《1. Introduction》
1. Introduction
China is the largest producer and user of ordinary Portland cement (OPC). In 2018, the production of 2.4 × 109 t of cement was estimated to consume 3.0 × 109 t of limestone, 7.2 × 108 t of clay, and 3.2 × 109 J of energy, while emitting 2.4 × 109 t of carbon dioxide (CO2). The CO2-emission reduction target [1] has driven the entire cement and concrete industry to look for cleaner production technology and alternative binding materials in the past ten years. Alkali-activated materials (AAMs), also known as geopolymers, are manufactured by chemical reactions between an alkaline activator and reactive aluminosilicate materials [2–4]. The reaction process, polymerization mechanism, and products of AAMs have been extensively studied [5–19]. This manufacturing process, which occurs at room temperature or at a slightly elevated temperature (60–80 C) is completely different from the traditional milling and calcination processes of OPC. Because the raw materials are widely available and include industrial wastes such as slag (SG), steel slag, fly ash (FA), and thermally activated clay (e.g., metakaolin (MK)), AAMs are much more environmentally friendly than OPC. Despite being greener, AAMs have been reported to have mechanical properties that are comparable to those of OPCs and, in most cases, to have superior durability [20].
Depending on the raw materials, different types of AAMs have very different reaction mechanisms, product composition characteristics, and mechanical properties. Zhang [21], Adamiec et al. [22], Lothenbach et al. [23], and Khatib [24] have classified AAMs into calcium-free, low-calcium, and calcium-rich, according to the calcium content in the aluminosilicate raw materials. Representative raw materials are MK, FA, and SG (Fig. 1). Attempts are being made to use many other aluminosilicate raw materials and industrial waste solids to produce AAMs, such as red mud, steel slag, and heated coal gangue. The products of calcium-free/lowcalcium systems are mainly zeolite-like (such as analcite, sodalite, etc.) gels, while those of calcium-rich systems are calcium aluminium silicate hydrates (C-(A)-S-H) gels with a low calcium/silicon (Ca/Si) ratio. Attempts have been made to use many other industrial wastes containing potential reactive components to manufacture AAMs; however, trace amounts of components present in the industrial wastes may affect the performance of AAMs, especially in terms of durability [25–27].
《Fig. 1》

Fig. 1. SiO2-Al2O3-CaO ternary diagram of the aluminosilicate materials MK, FA, and SG, which can be used in AAMs. A comparison of these materials with cement and lime stone is shown. Reproduced from Ref. [22] with permission of Chinese Society of Particuology and Institute of Process Engineering, Chinese Academy of Sciences, © 2008.
This review reports on recent studies on the durability of AAMs, particularly in comparison with OPC, for a better understanding of when and where AAMs can be used as OPC alternatives. Four aspects of AAMs durability are summarized: sulfate attack, acid corrosion, carbonation, and chloride penetration. This review provides critical information for further studies toward the development and applications of different AAMs under various conditions.
《2. Sulfate attack》
2. Sulfate attack
Sulfate attack is one of the most serious issues affecting the durability of concrete. The deterioration mechanism includes a set of complex physical and chemical reactions. External or internal sulfate ions of the concrete react with the hydration products of OPC, leading to expansion, cracking and, finally, disintegration of concrete elements [28–32]. C3A and C3S contents and concrete density are considered to be the main inter-factors affecting the sulfate resistance of concrete. C3A is considered to be a key factor in the formation of ettringite, as its hydration product supports the aluminum (Al) phase as a reactant. In addition, the ettringite phase is affected by the content of C3S, since its hydration product CH is one of the factors contributing to the formation of expandable gypsum and ettringite (Fig. 2) [33,34]. It has also been found that sulfate attack results in decalcification of calcium silicate hydrate (C-S-H) gel, mainly due to the continuous formation of gypsum under low pH conditions as CH is continuously consumed. Thaumasite usually forms when C-S-H gels react, and causes softening of concrete, reduction of strength, and even complete damage [35,36].
《Fig. 2》

Fig. 2. Microstructure of OPC mortars after sulfate attack in Na2SO4 solution. (a) Gypsum formation; (b) ettringite formation. Reproduced from Ref. [34] with permission of co-author KW Liu, @2010.
Calcium-free AAMs (e.g., MK-based AAM) contain totally different products from OPC systems [37]. Sulfate attack on calciumfree/low-calcium AAMs is an exchange process, in which cations exchange with the components of gels, resulting in increased porosity [38]. However, calcium-rich AAMs (e.g., alkali-activated slag (AAS)) contain C-(A)-S-H gel with a lower Ca/Si ratio than that of C-S-H in an OPC system [39]. The sulfate resistance mechanism of calcium-rich AAM is similar to that of OPC because of the similarity of the reaction (hydration) products. On the other hand, MKbased AAM has a different sulfate resistance mechanism because the main products are sodium aluminosilicate hydrate (N-A-S-H) gel. Therefore, the design and use of AAMs as sulfate-resistant alternatives to OPC should involve a careful consideration of the type of AAM.
Karakoç et al. [39] found that the compressive strength of AAS decreased with the increase of MgSO4 concentration and immersion time, while the appearance showed little change after 24 weeks of immersion. Alcamand et al. [38] adjusted the content of CaO and the matrix composition (in terms of the SiO2/Al2O3 molar ratio) of AAM by adding slag and silica fume into MKbased AAM. Ideally, this AAM structure should be stable enough to resist sulfate attack. However, the Ca2+ in (C, N)-A-S-H gel can be exchanged by external cations during sulfate attack. Therefore, the incorporation of Ca has a certain negative effect on sulfate resistance. It is evident from this research that N-A-S-H gel, as the main product in low-calcium/calcium-free AAMs, has a better resistance to sulfate attack (Fig. 3). In comparison, C-(A)-S-H gel, as the main product in calcium-rich AAM, has worse sulfate resistance to the formation of gypsum and ettringite [38,40,41].
《Fig. 3》

Fig. 3. Minerals formed on the surface of the specimens after 180 d of magnesium sulfate attack. (a) Sound AAM made with 100% MK; (b) 80/20 MK/silica fume; (c) 80/20 MK/ SG; (d) 60/40 MK/SG. M: magnesium sulfate; C: sodium carbonate; X: sodium silicate; S: sodium sulfate; O: silicon oxide; E: etringite; G: gypsum. Reproduced from Ref [38] with permission of Elsevier Ltd. and Techna Group S.r.l., ©2018.
In many previous studies on the durability of AAMs, it was highlighted that AAMs produced from MK do not produce sulfatealuminate minerals such as ettringite during the hydration process. Ettringite- and gypsum-type sulfate attack products are avoided, so calcium-free or low-calcium AAMs have better resistance to sulfate attack [37,42–48]. Tao et al. [49] investigated the sulfate resistance by measuring the strength retention rate; their results showed that the strength retention rate of MK-based AAM was much higher than that of OPC in Na2SO4 solution after 28 d. Hou et al. [50] found significant differences in the compressive strength and apparent density of FA-based AAM after 150 d of immersion in different sulfate solutions, and considered that the effect of the sulfate solution on the properties of FA-based AAM was related to diffusion. In addition, the durability of the samples in sulfate solution was related to the type of cation of the activator, the cation in the sulfate solution, and the concentration of the solution.
Tang et al. [51] found that during sulfate attack, both the compressive strength of FA-based AAM concrete and that of OPC concrete showed a decreasing trend first, followed by an increasing trend. The mass change of the attacked group was not large (as shown in Fig. 4) in 5% Na2SO4 solution. FA-based AAM concrete prepared with recycled aggregate showed high compressive strength rather than damage in 5% Na2SO4 solution with a drywet cycle [52]. Palomo et al. [53] and Bakharev [54] had similar conclusions. In a study of the performance change and microstructure evolution of FA-based AAM in a sulfate environment, Tang et al. [55] found that there were no crack or spalling phenomena after sulfate attack for 60 d. It was concluded that FA-based AAMs will not produce expansive products that are harmful to the structure in a sodium sulfate environment.
《Fig. 4》

Fig. 4. Compressive strength of (a) OPC concretes and (b) FA-based AAM concretes after immersion in 5% Na2SO4 solutions for 27–59 d. Reproduced from Ref. [51] with permission of Material Reports, © 2015.
Dzˇunuzovic´ et al. [56] studied the effect of Na2SO4 solution on the durability of alkali-activated FA–SG blended materials (Table 1). The composition change of the Na2SO4 solution was analyzed. The Si found in the solution may be from the dissolution of the unreacted alkaline activator component of the AAM or the silicon-rich component of the gel structure. The increase of Ca concentration in the solution indicates that the calcium-containing hydrates such as C-S-H gel and C-(A)-S-H gel have poor sulfate attack resistance compared with the N-A-S-H gel. Ismail et al. [57] suggested that dissolution of Ca in AAM may be due to the reaction with Na2SO4 or ion exchange in sulfate solution. Zheng et al. [58] studied the sodium sulfate resistance of AAM mortars and cement mortar under dry-wet cycling conditions. It was found that the coefficient of compressive strength of AAM mortars was better than that of OPC mortars after 75 cycles. AAM mortars contained only the sodium sulfate phase as corrosion products.
《Table 1》
Table 1 pH and composition analysis of the 5% Na2SO4 solution before and after interaction with alkali-activated FA–SG materials.
Elyamany et al. [59] found that the water absorption and porosity of FA-based AAM decreased with the increase of curing temperature and molar concentration of the activator, and that the corrosion resistance to MgSO4 was therefore improved. However, gypsum crystals were detectable in the AAM samples after MgSO4 corrosion, and the microcracks after corrosion were attributed to the formation of gypsum. Jin et al. [60] found that the compressive strength of FA-based AAM first decreased and then increased in 5% MgSO4 solution, which indicated that the diffusion of Mg2+ into AAM concrete and the migration of alkali metal cations into the solution occur simultaneously. It can be inferred that the two processes will eventually reach equilibrium after a long period of twoway ion diffusion. This evidence shows that the Ca concentration of AAMs and the sulfate type (i.e., sodium or magnesium) are the two most critical factors affecting their sulfate resistance performance.
Sulfate attack is a dynamic process for both OPC and AAM, and the corrosion mechanisms depend on the nature of the hydration products. The attack on OPC mainly consists of a series of chemical reactions and physical impacts that generate extra crystals and internal stress. Normally, the softening of cement concrete is due to the decalcification of C-S-H gel, while expansion and cracking of the structure are caused by the formation of gypsum and ettringite [61]. Calcium-rich AAMs usually produce C-(A)-S-H gel, so the sulfate attack mechanism is more or less similar to that of OPC. For calcium-free or low-calcium AAMs, however, the ion exchange reaction occurs between the sulfate solution and the network-like structure. During the attack, the pores of the network structure of the N-A-S-H gel change, and microcracks are gradually formed, leading to deterioration of the AAM structure.
《3. Acid corrosion》
3. Acid corrosion
The acid corrosion resistance of OPC cements and concretes is rather poor because of the nature of high pH and the porous matrix. Acid can react with CH and C-S-H gel in cement concrete to form non-gelling or water-soluble substances, resulting in the destruction of concrete. Acid corrosion also causes the decomposition of calcium silicate hydrates and calcium aluminates hydrates, and thus destroys the cementitious binder and reduces the strength of the concrete [62]. Wang et al. [63] found that the surface of concrete soaked in acid solution (pH = 2) first formed a layer of white sticky substance, and then became softened. Ning et al. [64] found that acid corrosion began at the surface of the sample, and the extent of corrosion in the concrete was inversely proportional to the pH value of the solution. Alexander et al. [65] reported that CH on the surface of OPC was first consumed by the reaction with acid, which increased the porosity and allowed the acid to erode the interior part. With a drop in the pH of the pore solution below 12.4, C-S-H gel will decalcify, accompanied by the dissolution of Al2O3–Fe2O3-mono (AFm) and Al2O3–Fe2O3-tri (AFt).
AAMs usually show better acid resistance than OPC [66–68]. Table 2 summarizes recent research findings on different acids that attack AAMs [68–75]. Decalcification also occurs in calcium-rich AAM; however, because AAMs have lower permeability than OPC, this process is much slower. In addition, a dense layer of aluminosilicate gel can prevent corrosion [76,77].
Bouguermouh et al. [72] considered that the main factors affecting the acid resistance of AAMs were the mineral composition of the aluminosilicate raw materials and the types of alkali metal cation in the activators. The N-A-S-H gel in MK-based AAM was only slightly affected by acid corrosion (0.1 mol·L-1 HCl). Although cracks caused by shrinkage of the gel layer did exist, the material still maintained a good structure. Jin et al. [78] found that the appearance of MK-based AAM remained almost unaffected during the corrosion cycle of acid rain (molar ratio SO42-/NO3- = 3/4); X-ray diffraction (XRD) analysis showed no significant compositional change.
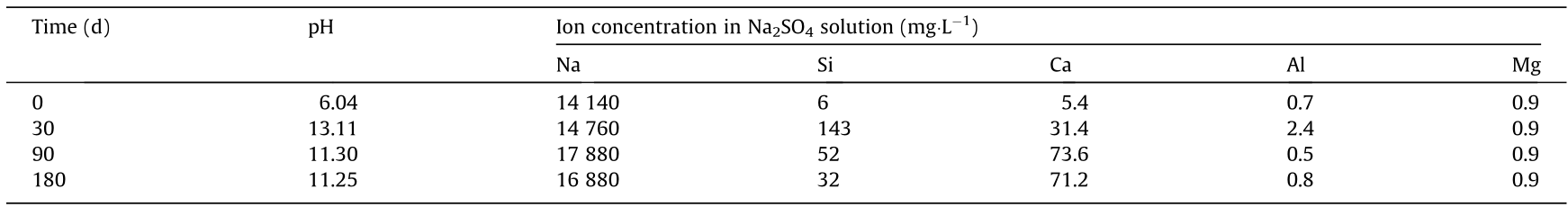
Zheng et al. [79] immersed FA-based AAM in 5% H2SO4 solution for 28 d, and found that the specimen still met the acid soaking safety index of the Chinese standard GB 50212–2002 “Specification for construction and acceptance of anticorrosive engineering of buildings.” Zhao et al. [80] reported that N-A-S-H gel, as the main product of FA-based AAM, has good HCl resistance, and that the structure was slightly affected by acid. When the concentration of calcium in the FA is high or when the acid type changes, the acid resistance may decrease. Mehta and Siddique [81] found that the SO42- in sulfuric acid will react with Ca2+ in the product to form CaSO4 (as shown in Fig. 5), in addition to H+ reacting with the body material in the corrosion process of FA-based AAM. The interaction of two corrosion processes aggravates the deterioration of the sample.
《Fig. 5》
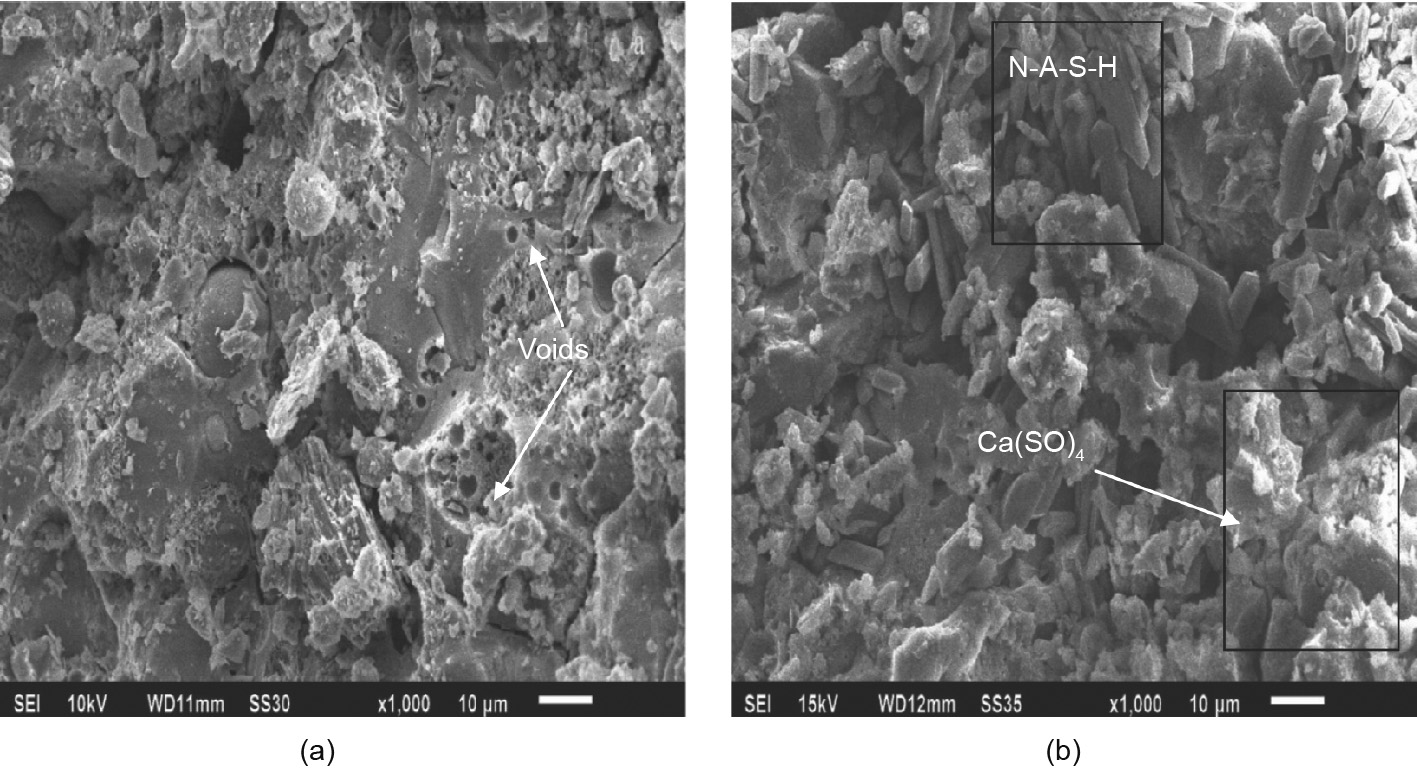
Fig. 5. Scanning electron microscope (SEM) images of FA-based AAM with the addition of 10% OPC (a) before and (b) after corrosion in 5% H2SO4 solution for 365 d. Reproduced from Ref. [81] with permission of Elsevier Ltd., © 2017.
Both AAMs and OPC are alkaline materials, and a neutralization process occurs when they come into contact with acid. The findings reported in Table 2 indicate that the acid resistance of AAMs is generally better than that of OPC due to their intrinsic composition and structural characteristics. Shi and Stegemann [82] and Beddoe and Schmidt [83] reported that the corrosion resistance of cement paste depended on the protective layer or on the hydration product itself, rather than on the pore permeability of hardened paste during the process of acid corrosion. Gutberlet et al. [84] studied the resistance of OPC to H2SO4 acid corrosion at pHs of 2, 3, and 4, respectively, and found that the surface of OPC will form a porous layer. The transition zone between the deteriorated layer and the non-corroded material is shown in Fig. 6. The surface layer and the transition zone are the key factors that determine the durability of materials. If the surface layer is relatively dense, it can effectively isolate and protect the internal material from further acid corrosion.
《Table 2》
Table 2 Research works on different types of acid corrosion of AAMs.
《Fig. 6》

Fig. 6. (a–c) Degraded OPC samples after storage in H2SO4 solution for 28 d at a pH of (a) 4, (b) 3, and (c) 2. (d–f) Micrographs of the corresponding cross-sections. Reproduced from Ref. [84] with permission of Elsevier Ltd., ©2015.
《4. Carbonation》
4. Carbonation
The carbonation of concrete refers to a neutralization process in which the acidic gas CO2 in the air reacts with the aqueous alkaline substance of the concrete, resulting in a decrease of alkalinity and a change in the chemical composition of the concrete [85]. Carbonation itself produces no obvious damage to OPC concrete. The main problem is the decrease of alkalinity causes damage to passivation film of protects the steel bar. With the participation of water and air, the steel will rust; eventually, the structure will be destroyed due to the internal expansion stress [86]. Shi et al. [87] pointed out that the carbonation reaction can lower the pH value of concrete from an initial value above 13 to roughly 8. It also causes changes in the pore size distribution and the porosity of hardened cement paste. Furthermore, it will affect the diffusion of harmful ions (i.e., Cl-, SO42-) in concrete. The C-S-H gel is preferably present as an irregular cluster structure instead of as a densely arranged structure [88]. The decrease in the pH of the pore solution during carbonation accelerates the decomposition of Friedel salts (calcium monochlorate aluminate) in hardened cement paste, which increases both the porosity and the diffusion coefficient of Cl- in concrete [89,90]. Papadakis [91] simulated the carbonation process of cement-based materials as follows: First, CO2 diffuses into the pores of cement-based materials, while the hydration product CH dissolves in the pore solution. Then, CO2 dissolves in the pore solution and reacts with CH to form CaCO3. Normally, the main products of natural carbonation for CH are calcite, nepheline, and aragonite [92]. The carbonation process is accompanied by microstructural changes, such as the formation of CaCO3, polymerization of decalcified C-S-H gel, and decomposition of ettringite [93]. C-S-H gel and CH, as the main cement hydration products, are prone to carbonization [94]. The process of C-S-H gel carbonation involves the decalcification of C-S-H gel and, finally, the formation of amorphous gel. The whole process is accompanied by an overall reduction in the volume of the products and an increase in porosity and pore size [95,96].
The carbonation mechanism of AAMs differs from that of OPC. Moreover, the reaction mechanisms of AAMs in calcium-rich and low-calcium systems are different. During the carbonation process of calcium-rich AAMs, CO2 dissolves into the pore solution to form carbonic acid, and directly reacts with C-(A)-S-H gel to form CaCO3. However, N-A-S-H gel, the main product in the low-calcium system, does not undergo the decalcification process, and carbonation of N-A-S-H gel in this case is mainly the transformation of the pore solution from high alkalinity to a high concentration of sodium carbonate [97,98].
Products of AAS are mainly C-(A)-S-H gels with low crystallinity and a uniform and dense amorphous feature. In carbonation, since the product does not contain CH, the dynamic equilibrium of Ca2+ is directly maintained by the decalcification of C-(A)-S-H gel [99]. Due to decalcification, C-(A)-S-H gel undergoes volume shrinkage and an increased degree of polymerization. Further carbonation transforms the gel into silica gel, resulting in greater shrinkage [100]. In the case of natural carbonation, the main carbonation product of AAS is Na2CO3 ·10H2O; under accelerated carbonation conditions, it is NaHCO3 [101]. Chen et al. [102,103] found that the carbonation rate of AAS mortar was higher than that of OPC mortar during accelerated carbonation with 20% CO2 concentration. Carbonation will cause a large shrinkage of mortar, which will result in microcracks around the aggregate, and will increase the porosity of the paste and the CO2 diffusion coefficient of the mortar. Yu et al. [104] reported that the amount of activator can affect the carbonation resistance of AAS. With an increase of sodium silicate, the carbonation resistance of AAS can gradually be improved under accelerated carbonation conditions with 20% CO2 concentration. Dong et al. [105] used mineral powder and Pisha sandstone as raw materials to prepare AAMs and conducted a carbonation study under accelerated conditions. They reported that the carbonation resistance was enhanced when the amount of NaOH was increased.
For low-calcium AAMs, Li ZG and Li S [106] found that the number of microcracks in FA-based AAM decreased significantly after the carbonation process under the conditions of 5% CO2 concentration, and the microstructure of the FA-based AAM became denser, as shown in Fig. 7. The pH of the pore solution decreased substantially to about 11 throughout the carbonation process. Bernal et al. [107] proposed that the main product of FA-based AAM, N-A-S-H gel, is stable during carbonation. However, the dissolution of NA-S-H will occur only when OH– from the pore solution reacts with carbonic acid. When calcium is added—for example, by blending SG in FA-based AAM [106] or blending SG in MK-based AAM [108]—the carbonation resistance of AAMs generally decreases.
《Fig. 7》
Fig. 7. SEM images of FA-based AAM (a) before and (b) after a carbonation test with 5% CO2 concentration. Reproduced from Ref. [106] with permission of Elsevier Ltd., © 2018.
According to Pouhet and Cyr [109], the carbonation reaction in the pore solution of calcium-free AAMs with natural CO2 content can be divided into two separate phases followed by stabilization of the pH: Phase 1 comprises the nearly complete carbonation of the pore solution during the first two weeks of exposure to atmospheric CO2, resulting in the formation of Na2CO3 and a pH value of around 12 being reached. Phase 2 comprises the evolution of the carbonate/bicarbonate phase equilibrium, resulting in the formation of bicarbonate (10% at 180 d) and a pH of around 10.5. The pH value of the pore solution is higher than the steel depassivation limit of 9. The compressive strength of MK-based AAM remains unchanged (Fig. 8).
《Fig. 8》

Fig. 8. pH evolution of the pore solution and compressive strength of MK-based AAM cured under natural CO2 content. Reproduced from Ref. [109] with permission of Elsevier Ltd., © 2016.
It can be concluded that the carbonation resistance of AAMs is not as good as that of OPC, but that the pH value of AAMs after carbonation can be maintained at above 10.5. However, there are a few conflicting findings. Huang et al. [110] reported that the strength of OPC improved after a carbonation test with 20% CO2 concentration, while the strength of FA-based AAM was not affected by carbonation. Sufian Badar et al. [111] prepared FAbased AAM under accelerated carbonation for 450 d, and found a remarkable decrease in the pH, a consequent increase in the total porosity, and a reduction in the mechanical strength properties.
The reported differences in the carbonation resistance of AAMs are probably related to differences in measurement methods. According to Huang et al. [112], phenolphthalein indicator and the pH value cannot effectively determine the carbonation resistance of FA-based AAM. Yu et al. [104] reported that the test method has an impact on the measurement of the carbonation resistance of AAMs. The results obtained by the electrochemical method and the borehole sampling method are more consistent than those obtained using phenolphthalein indicator. Natural carbonation and accelerated carbonation will also affect the experimental results. Bernal et al. [101] proved that the carbonation products of AAS by these two methods are different. The increase in CO2 concentration will directly affect the equilibrium of CO32-/HCO3- , and will thus have a significant influence on the pH value. When OPC is carbonized, the consequent pH value, mineral composition, and pore structure also depend on CO2 concentration [113].
In addition, the characteristics of the raw materials used to prepare the AAMs will affect the carbonation results. There is no standard, and just a few recommendations have been made; for example, FA-based AAM needs a high concentration of NaOH solution, and curing at 60 °C for 48 h will result in more complete polymerization [114,115]. The physical and chemical characteristics of the raw materials differ from one place to another, resulting in differences in the properties and microstructure of the AAMs [116]. Bernal et al. [117] considered that AAS microstructure development greatly depends on the chemical compositions and mineral compositions of SG. The type of activators and binder volume of AAS will exert a great influence on the carbonation resistance. Furthermore, the water content of the porous structure in AAS will affect the carbonation experiment. It has been shown that when the relative humidity (RH) of the OPC pore structures varies between 50% and 70%, the CO2 diffusion rate is promoted, whereas when the RH is higher than 70%, the CO2 diffusion rate will decrease because pores are filled with water [118]. These differences in the raw materials, experimental conditions, and internal water content will surely lead to different results in the carbonation resistance of AAMs.
Efflorescence is a process that involves carbonation. The efflorescence reaction is similar to the carbonation reaction of calcium-free/low-calcium AAMs (the corrosion process only consumes free alkali and the substrate will not cause damage) [119,120]. Kani et al. [121] pointed out that when the concrete column touched damp soil at the bottom, water migrated to the surface of the concrete through capillary action and evaporated, and soluble alkali dissolved, reacted with CO2 from the air, and eventually precipitated on the surface of the concrete with water evaporation. Pouhet and Cyr [109] pointed out that efflorescence did not cause real durability problems for OPC, but could affect the appearance.
AAMs contain large amounts of soluble alkali (e.g., NaOH and KOH). Provis et al. [122] reported that Na+ is not stable in the network structure and can be dissolved in the pore solution of AAMs. Several reasons for efflorescence have been determined, and include: ① Efflorescence can be caused by the open pores of AAMs and a low degree of polymerization; ② an excessive amount of alkali activator leads to high alkali concentration in the pore solution; and ③ Na+ has a poor binding ability with the network structure of AAMs [123–125].
Zhang et al. [119,126] considered that the main factors influencing the efflorescence of FA-based AAM involve the alkali content of the activator, the type of activator, and the curing temperature. Among these factors, the alkali content of the activator was considered to be the most important factor affecting efflorescence. Free alkali inside the AAM will cause efflorescence under partially dry and wet conditions (as shown in Fig. 9). The larger pores and higher total porosity in the internal structure of the AAM contribute to fast alkali leaching, causing more severe efflorescence. However, efflorescence on the surface of the sample will not change the mineral composition of the material itself.
《Fig. 9》

Fig. 9. (a) Deterioration of FA-based AAM after 7 d in contact with water at the bottom; (b) deterioration of low-temperature cured FA-based AAM after 90 d of exposure to simulated efflorescence conditions with the regular addition of water at the bottom; (c) schematic drying of FA-based AAM in contact with water and the consequent crystallization. Reproduced from Ref. [119] with permission of Elsevier Ltd., ©2018.
《5. Chloride penetration》
5. Chloride penetration
Chloride penetration is the major factor that leads to steel corrosion in concrete. Due to its significant effect, chloride penetration is an important issue for concrete durability. Corrosion damage of steel-reinforced OPC concrete structures under the attack of Cl– usually manifests as surface corrosion of reinforcement bars and the cracking and spalling of concrete cover due to the formation of expansive products during steel corrosion [127]. Cl– ions in concrete come from two sources: internal Cl– from the components of concrete mixtures and external Cl– from the environment. Partial Cl– dissolves in the pore solution as free chloride, and partial Cl– is combined in the cement hydration products by means of chemical/physical binding [128]. The main factor affecting chloride ion penetration is the porosity of the cement paste. The coarser the pore structure is, the more open the pores are, and the greater the penetration is. The capability of the cement binder to solidify chloride ions also affects the permeability of chloride ions in concrete [129,130]. Martı´n-Pérez et al. [127] pointed out that the chloride-binding capacity of cementitious materials has an important effect on the chloride migration rate and steel corrosion in concrete. Xie et al. [130] reported that steel corrosion occurred when free Cl– reached the surface of the steel bar at a certain concentration. Because carbonation will affect the pore structure of the cement paste, there is a close relationship (a coupling effect) between carbonation and chloride penetration. This is a complex process of Cl– migration in concrete, which simultaneously includes diffusion, capillary absorption, and infiltration [131]. The factors affecting the chloride resistance of OPC concrete in studies [129–137] are shown in Table 3.
《Table 3》
Table 3 Factors affecting the chloride penetration of OPC concrete.

AAMs have a dense microstructure and the chemical compositions of pore solution are complex; however, the relationship between chloride penetration and porosity follows a similar trend as in OPC systems, albeit with a lower penetration rate [138]. For example, the chloride penetration coefficient of FA-based AAM is one third that of OPC at the same strength grade [139]. At present, chloride ion transport test methods for OPC concretes include the natural diffusion method and various accelerated diffusion methods [132]. Further research is required to know whether these methods can be directly applied to perform chloride penetration testing on AAMs.
Researchers [140–148] systematically studied the factors influencing the chloride penetration of AAMs, which are summarized in Table 4 and Fig. 10. Zhou [149] found that when a NaOH solution with a higher concentration was used in the manufacture of AAM, a higher reaction extent of FA was achieved, and the porosity and chloride penetration were subsequently reduced. Calcium-rich AAMs are considered to have better anti-chloride permeability than OPC. With an increase in age, the durability advantage of AAS over OPC is more obvious [150]. Shi et al. [151] showed that the AAS reaction product, C-(A)-S-H gel and the hydrotalcite phase, could adsorb Cl–, and that there was less free Cl– in the early stage of chloride penetration, so the reinforcement had no obvious rust phenomenon in this period.
《Table 4》
Table 4 Factors affecting the chloride penetration of AAMs.

《Fig. 10》

Fig. 10. Langmuir isotherm fittings between free and bound chlorides depending on the activator type of AAS [141]. Cb : concentration of bound chloride; Cf : concentration of free chloride.
Chen et al. [152] discovered that the penetration of Cl– in hardened AAS paste mainly depended on connected pores. The utilization of silica fume in the AAS improved the resistance to chloride penetration, since silica fume effectively filled the pores and refined the pore structure. Zhang et al. [153] added lithium SG into AAS and obtained reduced chloride permeability, mainly because of the adsorption of Cl– onto the structure and the filling effect of lithium SG particles. In addition, Khan et al. [154] found that due to the formation of the hydrotalcite-like phase, AAS binders have a better capacity for Cl– adsorption than OPC. It was reported that the two types of layered double hydroxides (LDHs)—that is, the magnesium–aluminum (Mg-Al) hydrotalcite-like phase and strätlingite—that are present as the two reaction products in AAS both have Cl– uptake capacity, but their mechanisms are different: Chloride uptake in hydrotalcite structures is governed by surface adsorption, while that in strätlingite shows the formation of a hydrocalumite-like phase and ion exchange [155].
《6. Conclusions and perspectives》
6. Conclusions and perspectives
This paper presents the most recent research results from durability studies on AAM materials and OPC systems in terms of sulfate attack, acid corrosion, carbonization, and chloride penetration. The following conclusions can be drawn based on the results and discussion:
(1) In general, AAMs have better durability than OPC. However, calcium-free/low-calcium AAMs and calcium-rich AAMs show significant differences in specific durability studies due to the difference in reaction products. The mechanisms of strength loss, pore structure change, cracking due to chemical attacks on N-A-S-H in calcium-free AAMs, and cracking due to chemical attacks on C-(A)-S-H in calcium-rich AAMs are substantially different. Furthermore, because of the similarity of the products of calcium-rich AAMs (e.g., AAS) and OPC, the degradation mechanisms of binders and their concretes that are exposed to carbonation, acid, and sulfate attack are more or less the same.
(2) The raw materials used to manufacture AAMs are no longer only MK, FA, and SG. Attempts are being made to use a wide range of solid wastes. At present, the core aim of using solid wastes is to recycle. Thus far, not much effort has been spent on relevant standards to guide this utilization. Some minor components (e.g., MgO) in raw materials may have unknown effects on the durability of AAMs. This raises concern in industry, and deserves more research in the future.
(3) Durability tests of AAMs are based on the testing methods for OPC cements and concretes. AAMs and OPC-based materials differ in terms of reaction mechanism, products, and microstructures, and it is still controversial whether such testing methods are applicable or reasonably reflect the true trend of durability. This topic requires further validation in both laboratory and field study (which will only be practicable when a certain volume of AAMs is used in the future).
(4) Some upgrading technologies can improve the corrosion resistance of AAMs, mainly by achieving a higher degree of reaction, refined pore structure, and an improvement of density. The utilization of an appropriate amount of nano-SiO2 in AAMs can improve the microstructure of the products and the carbonation resistance. LDHs have interlayer ion exchangeability, which can uptake chloride ions and other harmful ions that penetrate into cementitious materials. The incorporation of LDHs into AAMs holds great potential for enhancing their durability.
《Acknowledgements》
Acknowledgements
Financial support from the National Natural Science Foundation of China (51778003, 51878263, and 51608004), Opening Foundation of State Key Laboratory of High Performance Civil Engineering Materials (2018CEM002), Anhui Provincial Education Department (gxfxZD2016134), and Anhui Province Higher Education Revitalization Program ([2014]No.11).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Aiguo Wang, Yi Zheng, Zuhua Zhang, Kaiwei Liu, Yan Li, Liang Shi, and Daosheng Sun declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




