《1 Introduction》
1 Introduction
Vaccines are crucial for the prevention and control of infectious- and some non-communicable diseases, such as different types of cancers. Vaccination has eliminated diseases like smallpox and polio but cannot effectively prevent and control infectious diseases such as human immunodeficiency virus (HIV) infection and malaria that pose a major threat to human health. Some novel infectious diseases, such as infectious atypical pneumonia, and infections due to highly pathogenic avian influenza, novel influenza A, Ebola, and the Zika virus, spread rapidly, leading to high mortality rates. No specific drugs are available worldwide for the treatment cure of these diseases, emphasizing the need for various candidate vaccines to aid their prevention and control. China, therefore, needs to accelerate the research and development (R&D) of vaccines to improve technical vaccine reserves to pre-emptively manage any major future challenges [1,2].
In recent years, based on the traditional attenuated vaccine and inactivated vaccine technology, a variety of novel vaccines were recently developed using biochemical synthesis, artificial mutation, molecular microbiology, genetic engineering, and other modern biotechnological techniques. The representative vaccines include genetically engineered subunit, recombinant, virus-like particle (VLP), polysaccharide binding, synthetic peptide, genetically engineered vector, and nucleic acid vaccines (DNA and RNA vaccines). Nucleic acid vaccines have been thoroughly studied, especially the development of DNA vaccines in the 1990s [3], but they still require further investigation in terms of clinical application. The coronavirus virus disease 2019 (COVID-19) pandemic is still ongoing, and messenger ribonucleic acid (mRNA) vaccines have played an important role in its prevention and control. Because of the improved efficiency of mRNA vaccines compared to that of traditional vaccines, mRNA vaccines have become popular in the field of vaccine R&D. Some previous studies have reported that nucleic acid, viral vector, and protein subunit vaccines, and other technical vaccine routes are widely used in the development of vaccines for COVID-19 [4]. The key technologies and product development processes involved in DNA and mRNA COVID-19 vaccine production have also been reviewed [5,6]. Additionally, the potential biological mechanisms of mRNA vaccines and their applications in some infectious diseases and cancers have also been reviewed [7]. We analyzed the SCI publications related to DNA, RNA, conjugate, subunit, and VLP vaccines and found that nucleic acid-based and other novel vaccines have attracted considerable attention over the last 20 years. RNA vaccine technology has developed immensely since 2000, although it is still progressing and has gained significant attention since 2020 because of the COVID-19 pandemic. Conversely, DNA vaccines have reached a mature stage with respect to R&D, showing a downward trend since 2010 (Fig. 1).
《Fig. 1》

Fig. 1. The publication trend of novel vaccine research.
Note : Data obtained from PubMed, as of June 30, 2021.
According to published reviews, the overall development trajectory of nucleic acid vaccines and the correlation and evolution between DNA and RNA vaccine R&D have not been compared and analyzed. Additionally, comparative analyses of systematic research output data have also not been performed. Therefore, in this study, we focused on the development process, application situation analysis, and development trends of nucleic acid vaccines. We also provide some suggestions for the development of nucleic acid vaccines in China, providing a basic reference for R&D layout and management policy research.
《2 Overview of nucleic acid vaccine technology》
2 Overview of nucleic acid vaccine technology
Nucleic acid vaccines are also known as genetic vaccines. The exogenous genes (DNA or RNA) encoding certain antigen proteins are directly introduced into animal somatic cells, via methods such as intramuscular injection, microprojectile bombardment. The antigen proteins are then synthesized through the host cell expression system, inducing the host to produce an immune response to the antigen proteins to prevent or treat diseases. According to the various main components, nucleic acid vaccines are divided into DNA and RNA vaccines, of which RNA vaccines mainly refer to mRNA vaccines.
《2.1 DNA vaccine technology》
2.1 DNA vaccine technology
DNA vaccines are further divided into “naked vaccines” that do not require chemical vectors, and DNA plasmids that recombine genes encoding a protein antigen into eukaryotic expression vectors [5]. Cellular uptake (tissue cells, antigen-presenting cells, or other inflammatory cells) can be expressed in the intracellular pathogen protein antigen by stimulating the body to produce cellular and humoral immune responses, either directly or after host body packaging.
Compared to that of the traditional protein vaccines, the immunogenicity of DNA vaccines is limited by the low antigen expression level, and the results of human clinical trials are not ideal in the development process of more than 30 years. Novel technologies will likely improve the efficacy of DNA vaccines, such as improving the immunogenicity of vaccines through codon optimization, gene modification, adjuvants, and complex initiation-promotion schemes, and by adopting better vaccine delivery technologies, such as electroporation and lipid nanoparticles.
《2.2 Messenger RNA (mRNA) vaccine technology》
2.2 Messenger RNA (mRNA) vaccine technology
An mRNA vaccine is an mRNA sequence containing specific antigens synthesized in vitro. After being injected into the body, the vaccine first needs to enter the cells through cell membrane. Protective measures are needed to prevent mRNA degradation due to its unstable structure and the presence of nucleic acid hydrolases inside and outside the cells. Efficient transmembrane proteins are also required. Messenger RNA vaccines generally enter cells via the endosomes, which release them into the cytoplasm. Messenger RNA translation also requires regulation to prevent further premature degradation [8].
Currently, mRNA vaccines mainly include traditional or self-amplified mRNA vaccines. The open reading frames (ORF) of the former only contains genes encoding antigens, whereas the ORF of the latter contains genes encoding antigens and non-structural proteins required for mRNA amplification, thereby increasing target antigen expression. Both vaccine types also contain a 5′-untranslated region (5′-UTR), 3′-untranslated region (3′-UTR), 5′-cap structure (Cap), and 3′-polyadenylate structure (Poly-A). Through the artificial modification of mRNA, the stability of mRNA can be enhanced, the accuracy and efficiency of mRNA translation can be improved, and the immunogenicity of mRNA can be reduced, thereby improving the safety.
Delivery vectors also play an important role in the stability and translation efficiency of mRNA vaccines and can be characterized as viral or non-viral vectors. Viral vectors also exhibit immunogenicity, which may interfere with antibody production and affect mRNA vaccine efficacy. Liposomes are the most widely used non-viral vectors for mRNA vaccine delivery. The main advantages of this delivery system are as follows. Liposomes are cystic, and mRNA can be enclosed in the cavity by lipid nanoparticles to avoid contact with nucleic acid hydrolases, preventing degradation. Liposomes have properties similar to those of cell membranes, which promote the fusion of carriers and cell membrane, and improves the efficiency of the entry of the mRNA into the cells. Liposomes promote mRNA release from endosomes to the cytoplasm, greatly improving mRNA expression efficiency in vivo, which can easily be quantified and applied to mass production of vaccines.
《2.3 Characteristics of nucleic acid vaccines》
2.3 Characteristics of nucleic acid vaccines
Nucleic acid vaccines have remarkable advantages over traditional inactivated and subunit vaccines [5,9]. Theoretically, nucleic acid vaccines can synthesize and express almost all protein antigens, providing great flexibility in antigen design. Data mining technology can be used for antigen optimization, the design of gene-independent vaccines, and the optimization and personalization of nucleic acid vaccines. The advantages include: (1) Effective immune protection. The protein is expressed in host cells after inoculation and directly binds to histocompatibility complex I or II molecules, providing cellular and humoral immunity; (2) The preparation is simple, efficient, and cost-effective, as only antigen-coding genes are to be designed and cloned. A universal production process and quality control system can be used without changing the antigen-coding genes or in vitro protein expression and purification, enabling rapid vaccine development during epidemic outbreaks; (3) The immune response is relatively persistent. For example, a considerable number of exogenous genes can still be detected after 19 months [10]; (4) Cross-protection of homologous strains. Nucleic acid sequences encoding conserved pathogen proteins were previously selected as vaccines, and cross-immunity was generated for the same pathogen because they did not mutate; (5) Effective preventive and therapeutic effects. For example, in cancer prevention and treatment, the inoculation of gene vaccine can induce a cytotoxic T lymphocyte immune response, monitor the malignant transformation of cells, and produce an immune response to cancer cells; and (6) Simple storage and transport, mainly referring to DNA vaccines that are relatively stable at room temperatures.
Nucleic acid vaccines also present some limitations. The antibodies induced by DNA vaccines may not be antigen-specific but they target the double-stranded plasmid DNA molecules or mRNA, promoting the development of autoimmune diseases and leading to immune tolerance in the body. Exogenous DNA can potentially be integrated into the host genome. DNA vaccines can produce obvious effects in small animals, but their immunogenicity in large animals and humans is relatively low. Additionally, the recently developed RNA vaccines have the advantages of having immune response mechanisms like live viruses, simple and rapid chemical synthesis, thermal stability, no genomic integration, and no risk of genomic transcription interference. However, adverse reactions with unknown causes are occasionally reported [5,11]. Researchers may miss important new antigens during screening. Additionally, antigens that are inefficient or off-target may lead to potential safety problems. Mutation cloning may also vary greatly, and determining the antigen demand for a sufficient antigen immune response may prove challenging. Thus, the synthesis of unusual sequences and a reduction in the production cost of related reagents are some of the current challenges [7].
《3 Development and current trends in nucleic acid vaccine production》
3 Development and current trends in nucleic acid vaccine production
《3.1 DNA vaccine R&D is relatively stable, while mRNA vaccine R&D has increased》
3.1 DNA vaccine R&D is relatively stable, while mRNA vaccine R&D has increased
Nucleic acid vaccine research originated in the 1980s and the 1990s, after inactivated, attenuated, subunit, and various other vaccines underwent extensive development, leading to their practical application. Wolff et al. (1990) constructed an exogenous recombinant plasmid that could be absorbed by cells and that stably expressed the encoded protein in vivo after inoculation into mouse muscle [3,10]. Williams et al. (1991) found that in vivo expression of exogenous genes could induce an immune response [12]. Tang et al. (1992) found that in vivo expression of exogenous plasmids could induce antibody production against the expressed gene products, establishing the concept of a genetic vaccine [13]. Ulmer et al. (1993) injected a recombinant plasmid containing influenza A virus nucleoprotein into mice muscles tissues, which effectively protected the mice against the infections due to the heterologous strains of the influenza virus [14]. Previous animal experiments have shown that introduced foreign DNA can cause cellular and humoral immunity under appropriate conditions. These vaccines were named the nucleic acid vaccines at a conference in Geneva in 1994; they are also known as the “third-generation vaccines,” following inactivated/attenuated vaccines and subunit vaccines.
Wolff et al. (1990) injected in vitro synthesized mRNA into murine skeletal muscle tissues and discovered specific protein production and immune responses in the skeletal muscle cells of mice, demonstrating the therapeutic potential of mRNA [3]. However, mRNA is easily degraded by ubiquitous RNA enzymes and is inherently unstable. Thus, its practical application was considered unviable, haltering the development of mRNA vaccine and drugs for many years. However, recent RNA stabilization technology and delivery systems have markedly improved the R&D of RNA vaccines. For example, a research team at the University of Mainz in Germany developed a personalized mRNA vaccine containing multiple cancer antigens for different melanoma patients [15]. The research on DNA vaccine technology has plateaued over the years, while the number of papers and patent applications for RNA vaccines has increased significantly since 2014 (Figs. 1 and 2). RNA vaccine technology is gradually improving and meeting the need for rapid and efficient vaccines against the COVID-19 pandemic. The R&D of mRNA vaccines has progressed substantially after rapid verification of the efficacy of the two mRNA-based COVID-19 vaccines in 2020 (Fig. 1).
《Fig. 2》
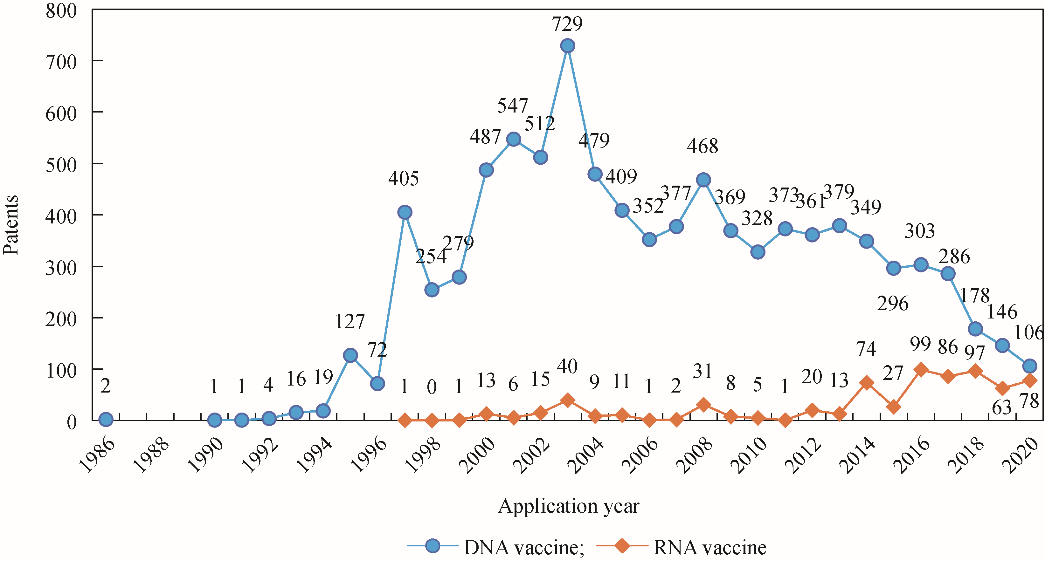
Fig. 2. Trends of patent applications for DNA and RNA vaccines.
Note : Data was obtained from the IncoPAT patent database as of 2020. Due to the delay in patent disclosure time, the data for 2019–2020 are not included in the final report.
Significantly fewer studies on RNA vaccines than on DNA vaccines were funded by national research funds before 2020. The National Natural Science Foundation of China (NSFC) and US National Institutes of Health (NIH) are the major global institutions funding nucleic acid vaccine research. Publications on DNA vaccine studies funded by the NSFC and NIH accounted for 80%–90% of the total publications on DNA vaccine studies with funding sources. Publications on RNA vaccine research funded by the NSFC and NIH were less than 1/10 of those on DNA vaccine research.
《3.2 The COVID-19 pandemic accelerated mRNA vaccine research》
3.2 The COVID-19 pandemic accelerated mRNA vaccine research
Several nucleic acid vaccine R&D studies entered phase III clinical trials before 2020, but all failed, and no nucleic acid vaccines were approved for human use. Only seven veterinary DNA vaccines have been approved to date. West Nile Innovator DNA was the first DNA vaccine approved to prevent equine West Nile virus infection.
After the COVID-19 pandemic began, the demand for rapid and effective vaccines, has significantly promoted the development process of nucleic acid vaccines; nucleic acid vaccine research now accounts for more than 20 % of the current vaccine research. A total of 707 nucleic acid vaccines have been described as of June 30, 2021. New products increased by 18 % within a year and a half (since the beginning of 2020) compared to the total number of products developed in the previous 24 years. Of the 707 products, 394 (including 356 DNA vaccines and 38 RNA vaccines) were terminated. There are currently 313 vaccines (including 190 DNA vaccines and 123 RNA vaccines) that are being subjected to active R&D, of which approximately 10 % are in the drug discovery stage, and about 88 % are in the pre-clinical or clinical research stages. Two products were suspended due to financial or data problems. One product was in the pre-registration stage, and two mRNA products (mRNA-1273 and BNT162b2) were approved for emergency use in the United States.
Sixty-six (approximately 60%) newly launched nucleic acid vaccines have been developed for COVID-19 since 2020, 44 of which are mRNA vaccines. Additionally, 41 preventive mRNA vaccines and 23 preventive DNA vaccines for COVID-19 are in the active stage of research. A total of 44 vaccines for infections caused due to influenza virus, HIV, hepatitis B virus (HBV), Zika virus, and other viruses, cancers, and other non-infectious diseases are under active research. Messenger RNA vaccines can be developed and approved for emergency use in only one year, substantially improving the conventional 8–10 year vaccine R&D cycle and enabling rapid access to preventive vaccines for emergencies and re-emerging infectious diseases.
《3.3 Nucleic acid vaccine development varies with each country》
3.3 Nucleic acid vaccine development varies with each country
Patent applications are indicators of the intensity of product development. Based on the patent applications for nucleic acid vaccines, the United States currently leads the field of DNA and RNA vaccine R&D (Table 1 and Fig. 3), with particular emphasis on RNA vaccines since 2015. The R&D department of mRNA vaccines in Germany has also increased significantly over the last 10 years. Therefore, the United States and Germany have good technical reserves, for the development of mRNA vaccines, enabling rapid mRNA vaccine development and establishing their lead after the COVID-19 outbreak. The number of COVID-19 mRNA vaccines in the United States and Germany accounts for 10 % and 5 % of the world’s total mRNA vaccines, respectively.
《Table 1》
Table 1. Distribution of main patent application countries/regions for nucleic acid vaccines.
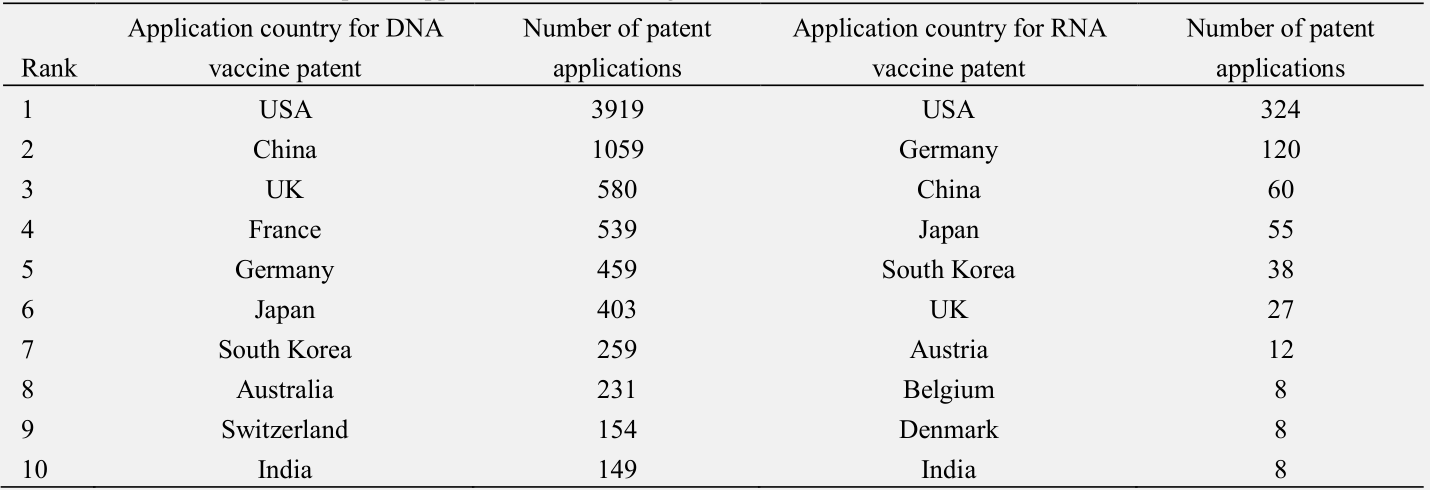
Note : Data came from the IncoPAT patent database as of 30 June 2021.
《Fig. 3》
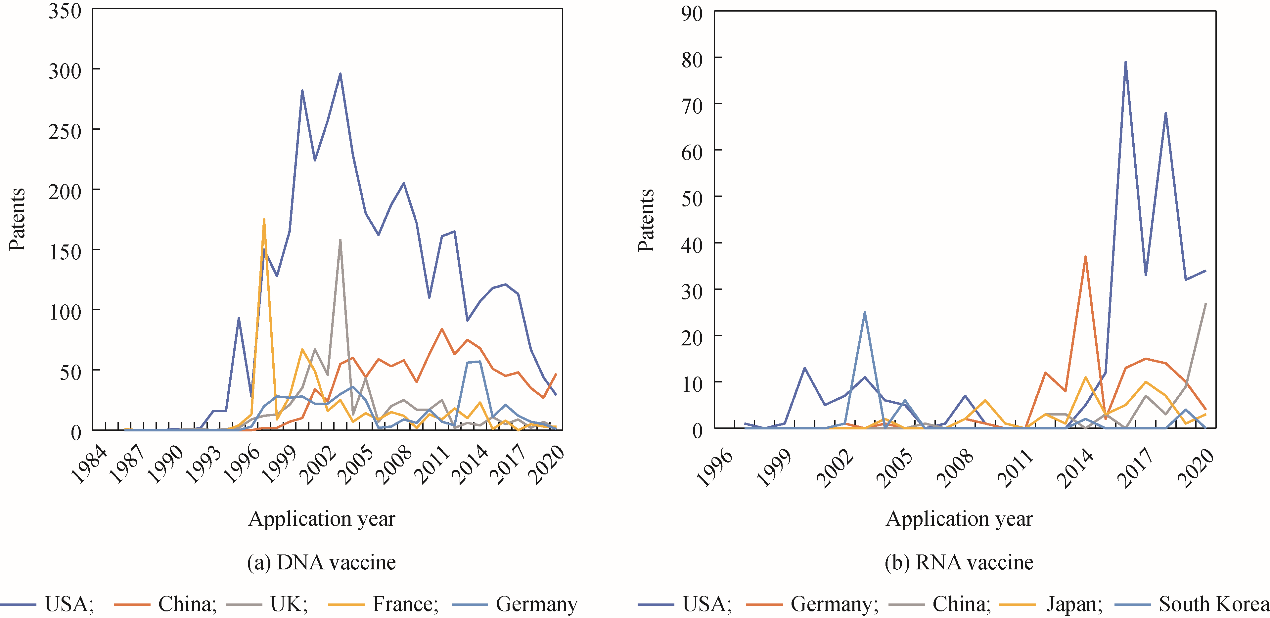
Fig. 3. Trend of patent applications for nucleic acid vaccines.
Note : Data was obtained from the IncoPAT patent database, sorted according to the top five applications.
Given the high risk associated with the R&D of RNA vaccines, China has focused on subunit, viral vector, and DNA vaccines with relatively mature technologies. In the past 20 years, China has become a significant stakeholder in the R&D of DNA vaccines. The number of patent applications for DNA vaccines in China is second only to that of the United States, although the R&D of RNA vaccines is still lagging (Tables 1 and 2, and Fig. 3). However, Chinese laboratories have also started focusing on the R&D of RNA vaccines after their clinical application potential was proved during the COVID-19 pandemic. China surpassed Germany in terms of the number of relevant patent applications, becoming the second-largest RNA vaccine R&D entity globally (Fig. 3). However, the nucleic acid vaccine production rate in China is slower than the number of patent applications that are filed. China will likely take longer than developed countries to realize the transformation of technology into products. China’s leading DNA vaccines currently account for approximately 2 % of the global supply, while RNA vaccines account for approximately 9 %.
《Table 2》
Table 2. Distribution of main research initiator countries/regions for nucleic acid vaccines.

Note : Data came from the IncoPAT patent database as of 30 June 2021.
《3.4 Nucleic acid vaccines mainly target infection and cancer for clinical application》
3.4 Nucleic acid vaccines mainly target infection and cancer for clinical application
The fundamental research on nucleic acid vaccine development is mainly aimed at highly pathogenic viruses (such as HIV, HCV, Zika virus, and the West Nile virus) and cancers (such as cervical, prostate, and breast cancer). The research involves vaccine structure and preparation, immune mechanisms, effectiveness, safety/toxicity, etc.
Nucleic acid vaccine application is also focused on viral infection and cancer prevention and treatment; the viruses include influenza virus, HBV, HCV, HIV, and SARS-CoV-2, and the cancers include breast, ovarian, prostate, and non-small cell lung cancer, and melanoma and glioblastomas. A few nucleic acid vaccines are also used to prevent and treat non-communicable diseases, such as immune and neurological/psychiatric diseases and diabetic retinopathy. In contrast to other applications, COVID-19 vaccines are mainly mRNA-based. The successful application of these vaccines also promoted the development of mRNA vaccines against influenza virus (Table 3 and Fig. 4)
《Table 3》
Table 3. Distribution of disease types prevented or treated by nucleic acid vaccines.

Note : Data are from the Cortellis database as of 30 June 2021.
《Fig. 4》
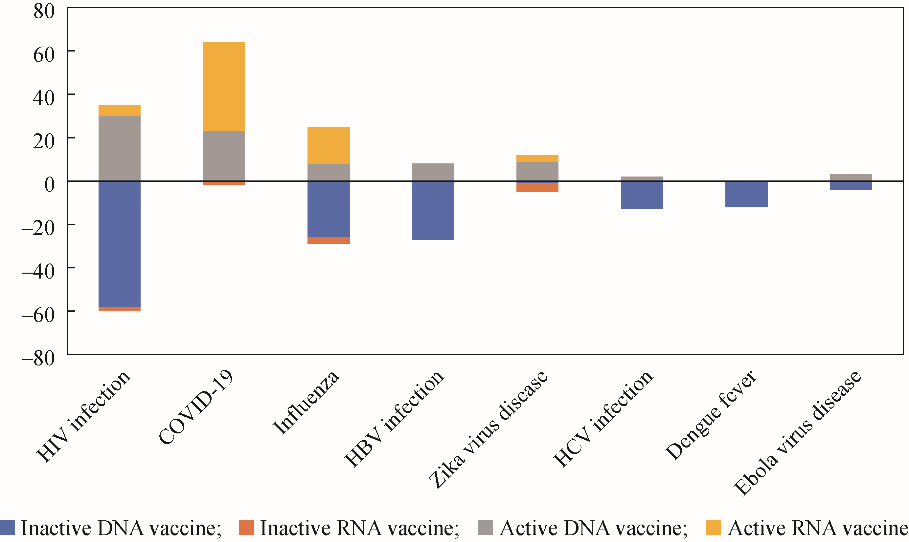
Fig. 4. Comparison of the number of nucleic acid vaccines currently described for a few viral infections.
Note : Positive numbers represent ongoing research projects, and negative numbers represent terminated projects.
《4 Suggestions for nucleic acid vaccine development》
4 Suggestions for nucleic acid vaccine development
The successful clinical application of nucleic acid vaccines, particularly mRNA vaccines, has significantly enhanced the response to and control of emerging infectious diseases. And from the perspective of stimulating human autoimmune responses, it has also enhanced the treatment of classical and re-emerging infectious diseases and cancers. Collaborative work on the development of technology, safety supervision, and prospective layouts is necessary to overcome the technical maturity and safety challenges associated with nucleic acid vaccines. This way, we can effectively deal with acute infectious disease outbreaks and promote the development of vaccines from preventive to therapeutic application, thereby improving human health and economic and social development.
《4.1 Suggestions for technical development》
4.1 Suggestions for technical development
4.1.1 Improving target gene expression
Nucleic acid vaccine research has entered the precise design stage. Interdisciplinary technologies, such as conservation genomics, structural vaccine science, and synthetic biology, can be applied to innovative research ideas, predict targets/target points, and redesign new antigens. By prioritizing the use of optimal codons and avoiding rare codons and methylated bases, the skeleton can be modified or an immune stimulation sequence can be added to optimize the target gene. Optimizing transcription regulatory elements, such as the promoters, enhancers, and introns, of plasmid vectors can improve the expression of exogenous genes.
4.1.2 Improving delivery systems
Introducing exogenous DNA into target cells can overcome low plasmid transfection efficiency and produce effective immune responses in the human body. Additionally, new physical or chemical methods have been adopted to improve transfection efficiency, such as electroporation, gene guns, biological injectors (needleless injection), and microneedle arrays. Novel mRNA vaccine carrier methods, such as liposome-, polymer-, and liposome and polymer-based nanoparticle delivery platforms can be used to efficiently deliver and protect mRNA from enzyme degradation.
4.1.3 Improving immune responses
Adding an immune adjuvant is an effective way to enhance the body’s immune response to antigens or alter the type of immune response. Research has also been conducted on cytokines, chemokines, signaling molecules, pattern recognition receptor ligands, and other immune adjuvant types to purify transcripted mRNA in vitro. The introduction of modified nucleoside groups into mRNA molecules or the formation of mRNA-carrier molecular complexes can optimize the core sequence of mRNA, improve translation efficiency, extend mRNA half-life, and regulate mRNA immune stimulation.
4.1.4 Enhancing mRNA stability and viability
In-depth research is needed to maintain the activity and preservation periods of mRNA vaccines. The stability and translation efficiency of mRNA can be improved by designing synthetic mRNA elements, such as Poly-A sequences in 5′- or 3′-UTRs, and Cap regions and nucleoside triphosphates (NTP) for in vitro transcription, or by optimizing mRNA through separation and purification technology. Furthermore, the delivery system should be improved and an efficient mRNA delivery vector should be used to significantly improve the stability and translation efficiency of mRNA vaccines. Further research is required on the effects of storage on mRNA combined with vector molecules under freezing conditions, to prolong the preservation period of the vaccine [16].
《4.2 Recommendations for industry policies》
4.2 Recommendations for industry policies
4.2.1 Strict supervision of the safety and effectiveness of nucleic acid vaccines
Although nucleic acid vaccines have been approved by foreign regulators due to their efficiency in the COVID-19 pandemic, the long-term effects of these vaccines need further investigation. Based on the effectiveness and short-term safety of nucleic acid vaccines, it is suggested that China’s regulatory authorities should follow a strict approval system and steadily promote the transitional application of nucleic acid vaccines to gradually improve long-term safety verification. Nucleic acid vaccines were not formally approved for human use before the COVID-19 pandemic. Therefore, China’s regulatory authorities should pay close attention to and follow up on nucleic acid vaccines that have been approved for human use in the context of epidemic prevention and control to assess the long-term safety accurately, and evaluate R&D investment and comprehensive output benefits.
4.2.2 Focus on progressive technology development and promote its transformation
Since nucleic acid vaccines are a new type of vaccine, technologies for their development are still evolving. However, some challenges surrounding vaccine structure, delivery, immunogenicity, and stability remain. Foreign institutions have adjusted their R&D priorities and have made breakthroughs in the development of mRNA vaccines to satisfy the demand for safe and effective vaccines against infectious diseases. It is suggested that China’s industry management agencies remain progressive and solution-oriented. Chinese institutions should work according to a progressive layout for solving urgent public-health problems (infectious disease prevention and control, cancer treatment) and actively manage and support high-risk research on nucleic acid vaccines, while also considering a mature R&D direction. Chinese institutions should also focus on accumulating and strengthening basic technical reserves, while emphasizing technology transformation and application efficiency, improving the batch production capacities, and steadily improving the comprehensive strength of vaccine R&D in China.














 京公网安备 11010502051620号
京公网安备 11010502051620号




