsible for relevant studies of this technology. Currently, KAERI has developed the required process technology, constructing a system with a 50 NL/h yield of hydrogen, and a closed-cycle experiment has been demonstrated. Many research institutes and enterprises in South Korea have joined to form an industrial alliance focused on nuclear hydrogen. They are studying the feasibility of nuclear hydrogen production via hydrogen-rich reduction and pure hydrogen reduction reactions in the steel industry.
3.1.4 Canada [11]
A fourth-generation national plan was formulated by the Canadian Natural Resource Committee that stipulates the development of a supercritical water reactor (SCWR). Hydrogen production is one part of this plan. The mediumtemperature thermochemical copper chlorine cycle, which is feasible considering the high outlet temperature of a SCWR, is the main hydrogen production technology that is being considered. Moreover, Canada is studying and improving the IS cycle to adapt this process to the lower outlet temperature of SCWRs. At present, the University of Ontario Institute of Technology is responsible for studying the copper chlorine cycle with the assistance of the Canada National Laboratory (CNL) and Argonne National Laboratory. In addition, CNL is constructing an HTSE model and performing primary research on electrolysis. The four-step copper chlorine cycle is shown in Table 1.
《Table 1》
Table 1. The four-step copper chlorine cycle and the involved conditions.

3.1.5 International organizations [12]
International cooperation projects involving nuclear hydrogen production are ongoing. The very/hightemperature reactor (V/HTR) system presented by GIF provides a hydrogen production project management board that regularly holds conferences to discuss the progress of and issues involved in R&D. Currently, Tsinghua University participates in activities involving reactor and V/HTR systems, among other topics, on the behalf of China. The International Atomic Energy Agency (IAEA) has coordinated a program related to the techno-economic feasibility of nuclear hydrogen production. More than 10 countries have participated in this program. INET has received a contract for this research topic and has participated in relevant studies.
《3.2 R&D progress of nuclear hydrogen production in China》
3.2 R&D progress of nuclear hydrogen production in China
3.2.1 IS cycle [13,14]
In China, nuclear hydrogen production started in the early stages of the 11th Five-year Plan. Intensive fundamental studies on the mainstream technologies of nuclear hydrogen production, including the thermochemical water splitting process and high-temperature steam electrolysis, have been performed. Facilities to verify these principles have been constructed and operated. The feasibility of these technologies for nuclear hydrogen production has been verified.
During the 12th Five-year Plan, R&D on hydrogen production based on HTGRs was supported by the National Science and Technology Major Project known as the High-temperature Gas-cooled Reactor Demonstration Plant. Meanwhile, direct cycle power generation of helium turbine and hydrogen production based on high-temperature reactors were studied. These studies allowed for the development of fourth-generation nuclear power technologies. This research was also intended to permit the understanding and application of key technologies in the IS cycle and high-temperature steam electrolysis, construct an operational IS cycle in an integrated laboratory-scale facility, and achieve continuous closed-cycle operation. In addition, high-temperature electrolysis facilities were built to perform electrolysis experiments.
The INET of Tsinghua University has carried out a comprehensive study on the chemical reactions and separation involved in the IS cycle, including the heterogeneous reaction kinetics, phase equilibrium, catalysts, electrolysis dialysis, and reactive distillation, among many other topics. As a result, many engineering challenges involved in this closed-cycle operation have been overcome, such as the simulation and optimization of the process, transportation of the strongly corrosive and high-density slurry, capability for online measurements, and control of the system. Many outstanding findings concerning these key technologies have been made, and these will now be discussed. First, a tetrahedral phase diagram of the four-element system of the main species involved in the IS cycle was constructed, and the phase-state determination criteria were proposed. A prediction model was then constructed and software that may be used to determine the involved phases was developed. These are useful tools for predicting the phases and composition at each stage in a closed-cycle operation. The second outcome was the development of high-performance catalysts for the decomposition of sulfuric and hydroiodic acids in a high-temperature and strongly corrosive environment. These catalysts allowed for the efficient decomposition of both sulfuric and hydroiodic acids. Moreover, the catalysts showed no significant decrease in performance after 100 h of use. Third, an electrolysis dialysis stack for the concentration of hydroiodic acid, as well as models and software for predicting the properties of the involved materials, mass transfer, and calculating the operating voltage, were developed. This has successfully been applied for solving difficult hydroiodic acid concentrations. A fourth outcome involves the construction of a full-process simulation model, and this was further developed into steady-state simulation software. The reliability of this model and software has been verified experimentally. This software can be applied in the optimization of an IS cycle and can be used to evaluate the process efficiency. The final outcome was the construction of an integrated laboratory-scale facility with a 100 NL/h hydrogen-producing capacity. An operation strategy considering multiple aspects (e.g., the system on-off state, steady-state operation, and typical troubleshooting) was proposed. In addition, 60 hrs of continuous and stable operation with a hydrogen output of 60 NL/h was achieved, thereby verifying the reliability of the developed IS cycle.
3.2.2 HyS cycle [15]
The HyS cycle is another nuclear hydrogen production technology with promising industrial applications. The sulfuric acid decomposition involved in this process is same as that in the IS cycle. Following this decomposition, sulfuric acid and H2 are produced via SO2 depolarized electrolysis(SDE), thereby forming a closed-cycle. Systematic fundamental studies on SDE have been conducted to better understand this process. The influences of the preparation conditions of the membrane electrode components and the electrolysis parameters on the process of electrolysis have been analyzed, thus providing an optimal preparation technique for the membrane electrode assembly. To determine the anode polarizing potentials that occur during electrolysis and the composition of species present at the anode, an in-situ, electrochemical impedance spectral technique capable of analyzing the SDE of a liquid feed was developed. The different polarizing impedances in the electrolysis process were analyzed via experimentation and analysis. According to the research results, an anodized overpotential accounts for a high proportion of the operation voltage and the electrolysis reaction kinetics are controlled by different processes. The electrochemical polarization was determined to be the rate-determining step in SDE under low decomposition voltages, while at higher decomposition voltages, the electrochemical polarization and the concentration polarization represent the rate-determining step. To reduce the anode overpotential and lower the cost of the catalyst, new catalysts for SDE were investigated. A series of activated carbon-supported, Pt-based bimetallic catalysts were prepared by an impregnation-reduction method. The structure, morphology, and electrochemical properties of the catalysts were then analyzed. In addition, the efficiency of SDE under different conditions was discussed. The presence of SO2 membrane diffusion and side reactions in the electrolysis system were confirmed by analyzing the gas products and production rate at the cathode outlet of the electrolysis tank. The efficiency was analyzed using different conditions and resulted in the conclusion that the electrolysis tank is close to 100% efficient when operated at a low temperature and high current density.
The authors built a semi-empirical model for the electrolysis calculations, and the overall simulation of the HyS cycle was realized using this model in combination with Aspen Plus®. Material equilibrium calculations, a sensitivity analysis, and the determination of the hydrogen production efficiency in the HyS cycle were performed using this model. The results of this simulation provided important references and effective tools for the design and optimization of this process.
On this basis, SDE facilities with a hydrogen output of 20 NL/h were constructed. Additionally, electrolysis experiments have been carried out successfully.
3.2.3 High-temperature steam electrolysis [16]
High-temperature steam electrolysis is a simple and highly efficient process. The solid oxide electrolysis cell is a main component of HTSE technology, and it is composed of a ceramic electrolysis cell, metallic sealing frame, bipolar plate, afflux network, base plate, and a roof. The material, chemical, physical, and mechanical properties of these components differ. This device operates at a high temperature (700−850 ℃) and a high humidity (steam content > 70%). Based on intensive studies of HTSE characteristics, many technological challenges, such as adjusting the thermal expansion coefficients of the stack components, sealing, improving the electrical performance of the stack, and mechanically positioning the stack, have been overcome by innovative structural designs, the screening of key materials, and technological investigations. Additionally, an electrolysis cell with excellent performances has been designed and manufactured. An HTSE system for hydrogen production at a laboratory-scale has been designed, manufactured, and tested through operation. Some issues including the provision of a stable supply and accurate control of the steam have been solved, and a program for the long-term stable operation of HTSE has been realized. At this scale, the highly efficient, continuous (115 h), and stable operation of 10 cells (with an effective area of 10 cm ×10 cm) has been realized. The hydrogen output of this system was stable for 60 h and the hydrogen output rate was 105 L/h. The developed stack can operate at a high temperature and humidity, and it is suitable for use in an HTSE. The structural design of the SOEC is innovative, and its operation is reliable, stable, and controllable.
《4 Safety of nuclear hydrogen production》
4 Safety of nuclear hydrogen production
It is important to manage nuclear hydrogen production safely to protect public health and safety, as well as the environment. There are three types of safety issues related to using a nuclear reactor for hydrogen production: first, accidents and hydrogen leakage in hydrogen production plants may occur. It is important to consider the potential damage that may be caused by chemical leakages towards the system, structure, and components of nuclear facilities, including explosive impact waves, fires, and chemical corrosion. Second, events and failures that compromise the safe operation of the system may occur in the heat exchange system. Nuclear hydrogen production involves the use of an intermediate heat exchanger (IHX) that connects the coolant from one loop of the reactor system to the hydrogen production facilities. Failure of the IHX might result in the release of radioactive substances or leakage of fluid from the intermediate loop into the reactor core. Lastly, events that occur in the nuclear facilities may influence the hydrogen production plant and result in radioactive leakage. Tritium (T), which is produced in the reactor, may migrate through the IHX and enter the hydrogen production plant, potentially being included in the final products of this system. In the design of safe nuclear hydrogen production facilities, factors that should be considered include the safe layout of the nuclear reactors and hydrogen production plants, coupling interface between the two facilities, safe design of the IHX, operational optimization between the nuclear reactors and hydrogen production plants, and risk of T leakage [17].
In the conceptual design of nuclear hydrogen production plants, the reactors and hydrogen production facilities are fully separated. This layout protects the reactor from explosions and chemical leakages that may occur in the hydrogen production plant. It also assures the level of radioactivity in the hydrogen production plants is low, thereby classifying the hydrogen production plant as a non-nuclear system. In this design, the pressure of the secondary loop is slightly higher than that of the primary loop, which aids in the separation of the nuclear and hydrogen production systems. H, deuterium (D), and T are isotopes of H and that can penetrate through metals. To prevent H penetration into the primary loop and prevent the penetration of T from the reactor core into the secondary loop, these processes have been investigated. It must be determined whether the radioactive substances should be processed according to the permission standards outlined in the national criteria for civil gas (natural gas).
《5 Economics of nuclear hydrogen production》
5 Economics of nuclear hydrogen production
The potential for the commercialization of nuclear hydrogen production is determined by technological developments, the overall efficiency of the process, and the response of the market to the price of hydrogen. Considering the latter, the future market price of hydrogen has attracted much attention, even though nuclear hydrogen production technologies are in their early stages. Countries emphasizing the development of technologies for nuclear hydrogen production (e.g. America, France, and Japan) and the IAEA have studied the techno-economics of nuclear hydrogen.
The United States Department of Energy evaluated the techno-economics e of nuclear hydrogen production under the NHI program, concluding that the cost of H2 ranged between 2.94–4.40 USD/kg. The IAEA developed an economic evaluation program for hydrogen and the countries participating in the development of this program carried out scenario analyses of the cost of hydrogen production. It was reported that the cost of H2 in these different scenarios ranged between 2.45–4.34 USD/kg. Moreover, the composition of these costs were analyzed. Compared with the hydrogen production by electrolysis based on pressurized water reactor, hydrogen production based on the HTGR has significant cost advantages, which could be attributed to the high efficiency of the thermochemical cycles or the HTSE [18,19].
Although techno-economic analyses have been performed, further studies are being carried out due to large uncertainties involving the choice of reactor, technical procedures, and simulation models. Economic evaluations can provide important references to guide the development and commercial applications of nuclear hydrogen production technologies.
《6 Development and potential applications of nuclear hydrogen production technology based on HTGR in China》
6 Development and potential applications of nuclear hydrogen production technology based on HTGR in China
《6.1 Evaluation of hydrogen production technologies coupled with an HTGR》
6.1 Evaluation of hydrogen production technologies coupled with an HTGR
Considering the characteristics and advantages of different nuclear hydrogen production technologies, a hightemperature reactor may be used to for the large-scale production of concentrated amounts of hydrogen with zeroemissions. Therefore, the hydrogen production technologies that are selected to couple with high-temperature reactors should also possess these characteristics.
In the transformation of nuclear energy to hydrogen energy, electrolysis is the most mature technology that can be applied with the use of surplus nuclear power from nuclear reactor to produce hydrogen. The nuclear-assisted reforming of fossil fuels can replace the fossil fuel used as heat source with nuclear-derived heat, which reduces the use of fossil fuels and lowers emissions. For example, hydrogen production technologies based on natural gas reforming with the assistance of heat generated from an HTGR reduces the amount of natural gas used by 30% and decreases CO2 emissions by 30%. This technology can be used as a transition technology for nuclear hydrogen production in the coming years. The coupling of the reactor and a hydrogen production plant, studies on the safety of nuclear hydrogen, application of relevant licenses, and economic evaluations of nuclear hydrogen production technologies must be further explored and promoted.
In the long run, the high-temperature process heat derived from the nuclear reactor should be used as the heat source for the thermochemical cycle and HTSE. Moreover, water should be used as the raw material from which hydrogen is produced such that CO2 emissions are minimized. Technological characteristics (e.g., hydrogen output, H2 purity, end use, and waste management), costs (e.g., H2 price, applicability of a hypothesis for techno-economics, and R&D) and risks (e.g., the development status and maturity of technologies, as well as risks involved in R&D) will be considered as nuclear hydrogen production technologies are developed. Considering the characteristics of nuclear hydrogen production technologies, industrial fields that require the large-scale production of clean hydrogen, such as ammonia synthesis, hydrogen metallurgy, petroleum refining, coal liquefaction, and biomass refining, represent appropriate end uses. The scale of hydrogen production plants should be in the order of 1×105 m3 /h of hydrogen. Both the thermochemical cycle and HTSE are highly efficient and have zero carbon emission. The former mainly involves chemical engineering technologies and complicated processes, but is easy scale-up and applicable to large-scale hydrogen production. The latter mainly involves challenges involving the required materials and simple processes; it is applicable towards medium and small-scale hydrogen production.
《6.2. Application prospects of nuclear hydrogen production with HTGRs》
6.2. Application prospects of nuclear hydrogen production with HTGRs
HTGRs have a unique advantage considering their high-temperature process heats. Since the early development stages of this reactor, coupling with nuclear hydrogen production has been considered. The proposed applications of the produced hydrogen include direct reduction iron-making, ammonia synthesis, coal liquefaction, and refining [20]. Because HTGRs can simultaneously provide large-scale H2 production, electricity, and heat, the produced energy is well-utilized in its operation. Hence, this technology is especially applicable to industries with such demands.
The effects of H2-based direct reduction iron-making on the hydrogen production process based on an HTGR have been preliminarily analyzed. Fig. 5 shows the coupling between an HTGR and the iron-making process, and this coupling has been shown to significantly reduce the emission of CO2 and other harmful substances during this process.
《Fig. 5》
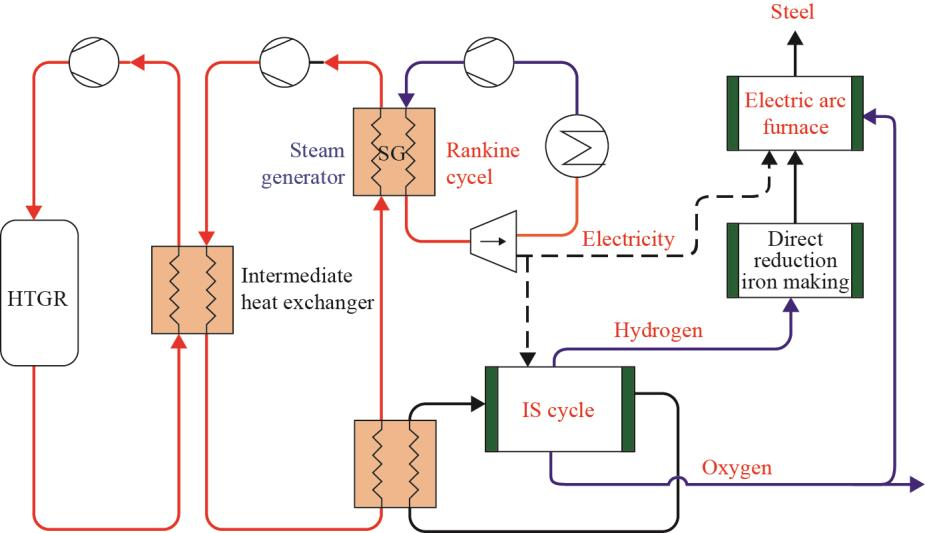
Fig. 5. Principle of nuclear hydrogen production coupled with direct reduction iron-making.
《6.3 Development of nuclear hydrogen production with HTGRs》
6.3 Development of nuclear hydrogen production with HTGRs
The overall objective of nuclear hydrogen production involving HTGRs is to demonstrate the feasibility of nuclear hydrogen production and its applications in related industries. HTGRs may be used to provide a large-scale supply of heat, electricity, hydrogen, and oxygen to other industries. They represent an important technological basis for energy-saving and emission-reducing processes, and allow for the advancement of certain products in China.
According to developmental laws related to nuclear hydrogen production technologies based on HTGRs and international R&D plans, the following course of development has been proposed: verification of the system principles and system integration; R&D of required materials and components; engineering tests; and finally, commercial demonstration. Thus far, the first stage of principle verification and system integration has been accomplished. The subsequent plan for the second stage, R&D, is described below.
By 2020, research on key components for nuclear hydrogen production will have been accomplished. In particular, the R&D of engineering materials for the IS or HyS cycles, the development of key components, as well as the construction and experimental verification of a system that simulates the supply of helium heating by HTGR will be undertaken. Based on these findings, pilot-scale tests of nuclear hydrogen production may be performed. Currently, relevant R&D activities are being carried out in major national scientific and technological projects.
By 2025, the pilot-scale testing of nuclear hydrogen production will be performed. At this stage, the R&D of key components and design of hydrogen production plants, along with the construction of a pilot-scale hydrogen production plant with a hydrogen output of 1000 m3 /h, will be undertaken. The conceptual design of a demonstration plant for nuclear hydrogen production based on a commercial HGTR will also be accomplished.
By 2030, the use of a very/high-temperature reactor for nuclear hydrogen production coupled with hydrogen metallurgy will be performed.
《7 Conclusions》
7 Conclusions
Nuclear hydrogen production is an efficient, clean, large-scale hydrogen production technique. It is expected to play an important role in providing a large-scale supply of hydrogen in the future. The comprehensive utilization (involving the general supply of hydrogen, electricity, and heat) of heat derived from a high temperature reactor drives nuclear hydrogen production and provides an important method of technological reform for multiple industries in China. It may play an important role in updating current products, lessening pollution, and reducing carbon emissions.
Supported by the national major scientific and technological project known as the “Advanced Pressurized Water Reactor and High-temperature Gas-cooled Reactor Demonstration Plant”, research on nuclear hydrogen production has shown tremendous progress. Nowadays, key components for and prototypes of these systems are under development. A pilot-scale demonstration is planned during the 14th Five-year Plan and a nuclear hydrogen production/hydrogen metallurgy project based on an HTGR will be demonstrated during the 15th Five-year Plan.
《Acknowledgments》
Acknowledgments
This project was supported by the National S&T Major Project (Grant No. ZX06901)














 京公网安备 11010502051620号
京公网安备 11010502051620号




