《1. Introduction: Salinity gradient energy》
1. Introduction: Salinity gradient energy
Salinity gradient energy (SGE), sometimes known as blue energy, is an energy source that was first identified in the 1950s [1]. This energy source relies on the energy that dissipates when two solutions with different salinities mix. It is a renewable energy source that is directly linked with Earth’s complex water cycle. In this cycle, water evaporates from bodies of water, mainly due to solar radiation. Water-based solutions with low salt concentrations are transported in the form of clouds, eventually reaching the ground once more as precipitation. Rain or melted snow run-off becomes slightly enriched with minerals as it travels, usually in the form of rivers, to reach the ocean.
One of the difficulties in communicating about salinity gradient energy is that this energy source relies on a complex concept of mixing energy. For example, at a point where the fresh water of a river meets the salt water of an ocean, the concentration difference creates an energy potential that is equivalent to that of a 140−240 m high dam (depending on the concentration difference between the ocean and fresh water).
To better understand SGE, one must examine the definition of the Gibbs free energy of mixing. For an ideal dilute solution ( ΔmixH=0), the Gibbs free energy of mixing can be expressed as:
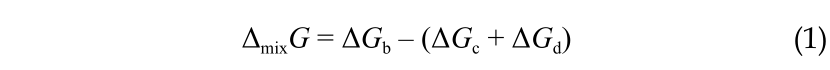
where the subscript c represents the concentrated solution; the subscript d represents the dilute solution; and the subscript b represents the brackish solution that results from the mixing.
The link with the entropy of mixing ΔmixS can be obtained by expressing Eq. (1) in the following way:

where n is the amount of particles (mol); Tis the temperature (K); andΔmixS is the molar entropy of mixing (J∙(mol∙K)−1) that can be expressed as:
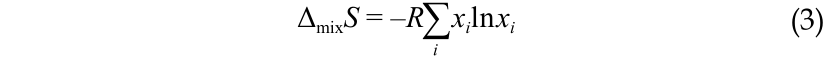
where R is the universal gas constant (8.314 J∙(mol∙K)−1); x i is the molar fraction of component i (in the case of ocean and fresh water, i is mostly Na, Cl, and H2O).
Using these equations, it is possible to calculate the potential energy that may be recovered from any river mouth. For example, mixing 1 m3 of sea water at 0.5 mol∙L−1 of NaCl with 1 m3 of river water at 0.01 mol∙L−1 of NaCl at a temperature of 293 K leads to a theoretical maximum amount of extractable energy of 1.4 MJ. Based on these calculations, the maximum theoretical worldwide potential for SGE technology is usually considered to be between 1.4 TW∙h and 2.6 TW∙h [2]. Of course, this value assumes ideal conditions and the exploitation of all possible sites, which is not always the case.
《2. SGE technologies: Pressure-retarded osmosis》
2. SGE technologies: Pressure-retarded osmosis
Based on the large potential output of such an energy source, several technologies have been considered for the utilization of SGE. Thus far, only two approaches have reached the pilot phase. The first of these two approaches is called pressure-retarded osmosis (PRO) [3]. This technology relies on semi-permeable membranes that permit only water molecules to pass through. In this approach, water flows from the dilute solution to the concentrated solution in order to bring the chemical potentials on both sides of the membrane to equilibrium. This movement of water can be used to feed turbines that transform the mechanical energy into electricity. Because this approach is similar to hydropower technology, it has been developed and piloted by a Norwegian energy company with expertise in operating turbines. The first osmotic energy power plant based on PRO was opened in 2009.
However, the PRO approach is very sensitive to membrane fouling, which is the accumulation of matter (scaling of minerals, growth of organisms, etc.) on the membranes. For this reason, very clean water is necessary for system operation. Eventually, the company developing it decided to stop work on this technology, since the cost of its operation did not allow it to be competitive.
《3. SGE technologies: Reverse electrodialysis》
3. SGE technologies: Reverse electrodialysis
The second SGE approach that has reached the pilot phase is called reverse electrodialysis (RED). This technology relies on the use of ion-exchange membranes, which are membranes that either let negatively charged ions (in anion-exchange membranes or AEM) or positively charged ions (in cation-exchange membranes or CEM) pass through. The particularity of such membranes is that the separation of charge that they operate due to their chemical composition leads to the formation of an electrochemical potential. When several of these membranes are stacked together (Figure 1), with alternating anion- and cation-exchange membranes and water-based solutions of different concentrations on each side of the membranes, it is possible to build up a considerable electrical potential that can be used as a driving force to generate electricity. This electrical potential is converted into an electron flow through redox reactions that may be reversible (e.g., Fe2+/Fe3+ or ferricyanide/ferrocyanide [4]) or irreversible (e.g., reduction of H2O into H2 and oxidation of Cl− into Cl2 [4]), or through ion adsorption in capacitive electrodes [5].
《Fig. 1》
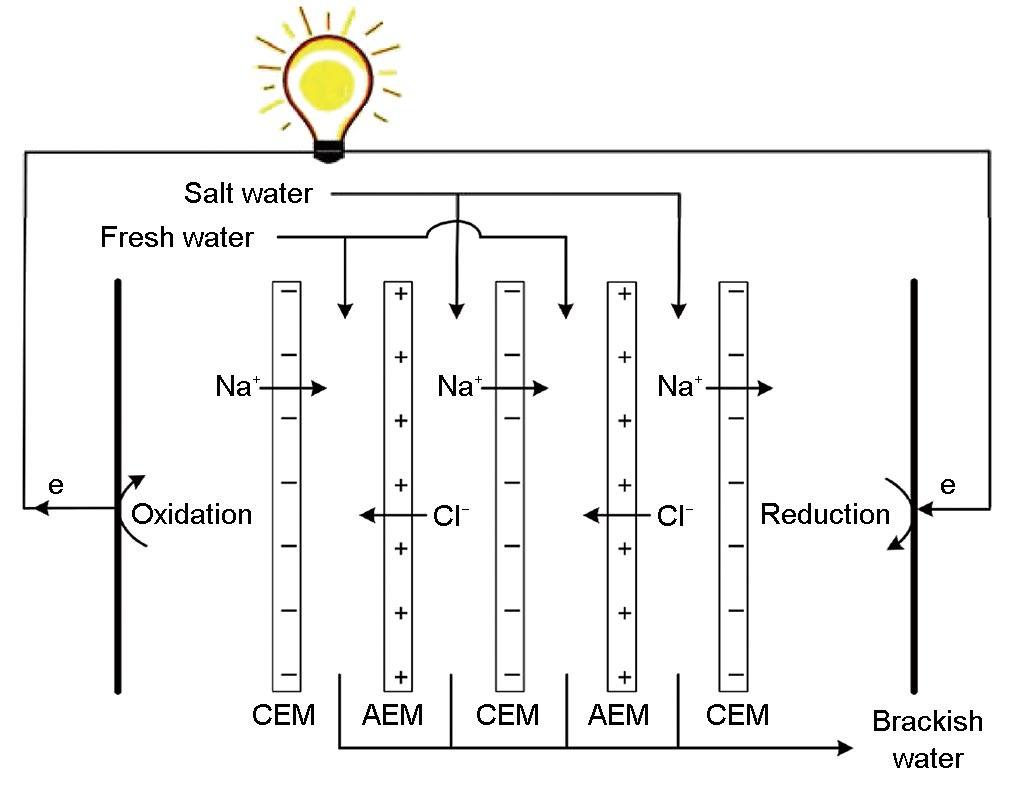
Fig.1 REDSTACK construction. In this specific case, redox reactions are used for the electron flow; however, these can be replaced by capacitive electrodes, where ions are adsorbed (negative ions at one side and positive ions at the other) to maintain electro neutrality.
This technology was matured in the laboratory of the Netherlandish research institute Wetsus. It eventually led to the creation of the spin-off company REDSTACK, which now ope-rates the first RED pilot plant, started in 2014. The pilot plant is located on the Afsluitdijk, a closing dike in the Netherlands where salt and fresh water are separated by a 90 m wide dike.
During this crucial pilot phase, REDSTACK engineers must confront RED technology in the real world, outside of the laboratory environment. A number of challenges have to be overcome in order to reach the 50 kW capacity aimed for by this pilot, before proceeding to a larger scale operation.
The challenges of RED technology essentially consist of its adaptation to a real environment in which the water-based solutions have more complex compositions than the simple NaCl solutions used in the laboratory. Real-world solution compositions are less controllable, leading to changes in concentration and temperature over time. Moreover, real sea or river water contains many impurities that can significantly hinder the functioning of a RED system.
《4. Challenges of RED》
4. Challenges of RED
The first challenge occurs at the inlet, where water-based solutions enter the system. Large volumes of salt water and fresh water must be pumped into the system as efficiently as possible, to ensure that the energy produced by the system is greater than the energy used by it. In addition, the pumping must conform to legislation regarding the protection of the natural environment. The pumping of large volumes of water can create sheer forces that are enough to harm certain animal or plant species; moreover, proper care must be taken to prevent larger animals such as fish from entering the pipe system.
Once the solutions are pumped into buffer tanks, they are filtered to remove large particles that may clog the system. Filtering is performed by big drum filters that can separate solid particles from the solutions. Different filter mesh sizes are being tested in order to optimize the tradeoff between the size of the particles allowed in the system and the energy required for filtration.
Once the solutions enter the system, several complications can occur. First of all, because it is an electrochemical system, the internal resistance must be minimized. To do this, the distance between each membrane is usually kept very small, on the order of 100 μm. This distance is traditionally maintained by a polymer-based spacer that allows water to flow between the membranes. The spacer tends to cover part of the membranes, creating a “shadow” that prevents the fully efficient use of the membrane.
One way to optimize the effect was proposed in 2011 [6]. This method uses structured membranes with ridges, as shown in Figure 2; the ridges can be of various shapes and depth. The ridges allow water to flow, and depending on the shape, can also optimize mass transport in the system (i.e., renewal of the boundary layer at the membrane interface). This method cannot be achieved with every membrane, as only thicker and more robust membranes can take the hot-pressing process that is required. New approaches are being developed to address this issue.
《Fig. 2》
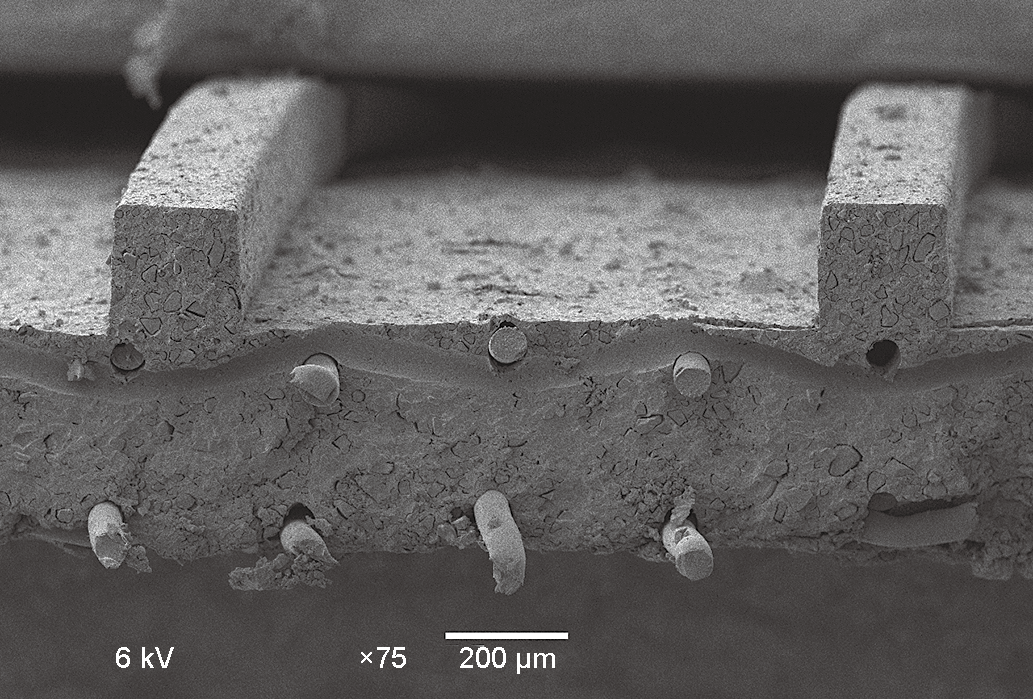
Fig.2 Scanning electron microscope image of a profiled membrane.
Another issue raised by real-world experiments involves the effect of multivalent ions on the performance of the system [7]. It has been observed that the presence of only a small amount of divalent cations, such as Ca2+ or Mg2+, can significantly lower the power output of the system (29%−50% lower power densities for a 10% molar fraction of Mg2+ salts). This situation can be explained through several phenomena.
(1) The membrane potential is defined with the Nernst equation below, where E is the electromotive force over the membrane (V), α is the apparent perm selectivity of the membrane, zi is the valence of the ionic species i, and αi is the activity of the ionic species i (mol·L−1).
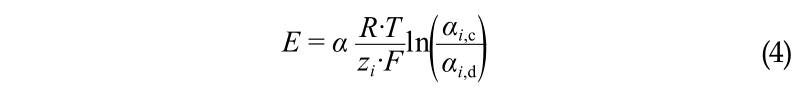
Eq. (4) clearly shows that the relative contribution to the membrane potential decreases as the valence increases.
(2) It has also been shown that multivalent ions are subject to an “uphill” transport against their gradient, which leads to less power being extracted.
(3) Finally, due to the higher charge of multivalent ions, their affinity with the membranes’ structure is higher than that of monovalent ions. This difference in affinities leads to a lower exchange of monovalent ions, and thus a lower contribution of those ions to the membrane potential. One way to address these losses is to make the membranes more selective to monovalent ions, either by making membranes specifically selective to the ions of interest (Na+ and Cl−), or by applying a thin layer of ion-exchange material of the opposite charge to repel multivalent ions (e.g., a thin layer of anion-exchange material on top of a CEM to repel Ca2+ or Mg2+).
Another intrinsic issue involves water from the natural environment, which contains substances that can damage the membranes over time. It has been observed that charged species such as humic acids or negatively charged clay particles can accumulate on the surface of the membranes. This is particularly the case for AEMs, which have an affinity for negatively charged species. Moreover, the presence of living organisms such as bacteria can lead to biofouling (Figure 3); that is, the growth and accumulation of living biofilms on top of the membranes. To address these issues, several strategies have been tested [8], such as regularly switching the feed solutions (i.e., the sea-water compartment is filled with fresh water, and vice versa), which hinders the establishment of biofilms. Sparging air is also proved to be a successful approach to limit the accumulation of clogging material. Cleaning the system with concentrated brines was also considered, but this leads to a decrease of perm selectivity and thus a lower power output. The accumulation of humic acids and other negatively charged colloids remains an issue, but one that can be solved with better membranes such as those developed to counter the multivalent-ion effect.
《Fig. 3》

Fig.3 Scanning electron microscope image of an AEM that was exposed to sea water for eight days. Bacteria cannot be seen, but the remains of living organisms such as diatoms are visible at the surface.
《5. Conclusion: The future of SGE》
5. Conclusion: The future of SGE
Once the above-mentioned challenges have been addressed, RED technology will be ready for implementation on a larger scale, which is likely to happen in the coming decades. In parallel with the development of RED technology, new approaches for energy production from gradients have been developed in recent years. New technologies such as capacitive systems [9,10] or salt batteries [11] are being developed. New concepts and applications are being developed as well, such as the use of concentrated brines (natural or industrial) [12] and the use of new gradients, including thermal gradients [13,14] and gradients of other compounds (e.g., mixing gases containing different concentrations of CO2, such as exhaust gases and air) [15]. Research on SGEs is entering its golden age, with more and more diversity in gradient sources and new technical solutions to harvest the energy being dissipated on mixing these sources.













 京公网安备 11010502051620号
京公网安备 11010502051620号




