《1 Introduction》
1 Introduction
China is actively responding to climate change by striving to achieve carbon peak by 2030 and carbon neutralization by 2060. As a conscientious country, China has also put forward stricter requirements for energy consumption and transformation. Natural gas plays a key role in Chinese national energy strategy. With the rapid growth in China’s natural gas consumption in recent years, its dependence on foreign sources has also grown rapidly. Accelerating the development and utilization of offshore natural gas, particularly in the South China Sea, to combat climate change and achieve China’s goal of carbon peak and carbon neutralization is an energy transformation and development objective in line with national conditions.
China’s sea areas are rich in oil and gas. The South China Sea has a large reserve of natural gas resources, which is important for the development of China’s natural gas industry. However, the natural gas in the South China Sea contains high concentrations of CO2, and the corresponding composition is notably different from that in inland areas. The CO2 content of typical gas fields in the South China Sea is generally 20%–80%. This type of natural gas is commonly referred to as carbon-rich natural gas, which generally requires the removal of extra CO2 for production and domestic use [1]. Meanwhile, the traditional utilization of carbon-rich natural gas in the South China Sea causes a large quantity of greenhouse gas emissions, which is not conducive to carbon emission reduction. Therefore, there is an urgent need to develop new technologies for the utilization of carbon-rich natural gas to support the highquality development of China’s offshore oil and gas industry.
The large-scale natural gas chemical industry mainly uses synthesis gas (syngas) to produce methanol, urea, ethylene glycol, and other chemicals. For the two typical greenhouse gases of CH4 and CO2, the industry develops them primarily through the reforming/gasification into syngas (CO+H2), and which is further converted into chemicals, fuels, and other energy chemical products, with methanol being one of the main products of the syngas chemical industry. Combining this technology with high-efficiency utilization technology of syngas makes its largescale commercial utilization possible. Simultaneously, the production of high-value chemicals from syngas is rapidly developing. For example, syngas directly produces olefins/aromatics and is used for hydroformylation. Technologies of dry (CO2) reforming of methane (DRM) to syngas and chemical products synthesis during syngas formation have become research priorities, and extensive research has been carried out in China and abroad [2]. A newly developed catalyst provides a solid technological basis for the direct utilization of carbon-rich gases in the South China Sea. In line with the resource characteristics of these gases, this study analyzes the resource demand, investigates the current situation of the industry, refines the technical system represented by the key technologies of CO2-CH4 DRM, and puts forward suggestions for the development of technology and its application to provide a reference for the comprehensive utilization of South China Sea resources.
《2 Demand analysis for the direct utilization of carbon-rich natural gas in the South China Sea》
2 Demand analysis for the direct utilization of carbon-rich natural gas in the South China Sea
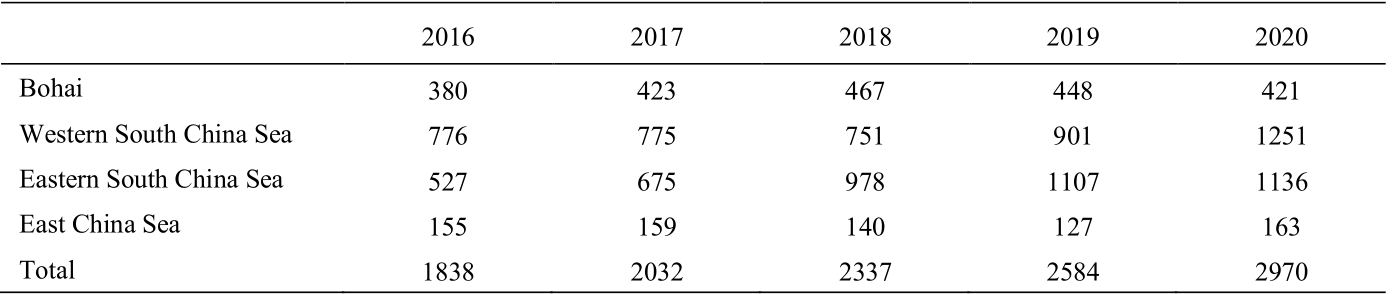
The global consumption of primary energy sources such as coal, oil, and natural gas is growing quickly along with rapid economic and societal development. Natural gas plays a key role in national energy strategy. In the past decade, China’s natural gas consumption has grown swiftly as has its dependence on foreign countries. For example, in 2018, China’s external dependence on natural gas was as high as 45.3%, approaching the upper limit of 50% in 2035, as estimated by China. Therefore, it is imperative to accelerate the development and utilization of natural gas resources in China. China’s sea area, which is rich in oil and gas resources, is approximately 3 million km2. For example, the amount of natural gas geological resources is 42.5 trillion m3 (including 10.7 trillion m3 offshore). China claims that the jurisdiction (hereinafter referred to as the South China Sea) is very rich in resources; it is the region with the most abundant domestic natural gas resources and production sources. Its gas geological resources are about 16 trillion m3 , which are more than one-third of China’s total oil and gas resources and equivalent to 12% of the world’s resources. As one of the four major oil and gas resource-rich seas in the world, the South China Sea has always been the main area for offshore natural gas exploration and development. Table 1 shows China’s offshore natural gas production from the China National Offshore Oil Corporation (CNOOC) 2020 Annual Results.
《Table 1》
Table 1. Domestic offshore natural gas production by CNOOC Ltd. during 2016–2020. (1×104 Nm3 /d)
According to the transportation requirements of commercial natural gas, the CO2 content of natural gas should not exceed 2% and that of liquefied natural gas should not exceed 0.2%. However, the CO2 content of natural gas extracted from gas fields in the South China Sea is generally high (Table 2); therefore extra CO2 must be extracted before further use. CO2 and CH4 are typical greenhouse gases, but they are also important carbon or energy sources [3]. The CO2 separation process leads to an increase in energy consumption and natural gas entrainment loss (using organic amine solvent entrainment loss is usually 2%–7% in industry), which remarkably increases the use cost of natural gas resources. This causes some carbon-rich natural gas resources to not meet the exploitation value or fail to be effectively utilized after exploration, resulting in idle or wasted natural gas resources. Therefore, the direct conversion of CO2 rich natural gas into high-value energy or chemical products without separation is one of the key technologies for achieving energy transformation and development.
《Table 2》
Table 2. Typical data of CO2-rich natural gas in the South China Sea.

《3 Development status of direct utilization of carbon-rich natural gas in the South China Sea》
3 Development status of direct utilization of carbon-rich natural gas in the South China Sea
《3.1 Current state of technological development》
3.1 Current state of technological development
At present, the world’s large-scale natural gas chemical industry reconstitutes natural gas via syngas into chemicals, such as methanol, urea, and ethylene glycol. In response to CO2 and CH4, the industry is committed to developing new technologies and processes to continuously improve the energy and carbon efficiencies of chemical systems [4,5]. The main method is to convert CO2 and CH4 into syngas using processes such as reforming and gasification and then into energy chemical products such as chemicals and fuels.
Technologies for the efficient utilization of syngas are primarily divided into two categories: upgrading existing processes and technological development of new products for high value. Methanol is one of the major products of syngas, and China, as the world’s largest producer of methanol, has a capacity breakthrough of 8.3×107 t/a, and the development of new processes and technologies for the synthesis of low-temperature methanol will effectively reduce the cost of methanol production. The low added value of chemical products downstream from conventional syngas makes it difficult to undertake carbon-rich natural gas feedstocks with a CO2 content ≥ 25%, low thermal value, and high cost. Developing technology for the direct preparation of high-value chemicals from syngas is the key to the resource utilization of carbon-rich natural gas, which has advanced rapidly in recent years, e.g., the direct preparation of alkenes and aromatics from syngas and the use of syngas for carbonylation reactions such as hydroformylation to prepare featured chemistries.
The CO2-CH4 DRM does not involve the participation of water vapor in the reaction; therefore, it has the potential to consume more CO2 during the reaction. Combining the DRM with syngas high-efficiency utilization technology makes it possible for large-scale commercial utilization. Related technologies are research priorities in the direction of syngas preparation, and the research institutes active in this field are the Lind group, BASF Company, and Shanghai Advance Research Institute of the Chinese Academy of Sciences.
《3.2 Application and development status》
3.2 Application and development status
In 2012, China’s National Development and Reform Commission promulgated the Natural Gas Utilization Policy, which pointed out the direction for the comprehensive development and utilization of natural gas with a high CO2 content. To solve the series of complications related to the CO2-rich natural gas in the South China Sea, China BlueChemical Ltd. (China BlueChem), under the CNOOC, has carried out successful chemical processing and utilization of CO2 gas. At the production base of China BlueChem in the Dongfang city of Hainan province, 3.5 billion m3 CO2-rich natural gas, of which, 25% constitutes CO2, can be used to produce 1.32 million tons of urea per year and 1.4 million tons of methanol per year. That means 442 thousand tons of CO2 per year can be chemically utilized by China BlueChem. Compared with fuel use, 3.367 million tons of CO2 were reduced annually.
For the utilization of CO2-rich natural gas with a CO2 content >25%, CO2 must be separated to meet the requirements of chemical production. If CO2-rich natural gas can be directly converted into high-value energy or chemical products, it will become a key technology for the realization of energy transformation and development. Domestic and foreign researchers are focusing on dry reforming and methanol production technology but have not yet achieved industrial applications. To address the CO2-rich natural gas in the South China Sea, Fudao Chemical Ltd. (hereinafter referred to as Fudao), a subsidiary of China BlueChem, combined with the Shanghai Advanced Research Institute of Chinese Academy of Sciences (hereinafter referred to as SARI) conducted the industrial demonstration construction of 10 000 m3 /h level dry reforming of CO2-rich natural gas with CO2 and low-carbon alkanes.
Compared with conventional method of converting natural gas to methanol where the ratio of hydrogen to carbon is generally controlled at (H2-CO2)/(CO+CO2)=2.05–2.15 and the actual industrial operation control ratio is higher, (H2-CO2)/(CO+CO2) =4–9. CO2-rich natural gas allows methanol synthesis by initial decarbonization, followed by its conversion into syngas by catalytic or non-catalytic reforming, which is further decarbonized again before entering the methanol synthesis section, finally yielding methanol. The first step is used for the efficient conversion of natural gas into syngas, and the second step is used to prevent carbon accumulation in the methanol catalyst. During the production process, the CO2 content in the syngas is maintained below 8% [3]. Therefore, CO2-rich natural gas is not suitable for conventional natural-gas-to-methanol conversion. Currently, the China BlueChemical Ltd. is working on core technologies for the direct utilization of CO2-rich natural gas. It is developing and constructing an industrialized large methanol plant, with a capacity of 1.2 million tons per year, which can directly use natural gas with a CO2 content of more than 30% as a raw material for a higher degree and larger amount of utilization. CO2-rich natural gas reduces production costs and improves the economic and environmental protection value of its development and utilization of CO2-rich natural gas.
《4 Key technologies for direct utilization of CO2-rich natural gas in the South China Sea》
4 Key technologies for direct utilization of CO2-rich natural gas in the South China Sea
《4.1 DRM technology》
4.1 DRM technology
4.1.1 Reaction mechanism and catalysts of DRM
The natural gas in the South China Sea is mainly composed of CH4 and CO2 and also contains a small amount (approximately 1%–3%) of ethane, propane, and other low-carbon alkanes. Using CO2, CH4 (or low-carbon alkanes) reforming to synthesize syngas will enable comprehensive utilization of CH4 (or low-carbon alkanes) and CO2 recycling, and set up a technical route for effectively using carbon and sources and converting greenhouse gases in a scaled manner. Subsequently, the syngas from the reforming unit can be utilized for further conversion to high value fuels, olefins, aromatics, and oxygen compounds [4–9]. Therefore, reforming CO2–CH4 (low carbon alkanes) to syngas is the most important platform technology and one of the most critical core technologies for the direct utilization of CO2-rich natural gas. Reforming reactions include steam reforming, partial oxidation reforming, and dry reforming [2]. The dry reforming reaction formula is expressed as follows:

No steam is involved in the above reaction, and DRM is the key core reaction for CO2-rich natural gas direct use. It has several advantages, such as no need for separation before CO2-rich natural gas application, no energy consumption for the separation process, and more importantly, two types of greenhouse gases can be transformed into high-value products simultaneously, thus, reflecting better environmental protection and economic value. The DRM reaction is a strongly endothermic reaction, and its energy is converted to “storage” in the form of syngas with a H2/CO ratio of 1 (or less than 1), which can be used to store and transport energy. In DRM, one CH4 molecule can reduce one CO2 molecule, which is usually defined as the methane reduction capacity (MRC) of CH4, which is 1. The core of this process is the catalyst, including noble metal catalysts such as Rh, Ru, Ir [10–12] and non-noble metal catalysts such as Ni. Noble metal catalysts usually have better performance but are expensive, whereas nonnoble metal catalysts are prone to carbon deposition and rapid deactivation.
Many researchers have studied the reaction mechanism of DRM process in the laboratory, although they have not figured out the precise mechanism yet. They reached a consensus that the carrier has a significant influence on the reaction mechanism [13–17]. Coking and deactivation of catalysts are common problems in DRM. According to the thermodynamic research on catalysts for coking under the operating conditions such as reaction temperature, reaction pressure, raw material proportion of CO2/CH4, and other oxidants, optimized conditions such as higher reaction temperature (> 850℃), lower reaction pressure, and higher ratio of CO2/CH4 are recommended to achieve higher conversion and less carbon deposition, but this is not consistent with the operating conditions (such as lower reaction temperature and higher reaction pressure) that are usually expected in industrial processes. Therefore, the development of industrial catalysts is a challenge.
Most researchers have used non-noble metal nickel-based catalysts for catalyst preparation and analyzing their performance. Therefore, adjusting the composition of metal particles and enhancing the interaction between the metal and carrier to enhance the anti-carbon deposition and anti-sintering properties of Ni nanoparticles is an important research priority and a discipline of DRM [18]. The strong coordination binding between the metal and carrier increased the positive charge of the nickel particles, leading to the existence of many ionic nickel species, thus reducing the DRM reactivity. To balance the activity and stability, the interaction between the microenvironment of nickel particles and carriers, additives, and alloys should be finely controlled. It was also found that the nanometer Ni clusters can inhibit the CH4 depth of cracking and easily produce reactive oxygen species to effectively avoid carbon deposition. However, under the condition of reaction with a highly active design, the preparation and high stability of the nano Ni clusters is still very difficult [19,20]..
4.1.2 Research on DRM of CO2-rich natural gas in the South China Sea
In view of the characteristics of the DRM process with a strong endothermic reaction, the loading of industrial catalysts in the reactor to avoid the existence of heat and mass transfer “dead zone” is another important aspect of DRM technology engineering and industrialization research. To adapt to the reality of CO2 content > 50% of CO2- rich natural gas in the South China Sea and maximize CO2 utilization, it is necessary to remarkably improve the MRC value of CH4 to change the reaction ratio in the DRM process. The idealized dry reforming equation is as follows[21]:

In the above idealized reaction, the MRC of CH4 is 3, which means that one CH4 molecule can reduce three CO2 molecules, implying that the limit at which CO2 in CO2-rich natural gas, such as that found in the South China Sea, can be directly converted to CO is 75%. However, thermodynamics restrict the idealized reaction, making it impossible to occur directly. Fig. 1 shows the thermodynamic equilibrium gas composition, equilibrium conversion of CH4 and CO2, and reducibility of CH4 at different temperatures when the CO2/CH4 ratio in the feed gas is 3.3.
《Fig. 1》

Fig. 1. Gas composition distribution diagram of thermodynamic equilibrium state at different temperatures.
For current conventional DRM reaction temperature (650–750 ℃), it is difficult for the MRC value of CH4 to exceed 1.5. To break through this limitation and achieve a significant improvement in CH4 reduction capacity, the concept of super DRM is proposed, which is a type of DRM at the temperature of 1000 ℃ [21]. As shown in the Fig. 2, the MRC of CH4 can reach up to 1.9 in the feed gas with 75% CO2 content, achieving an average reaction of 1.9 CO2 molecules per CH4 molecule and reaching the highest CO production rate of 2.9 mol CO/1 mol CH4.
《Fig. 2》

Fig. 2. DRM and high temperature oxidation–reduction process.
It can be seen that in the conventional DRM reaction, the carbon in CH4 mainly reduces CO2, while the H in CH4 generates hydrogen and does not participate in CO2 reduction. If all the four H in CH4 can participate in CO2 reduction, the goal of CO2 reduction capacity of CH4 approaching 3.0 can be achieved, which also requires remarkable improvement in the reactivity of H in CH4, and the formation of hydrogen overflow in the reaction process is an important means for achieving this improvement. The carrier and active species of the microenvironment determine the behavior of hydrogen overflow, and overflow can be optimized by changing the micro-environmental regulation catalytic properties of hydrogen [22]. CNOOC and the team of Professor Xiao of Zhejiang University found that using zeolite molecular sieve to encapsule Ni nanoparticles and strengthen the hydrogen overflow in DRM reaction, can significantly enhance the capacity of the reduction of alkanes: the CH4 reduction ability of CO2 can reach 2.6, and a high CO production rate is maintained (75.1 mol CO /mol Nih). The crystal structure of zeolite has good stability, and the zeolite encapsulation structure can effectively stabilize the metal nanoparticles and show excellent anti-sintering performance in the high-temperature reactions [23], which will effectively prevent the sintering of metal nanoparticles. This will help to improve the resistance and life of new DRM catalysts.
《4.2 Integration technology of methanol production from CO2-rich natural gas》
4.2 Integration technology of methanol production from CO2-rich natural gas
The technology of CO2-rich natural gas to methanol integration refers to the direct reforming of CO2-rich natural gas (i.e., DRM, steam reforming, and oxidation reforming) into a high hydrogen to carbon ratio syngas (H2 / (CO+ CO2) > 2) and the coupling performance of methanol synthesis catalysts such as nano-copper matrix composites in the direct synthesis of methanol catalyst technology. Therefore, CO2-rich natural gas to methanol integrated conversion technology is an important solution to reduce emissions and improve carbon efficient use of CO2-rich natural gas.
Compared with the conventional technology of producing methanol from natural gas, CO2-rich natural gas must be decarbonized and then converted into syngas (H2/(CO+CO2)>2) through catalytic or non-catalytic reforming. The obtained syngas must be decarbonized again and then entered into the methanol synthesis section to produce methanol. Therefore, CO2-rich natural gas is unsuitable for traditional methanol production from natural gas. The technology of conversion of CO2-rich natural gas to methanol integrated reforming to obtain syngas with the methanol synthesis. The reforming catalyst can be a means of resistance to carbon deposition and the technology of tube and tubular plate in tubular reactor to heat or direct CO2 into the rich syngas at the CO2-rich natural gas reforming unit, and then as a raw material gas after heat exchange by the rich carbon syngas synthesis to methanol under catalyst and tubular plate in a tubular reactor. The whole process does not require CO2 separation and can use CO2 as a raw material for the synthesis of syngas. Therefore, methanol production from CO2-rich natural gas has clear technical advantages and broad prospects for application. Fig. 3 shows the process flow diagram of the integrated technology for methanol production from CO2-rich natural gas in the South China Sea designed by CNOOC.
《Fig. 3》

Fig. 3. Process flow diagram of the integrated technology of methanol production from CO2-rich natural gas.
After the breakthrough of the dry reforming technology, the key to the integration technology is the hydrogenation of CO2 or the production of methanol from CO2-rich syngas. The main chemical reactions of CO2 hydrogenation are as follows:

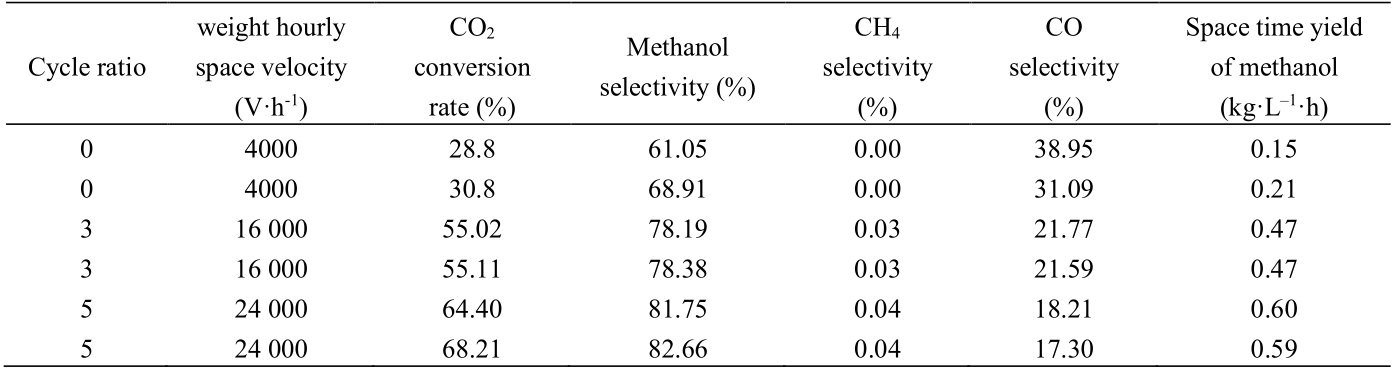
Fudao and SARI cooperated to conduct laboratory tests, pilot studies, and industrial demonstrations in this regard. The CO2 adsorption activation in the reaction process and the hydrogen dissociation bifunctional catalyst active sites are utilized to prepare highly active and stable copper-matrix composite nano catalyst using limited domain structure. When the reaction temperature is 230–250 ℃ and the pressure is 5 Mpa, the one-way conversion rate of CO2 exceeds 28.8%, and methanol selectivity exceeds 61.05%; under the condition of tail gas circulation, the CO2 conversion rate is higher than 55.02%. Based on nearly 5000 hours of continuous operation, kilogram-scale amplification and singletube test verification of the catalyst were completed. Table 3 shows partial data of the single-tube experiment of the catalyst used in the pilot and industrial demonstrations.
《Table 3》
Table 3. CO2 hydrogenation of single-tube experiments.
CNOOC’s industrial demonstration of the hydrogenation of CO2-rich syngas to methanol has been put into production since July 2020; since then, the unit has been running smoothly, and the product quality has been qualified, which has achieved the design purpose. According to calculations, the carbon efficiency and energy efficiencies of methanol production from CO2-rich natural gas in the South China Sea were 82.5% and 80.4%, respectively.
《4.3 Hydrogenation of CO2-rich natural gas to liquid fuel》
4.3 Hydrogenation of CO2-rich natural gas to liquid fuel
SARI has made strong progress in the preparation of liquid fuels from CO2 hydrogenation [24], using its original indium oxide/molecular sieve (In2O3/HZSM-5) bifunctional catalyst for CO2 hydrogenation. When the conversion rate was 13.1%, the selectivity of the C5+ component in the hydrocarbon components was as high as 78.6% while that of CH4 was only 1%. In reference to China’s carbon peak and carbon-neutrality goals, such products have practical significance for the production of carbon-neutral fuels and chemicals.
To meet the needs of the oil and gas industry developing in the South China Sea, CNOOC has established a major scientific and technological project for the direct conversion of carbon-rich natural gas and construction of a compound energy chemical system in the South China Sea. CO2-to-liquid fuel is an important component of China’s compound energy chemical system. Hydrogen is indispensable for the production of methanol and liquid fuels from CO2-rich natural gas. Hydrogen production using renewable energy is currently a priority. Using the electricity generated from renewable energy sources such as wind power, hydropower, solar energy, and nuclear power, to electrolyze water to obtain hydrogen is the main choice to achieve large-scale CO2 emission reduction and utilization [25]. CNOOC promotes the South China Sea CO2-rich natural gas direct conversion and hybrid energy and chemical system construction projects in Dongfang city in Hainan Province, which is rich in wind power, solar energy, and nuclear energy resources, and is suitable for using this energy to electrolyze water (seawater) to produce green hydrogen.
As per the Development Plan of the Marine Renewable Energy Industry in Hainan Province issued on November 2019, there will be 14 power sites in an offshore area with a water depth of 10 m–50 m, with a total installed capacity of about 17.37 million kilowatts. This will be a unit investment of about 17.5 CNY/W, with an annual average use of approximately 3000 hours, and an annual power output of approximately 50 945 GWH, equivalent to about 15% of Hainan’s annual electricity consumption at present. If one third of the power generated from wind is converted to hydrogen, 275 600 tons of hydrogen could be obtained. This sea area near the west side of Dongfang City is one of the high-quality places for wind energy resources in Hainan Province. The offshore distance of the wind field is approximately 16 km, with a north-south length of 18 km and width of 5.8 km. The measured average wind speed in two years is 7.84 m/s, which can meet demands to build a new 350 MW offshore wind farm. As shown in Fig. 4, offshore wind power can be combined with hydrogen production and the CO2 chemical industry to achieve a comprehensive assembly. CNOOC demonstrated the feasibility of using green oxygen to gasify carbon-rich natural gas and proposed its implementation.
《Fig. 4》

Fig. 4. Offshore wind power hydrogen production and CO2 Chemical utilization technology route.
《4.4 Key technologies for the direct production of fine chemicals from CO2-rich natural gas》
4.4 Key technologies for the direct production of fine chemicals from CO2-rich natural gas
Olefin is the most basic chemical raw material in the modern chemical industry and includes low-carbon olefins (ethylene, propylene, and butene) and long-chain olefins (C5+ ). Direct synthesis of olefins from syngas includes the use of the Fischer-Tropsch to olefins process with CO and H2 under the action of a catalyst. This process has the advantages of short processing time, lower energy consumption, strong competitiveness, and good prospects for future development [26]. Using CO2-rich natural gas from the South China Sea as a raw material, DRM technology was used to produce syngas with a low hydrogen/carbon ratio, using the cobalt-based catalyst at 260 ℃. CH4 in paraffin product selectivity was less than 7.5%, total olefin selectivity was up to 90%, and olefinic alkane ratio was high and had a very high commercial value, as shown in Table 4.
《Table 4》
Table 4. Technical parameters of FTO process for DRM of CO2-rich natural gas in the South China Sea.

The DRM product of CO2-rich natural gas in the South China Sea has a hydrogen-carbon ratio of 1 (or slightly less than 1), which is suitable for the production of high-carbon alcohols, aldehydes, and other chemicals through hydroformylation reactions with olefins [27,28]. CNOOC’s low olefin hydroformylation technology has a lowcarbon olefin conversion rate of more than 90%, and an aldehyde yield of more than 90%. The energy and material consumption are much lower than those of imported technology. Fig. 5 shows the process flow chart of the production of 2-propylheptanol (2-PH) by the first set of 70 000 t/a mixed C4 olefinic hydroformylation planned by CNOOC with its own technology.
《Fig. 5》

Fig. 5. Process flow chart of 2-propylheptanol (2-PH) produced by mixed C4 olefinic hydroformylation.
《5 Development proposals for direct utilization of carbon-rich natural gas in the South China Sea》
5 Development proposals for direct utilization of carbon-rich natural gas in the South China Sea
《5.1 Suggestions for technical development》
5.1 Suggestions for technical development
Due to the particularities of CO2-rich natural gas resources in the South China Sea, more attention should be paid to this research. First, we should focus on CO2-CH4 (low-carbon alkanes) dry reforming, CO2 hydrogenation, and the conversion of carbon-rich syngas to carbon-neutral chemicals (such as methanol). Further, we should overcome the difficulties of coupling syngas to olefins and hydroformylation of syngas and olefins to produce aldol chemicals, which can achieve the resource utilization of CO2 and CH4 to explore the economic and environmental value of
We recommend the establishment of an engineering platform for the comprehensive utilization of marine CO2- rich natural gas, covering the upstream, synthesis conversion, and high-value product technology of syngas; the research and development of new technologies for the comprehensive utilization of CO2-rich natural gas, pilot scaleups, and industrial demonstration should be promoted. This can also provide data support for engineering simulations, laboratory research grafting, and industrial applications, accelerating the industrialization of technology.
We must promote the sustainable development of CO2-rich natural gas in the South China Sea and closely integrate its development with renewable energy such as wind, solar, and nuclear energy. To continuously expand the use of CO2, we need to carry out in-depth research and development of catalytic materials continuously, to achieve higher proportions of CO2-CH4 dry reforming and other technological advances and accelerate the development of the integration of renewable energy sources such as wind, solar and nuclear energy. Coupling hydrogen production technology with water electrolysis continuously addresses the problems of engineering and industrialization to realize integrated development and large-scale development.
《5.2 Suggestions for application countermeasures》
5.2 Suggestions for application countermeasures
In the application area, we need to accelerate the industrial development of CO2-rich natural gas in the South China Sea to realize transformation and upgradation. We also need to intensify and accelerate the development and utilization of carbon-rich natural gas in the South China Sea, focusing on core technology research and development and large-scale industrial demonstration of carbon-rich natural gas resource utilization for CO2-rich natural gas in the South China Sea, and promote the development and utilization of natural gas reservoirs. It is necessary to establish a complete natural gas chemical industry chain, support the demonstration construction coupling renewable energy and nuclear energy with CO2-rich natural gas, and build a green and low-carbon natural gas chemical demonstration base.
We need to strengthen the cooperation between industry and technology and establish a strategic alliance between industry, university, research, and application. Strengthening domestic and international cooperation attracts domestic and foreign technology patentors and related product manufacturers to participate in the construction. Strengthening cooperation with upstream raw material suppliers and downstream consumer groups allows to establish strategic alliances and effectively integrate internal and external resources. Strengthening the raw material supply, fundraising, sales networks, technical services, and production management of the entire industry chain improves market competitiveness and promotes the development of CO2-rich natural gas chemical utilization.














 京公网安备 11010502051620号
京公网安备 11010502051620号




