《1. Introduction》
1. Introduction
The emission of greenhouse gases into the atmosphere during industrialization and urbanization has contributed to global warming and thus climate change. As the main source of greenhouse gases, the global CO2 emission reached 33.1 Gt in 2018, accountig for approximately 67% of total greenhouse gas emissions. This has significantly increased atmospheric CO2 concentration (approximately 412 parts per million (ppm)) [1,2]. Carbon capture, utilization, and storage (CCUS) is a potentially disruptive technology that could help against the challenge of climate change. CO2 can be captured from emission sources, such as power plants and industrial plants, as well as from the atmosphere. The captured CO2 can be utilized as a feedstock for chemical synthesis or injected into the deep subsurface for permanent and safe storage.
As one of the technologies that can deliver net-zero emissions at a large scale, CCUS (also biomass energy with carbon capture and storage (BECCUS) when using biomass) can be applied to existing coal- and gas-fired power plants and help provide low emissions generation capacity. In addition to contributing to the power supply sector, CCUS is possibly the only scalable and costeffective option for achieving deep decarbonization for certain industries such as steel, cement, glass, ceramics, as well as the manufacturing of chemicals that generate CO2 during the production processes. Analyses performed by the Intergovernmental Panel on Climate Change (IPCC) and the International Energy Agency (IEA) have shown that CCUS will be key in achieving ‘‘Net Zero” by 2050 which contributes one-sixth of global CO2 emissions reduction in order to limit the global temperature increase to within 1.5 °C, as stated by the Paris Agreement [3,4]. Without the successful deployment of CCUS, it will cost more to deal with climate challenge, for example, it will cost 25% more for China to achieve long-term climate change mitigation targets without CCUS [5].
In Section 2, carbon capture focusing on chemical absorption is discussed in detail. In Section 3, electrocatalytic CO2 reduction is selected as the main topic due to its great potential. Finally, Section 4 focuses on the fundamental CO2 trapping mechanisms which is of importance for carbon storage.
《2. Carbon capture》
2. Carbon capture
CO2 is emitted during power generation, industrial processes, and energy transformation. Carbon capture techniques are divided into three routes: post-combustion capture, oxyfuel combustion, and pre-combustion capture. There are various physical and chemical processes employed in capture technology, including solvent-based absorption, solid sorbents for adsorption/absorption, membranes, cryogenics, and chemical looping for CO2 separation [6–8]. Among these methods, chemical absorption is currently one of the most widely and commercially used techniques (e.g., 1 million tonnes CO2 (tCO2) per year Boundary Dam CO2 capture plants in Canada [9] and 1.4 million tCO2 per year Petra Nova carbon capture and storage (CCS) project in the United States [10]). The current cost for carbon capture projects globally is approximately 60–110 USD·t–1 , and it is forecast to decrease to approximately 30–50 USD·t–1 by 2030. This would enhance the promotion of this technology at a commercial scale [11].
Considering that post-combustion with chemical absorption requires minimal retrofitting of existing facilities, it has the largest potential to be commercially available in the near future. Chemical absorption involves various physical and chemical capture processes that utilize chemical solvents to absorb CO2. Current limitations, such as high energy consumption for solvent regeneration, corrosiveness, high toxicity, volatility, and high cost, are the main barriers for the deployment of capture technology. Currently, the energy consumption for capturing CO2 for Boundary Dam and Petra Nova projects is approximately 0.25–0.30 MW·h·tCO2–1 , which results in an energy efficiency penalty. It has been estimated that the net power generation efficiency of the plant, for example, a pulverized coal-fired supercritical power plant, will be reduced from 41%–45% to 30%–35% when the CO2 capture rate is 90%, and it is expected to reduce the energy consumption by 30%–40% for commercial applications [12].
To improve the capture efficiency and economic competitiveness, the development of novel solvents with high performance and effective process configuration modifications are attractive areas of research interest. Ideal CO2 absorbents, as the core in a chemical absorption process, should have the characteristics of high absorption rate, large absorption capacity, and low regeneration energy requirement. These are followed by safety, stability, environmental friendliness, low corrosion to equipment, and economic feasibility. Fig. 1 and Table 1 [7,13–38] summarize the different types of CO2 capture absorbents. Amine-based absorbents, including single amines, amine blends, biphasic solvents, and water-lean solvents, have been employed to achieve better efficiency [39]. The concept of biphasic solvents is to have an absorbent system of one phase feeding into an absorber and turning into immiscible CO2-rich and CO2-lean phases [40]. Water-lean solvents are mixtures of an organic diluent and an amine. These solvents have enhanced mass transfer properties, increased absorption capacities, and lower heat generation. For effective process configuration modification, potential improvements, including inter-cooling, rich solvent recycling, and lean solvent splitting, can be applied for the absorption process, while methods such as inter-heating, rich solvent splitting, and flashing stripping can be applied for the desorption process. These efforts could provide a critical foundation for reducing operating costs.
《Fig. 1》

Fig. 1. Various chemical solvents for CO2 absorption and associated absorptive capacity and absorption rate (see Table 1). g: gas; aq: aqueous; MEA: monoethanolamine.
《Table 1 》
Table 1 Absorptive capacity and absorption rate for various chemical solvents for CO2 absorption.

T: temperature; P: pressure; Q: flow rate; MDEA: N-methyldiethanolamine; AMP: 2-amino-2-methyl-1-propanol; DETA: diethylenetriamine; AEEA: N-(2-hydroxyethyl) ethylenediamine; ILs: ionic liquids; NDIL0309 and NDIL0230: types of micro-encapsulated CO2 sorbents
In addition to carbon capture from point sources, direct air capture (DAC) aims to directly remove low-concentration CO2 from the atmosphere. However, DAC techniques have not been well established, and the cost of CO2 capture is much higher than that of processes from high CO2 concentration emission sources. Currently, the cost of DAC at the pilot-scale is 94–232 USD·tCO2–1 , depending on the choice of technology. The overall cost is predicted to drop to approximately 60 USD·tCO2–1 by 2040, hastening the commercial viability of this technology [41].
《3. Carbon utilization》
3. Carbon utilization
CO2 utilization is proposed to elevate the economic competitiveness of CCUS technology through the profitable reuse of captured CO2. Generally, CO2 utilization includes the direct use of CO2 as dry ice, fire extinguisher, refrigerant, and in the food industry; other means include conversion of CO2 into high-value products through various chemical (e.g., chemical conversions into fuels and chemicals, mineralization) and biological (e.g., microalgae cultivation) processes. The scale of using CO2 to synthesize fuels ranges from 1.0 to 4.2 Gt CO2 per year [42]. Table 2 [43,44] summarizes the market status of the representative chemicals and the maturity of the CO2-derived techniques. Electrochemical CO2 reduction is a promising method for coupling CO2 to fuel processes with renewable energy.
《Table 2》
Table 2 Market status of representative chemicals and level of development of the CO2- derived technologies. Based on Refs. [43,44].

In recent years, electrocatalytic CO2 reduction driven by renewable electricity to synthesize fuels and chemicals has attracted significant interest (Fig. 2 [45–65]). Through careful design and screening of electrocatalysts, conversion of CO2 to two-electron reduction products, that is, carbon monoxide and formate, has been demonstrated with Faradaic efficiencies (FEs) > 95% [66]. In addition, the generation of deeply reduced products (electrontransfer number greater than two) with modest selectivity could only be obtained with copper-based electrocatalysts [67], but the stability of such systems still needs further improvement. Recently, the deployment of a gas-diffusion electrode architecture has enabled the operation of electrocatalytic CO2 reduction at high current densities (> 100 mA·cm–2 ), representing a significant step toward practical CO2 electrolyzer [68]. Furthermore, the formation of carbon-heteroatom (e.g., nitrogen) bonds coupled with electrocatalytic CO2 reduction could be a promising route for producing value-added chemicals under mild conditions [67]. With the rapid development of theoretical chemistry and data science, theoryand data-assisted catalyst design could markedly accelerate the discovery of high-performance CO2 reduction electrocatalysts [59]. In addition, CO2 is usually released into the atmosphere after consuming these products. Therefore, DAC could play an important role in further reducing the CO2 concentration in air.
《Fig. 2》

Fig. 2. Electrocatalytic CO2 conversion for fuel and chemicals production. (a) Summary of current sources and corresponding electrochemical reaction conditions of representative products potentially generated from CO2. (b) Trends of Faradaic efficiencies for representative CO2 reduction products achieved at current densities greater than 10 mA·cm–2 , including carbon monoxide [45–47], formate [48–50], methanol [51–54], methane [55–58], ethylene [59–62], and ethanol [63–65].  : the standard electrode potential.
: the standard electrode potential.
CO2 utilization has a great potential to reduce CO2 emissions. Although the utilization of CO2 has been proposed to reduce the cost of CCUS, many of the utilization technologies are not yet economically viable. Most chemical conversions of CO2 (except the acid-base neutralization reactions during mineralization) require the input of external energy, which also requires additional costs to drive the conversion processes. In this sense, the conversion of CO2 to certain products (e.g., methane) cannot compete with current petrochemical pathways in terms of price, even considering the projected performance improvement [69]. Therefore, CO2 conversion to high-value chemicals, such as polycarbonate and acrylate plastics [70], may be a feasible utilization pathway. Another factor that needs to be considered during the implementation of CO2 utilization is the logistics cost. Long-distance transportation between the CO2 emission sources, utilization facilities, and end-users should be avoided to reduce the overall costs of CCUS.
《4. Carbon storage》
4. Carbon storage
Carbon storage is a process that CO2 is injected and stored permanently in the subsurface, such as oil/gas reservoirs, nonmineable coal seams, and deep saline aquifers. IPCC and IEA both state that any plausible path to net-zero emissions to cope with climate change involves carbon storage at a global scale [3,71]. In recent years, enhanced oil recovery (EOR) in oil/gas reservoirs and enhanced coal-bed methane recovery (ECBM) in nonmineable coal seams have become attractive CO2 geologic utilization techniques. The injection of CO2 extracts extra oil and gas, while simultaneously storing CO2. The principle of CO2-EOR is to inject CO2 by either immiscible or miscible flooding into the pore space of the reservoirs, which can enhance the pore-scale displacement efficiency. Currently, this technique has been widely deployed, as it can offset some of the costs by recovering an additional 30%–60% of oil [72]. The mechanism for CO2-ECBM is based on the preferential adsorption of CO2 onto the coal micropore surface compared to methane (CH4). Currently, CO2-ECBM is not commercially available because of technical difficulties in injecting CO2 into unmineable coal seams with low permeability and additional costs for a good drilling. Carbon storage in deep saline aquifers has a large storage potential, but it is not yet commercially available. There are generally four types of CO2 storage in geological systems: stratigraphic trapping by impermeable cap rocks, solubility trapping where CO2 dissolves into the brine, mineral trapping where CO2 reacts with the host rocks, and residual or capillary trapping where CO2 is trapped by the surrounding fluids in the pore space as droplets (or ganglia) [73,74].
In the past decades, pore-scale imaging techniques have been developed to visualize and quantify multiphase flow in porous rocks at the pore scale [75]. The mechanisms of CO2 storage in deep saline aquifers and oil/gas reservoirs, which are associated with wettability, are now fully explained (Fig. 3 [76]). In saline aquifers, CO2 can be stored through capillary trapping: Water wets the rock surfaces and flows through wetting layers, leaving CO2, the nonwetting phase, stranded in the centers of the larger pores in disconnected blobs, and a significant amount of CO2 can become trapped in the subsurface. When storing CO2 in hydrocarbon reservoirs, the presence of hydrocarbons in porous rock over geological time also changes the wettability toward more oil-wet conditions, and concepts from CO2–water flow cannot be simply applied. It has been observed that wettability is dependent on the pore structure and fluid properties: CO2 may be the most non-wetting phase, occupying the largest pores, which facilitates flow and allows capillary trapping. In other cases, water becomes non-wetting, confining CO2 to low-permeability layers in the pore space but hinders capillary trapping.
《Fig. 3》
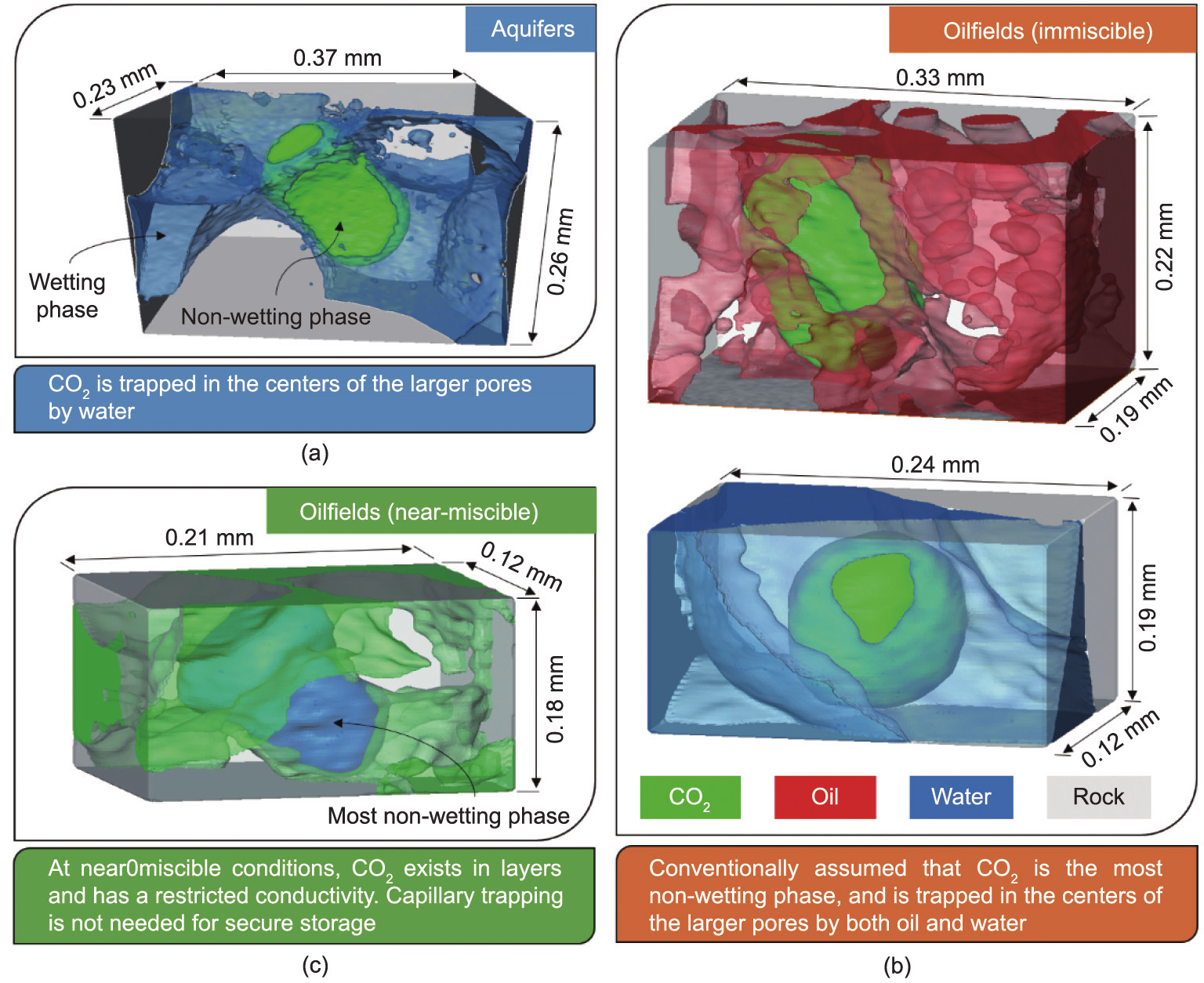
Fig. 3. Mechanisms of CO2 storage and the wettability status of stored CO2 in geological formation. (a) In a saline aquifer, CO2 is the non-wetting phase and can be trapped in the center of larger pores. (b) In an oilfield under immiscible conditions, CO2 is the most non-wetting phase and can be capillary trapped by either oil (top) or water (bottom). (c) In an oilfield at near-miscible conditions, water is the most non-wetting phase, followed by CO2 and oil. CO2 exists in layers surrounding the water phase, and its flow is restricted [76].
From a scientific perspective, while the concept and mechanisms of CO2 storage have been demonstrated, there is still concern over the storage efficiency and the long-term fate of CO2 in the subsurface when injected at the envisaged scales. The questions around geologic CO2 utilization and storage remain: How is CO2 trapped in the pore space and how does trapping cause changes in geological systems such as in sedimentary basins, depleted oil fields or hydrophobic formations, and in unconventional environments, for example, shale, coalbeds, and fractured rock? What is the impact of physical and chemical heterogeneity on storage? How should CO2 injection be designed to maximize storage security? How can CO2 storage be efficiently coupled with EOR and ECBM to provide permanent storage and efficient and economical fuel production? To answer these questions, a good understanding of three important aspects that can help design injection and storage strategies to enhance storage efficiency:
(1) The impact of geomechanics, such as the stress state and overburden pressure to the change of pore structure and flow properties such as permeability.
(2) Reactive transport (e.g., rock dissolution in the presence of CO2 in the pore space under reservoir conditions) and its consequence to the change in pore structure, flow path, and flow properties.
(3) The complex fluid mechanics of multiple fluid phases flowing in the pore spaces.
《5. Conclusions and perspective》
5. Conclusions and perspective
The increasing of CO2 emissions into the atmosphere is becoming a major environmental concern, pointing to global warming and climate change. Some specific technical aspects of CCUS have been discussed. For CO2 capture, chemical absorption is considered a potential candidate for commercial deployment. However, the cost of this technology is required to drop to 30–50 USD·t–1 with energy consumption for capturing CO2 lower than approximately 0.21 MW·h·tCO2–1 . To achieve this, absorbents with high efficiency and low regeneration cost are required to achieve a reduction in the capture cost for the successful deployment of this technology. For CO2 utilization, electrochemical conversion has the potential to convert CO2 into valuable chemicals. The future direction for this technique is to develop highly active, selective, and stable electrocatalysts and optimize the electrolyzer design to promote demonstrations at pilot scales, benefiting the assessment of the overall energy efficiency and cost of such processes. CO2 storage in the subsurface has great potential, where the storage of CO2 can be combined with energy production (e.g., EOR and ECBM) to bring economic benefits. Although the fundamental principles of CO2 trapping have now been explained, further studies of fluid mechanics, geomechanics, and reactive transport, as well as on how these processes could be coupled, are still challenging for optimized and safe storage requirements. This could be achieved using advanced and novel techniques, such as nondestructive imaging tomography techniques.
《Acknowledgments》
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51836006).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Qingyang Lin, Xiao Zhang, Tao Wang, Chenghang Zheng, and Xiang Gao declare that they have no conflicts of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




