《1. Introduction》
1. Introduction
Campylobacter spp. are Gram-negative thermophilic bacteria that are major etiologic agents for human foodborne illness (campylobacteriosis) worldwide [1,2]. Among all Campylobacter species, Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli) are the leading causes of human gastroenteritis [3]. Moreover, C. jejuni infection may lead to autoimmune conditions known as Guillain–Barré syndrome (GBS) and Miller Fisher syndrome [2]. Poultry, contaminated water, and raw milk are the most frequent vehicles for illness [4]. Chickens are the major host of Campylobacter due to their suitable intestinal environment, and thus play a critical role as the typical source of Campylobacter infections in industrialized nations [5].
Campylobacter infections are generally self-limiting and treated with supportive therapy, such as maintenance of hydration and electrolyte balance [6]. However, antimicrobial chemotherapy is recommended for patients with severe symptoms, extraintestinal infections, or acquired immune deficiency syndrome (AIDS) [6]. Fluoroquinolones and macrolides are the drugs of choice to treat campylobacteriosis [7], while tetracycline (TET) and gentamicin (GEN) are used to treat the systemic infection caused by Campylobacter [7]. However, widely used antimicrobial agents, such as ciprofloxacin (CIP) and azithromycin, have been challenged by drug resistance in both human and animal isolates. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria in 2017 showed that 57.7% of C. jejuni and 63.5% of C. coli of human origin were resistant to CIP [8]. Data from the National Antimicrobial Resistance Monitoring System (NARMS) report in the United States indicated that CIP resistance increased in C. coli from humans (34% in 2012 to 40% in 2015) and continued to increase or remained high in C. jejuni isolates from chickens (ranging between 22% and 28% during 2012–2015) [9]. Hence, the US Centers for Disease Control and Prevention (CDC) listed drug-resistant Campylobacter as a serious antibiotic resistance threat to public health in 2013 [10]. Similarly, the World Health Organization (WHO) listed fluoroquinolone-resistant Campylobacter species as high-priority pathogens in 2017 [11].
Unlike the United States and Europe, China lacks a complete antimicrobial resistance surveillance network for Campylobacter. Surveillance on Campylobacter in parts of China has shown that CIP resistance has reached a high level, with the most noticeable rates being observed in Jiangsu (85.2%) [12], Beijing (87.5%) [13], Shenzhen (89.7%) [14], and Shanghai (97.4%) [15]. Furthermore, the Campylobacter isolated from broiler chickens and swine in five provinces during 2008–2014 exhibited 100% resistance to CIP [16], suggesting that this drug may no longer be suitable for the treatment of Campylobacter infection in these areas.
The main mechanism of Campylobacter resistance to fluoroquinolones is mediated by point mutations in the quinolone resistance-determining region of GyrA and the tripartite multidrug efflux pump CmeABC [17,18]. CmeABC belongs to the resistancenodulation-division (RND) protein family, and consists of a membrane fusion protein (CmeA), an inner membrane transporter (CmeB), and an outer membrane protein (CmeC) [19]. As a predominant efflux pump for Campylobacter, CmeABC extrudes toxic compounds as well as structurally diverse antimicrobials including fluoroquinolones, phenicols, macrolides, and TETs [20]. It is notable that a potent variant of CmeABC, named RE-CmeABC, has recently been found in Campylobacter [21]. Using basic local alignment search tool (BLAST), the unique CmeB shares 80.5%–81.2% amino acid sequence identity with wide-type CmeB in RE-cmeABC-negative strains; and its differences mostly contribute to the enhanced resistance level to various antibiotics. Furthermore, RE-CmeABC not only promotes the emergence of fluoroquinolone-resistant mutants under selective pressure, but also confers exceedingly high-level resistance (minimum inhibitory concentration (MIC) of CIP ≥ 128 mg·L-1 ) to fluoroquinolone in the presence of GyrA mutations [21].
Since the RE-cmeABC genes were first identified in 2014, no surveillance of RE-cmeABC genes in Campylobacter collected from the same regions has been conducted. We therefore investigated the presence and antimicrobial susceptibility of RE-cmeABC-positive Campylobacter isolates of food-animal origin from slaughterhouses and farms in three representative regions of China during 2014–2016.
《2. Materials and methods》
2. Materials and methods
《2.1. Isolation, screening, and mutation detection of RE-cmeABCpositive Campylobacter isolates》
2.1. Isolation, screening, and mutation detection of RE-cmeABCpositive Campylobacter isolates
We analyzed the presence and epidemic trend of RE-cmeABC in Campylobacter over three successive years (2014–2016) in three regions of China (Guangdong, Shandong, and Shanghai). A total of 1088 Campylobacter isolates (931 C. coli and 157 C. jejuni) were recovered, of which the isolates from 2014 have been published previously [21]. These Campylobacter strains were isolated from the cecal contents, carcasses, feces, and retail meat of swine and chickens under our laboratory’s annual antimicrobial resistance surveillance program during 2014–2016. All of the Campylobacter strains were grown on Mueller–Hinton (MH) agar (Sigma–Aldrich, Inc., USA) at 42 °C under microaerobic conditions (5% oxygen, 10% carbon dioxide, 85% nitrogen) for 18–24 h. All isolates were screened for the presence of the RE-cmeB gene using the primers RE-cmeBF (5' -CGTATTGCACGATTATTTGGAC-3' ) and RE-cmeBR (5' -ATCGTTATCAAACCCTCTATGTGCC-3' ). To investigate whether the high-level resistance to CIP was relevant to the coharboring of the DNA gyrase gyrA mutation and RE-cmeABC, multiplexmismatch amplification mutation assay-polymerase chain reaction (MAMA-PCR) was used to detect the single nucleotide mutation (C257 to T) that is frequently observed in the gyrA gene of all REcmeABC-positive strains [22].
《2.2. Antimicrobial susceptibility testing》
2.2. Antimicrobial susceptibility testing
The standard agar dilution method recommended by the Clinical & Laboratory Standards Institute (CLSI) M45 (2016) was used to determine the MICs of various antimicrobial agents for all Campylobacter isolates [23]. The tested antimicrobial agents included: GEN, erythromycin (ERY), CIP, TET, and clindamycin (CLI). Standardized MIC breakpoints for phenicols were not available for Campylobacter from the CLSI. Therefore, we utilized the breakpoint as recommended by the NARMS [24]. C. jejuni ATCC 33560 was used as the quality-control strain.
《2.3. Molecular typing》
2.3. Molecular typing
A total of 97 representative Campylobacter isolates containing RE-cmeABC were genotyped by pulsed-field gel electrophoresis (PFGE), conducted with a CHEF-DR III apparatus (Bio-Rad Laboratories, Inc., USA) in accordance with the protocol for Campylobacter [25]. The DNA of Campylobacter was digested with SmaI, while Salmonella H9812 was digested with XbaI used as the reference marker. The results were analyzed with InfoQuest FP software version 4.5 (Bio-Rad Laboratories, Inc., USA).
《2.4. Data collection and statistical analysis》
2.4. Data collection and statistical analysis
A descriptive analysis of the percentage and prevalence (together with 95% confidence intervals (CIs)) was performed using the functions provided in Excel 2016 (Microsoft, USA). Univariable analysis among RE-cmeABC, Campylobacter species, and MIC values of antimicrobial agents was conducted using Prism 7.0 (GraphPad Software, USA), which was adopted to select variables with P ≤ 0.05. The association between RE-cmeABC positivity and the variables was examined by odds ratio (OR). The differences between the rates were tested by Χ2 or Fisher’s exact test, if appropriate.
《3. Results and discussion》
3. Results and discussion
《3.1. Presence of RE-cmeABC in Campylobacter》
3.1. Presence of RE-cmeABC in Campylobacter
Among the 1088 Campylobacter isolates tested, 122 (11.2%, 95% CI: 9.4–13.2) RE-cmeABC-positive isolates, all from chicken, were detected. These included 111 (111/157, 70.7%, 95% CI: 62.9–77.7) C. jejuni and 11 (11/931, 1.2%, 95% CI: 0.6–2.1) C. coli. RE-cmeABCpositive C. jejuni were significantly more prevalent compared with RE-cmeABC-positive C. coli (70.7% versus 1.2%, P < 0.0001) over three years (Fig. 1(a)). The prevalence difference between C. jejuni and C. coli was similar to a previous study that examined Campylobacter isolates from 2012 to 2014 [21]. Furthermore, the prevalence of RE-cmeABC in C. jejuni isolates from the two studies increased from 34.7% (189/544) to 70.7% (111/157) (P < 0.0001), while RE-cmeABC-positive C. coli isolates decreased from 3.2% (47/1458) to 1.2% (11/931) (P < 0.0001). This result suggested that the transformation of RE-cmeABC in C. jejuni is more frequent than in C. coli. This finding indicated that C. jejuni has become the dominant reservoir for RE-cmeABC. Moreover, all RE-cmeABC-positive Campylobacter were isolated from chicken samples, possibly because C. jejuni is the dominant species in poultry [26]. Due to the low isolation rate and limited number of RE-cmeABC-positive C. coli, we only compared RE-cmeABC-positive C. jejuni from various regions and years.
《Fig. 1》
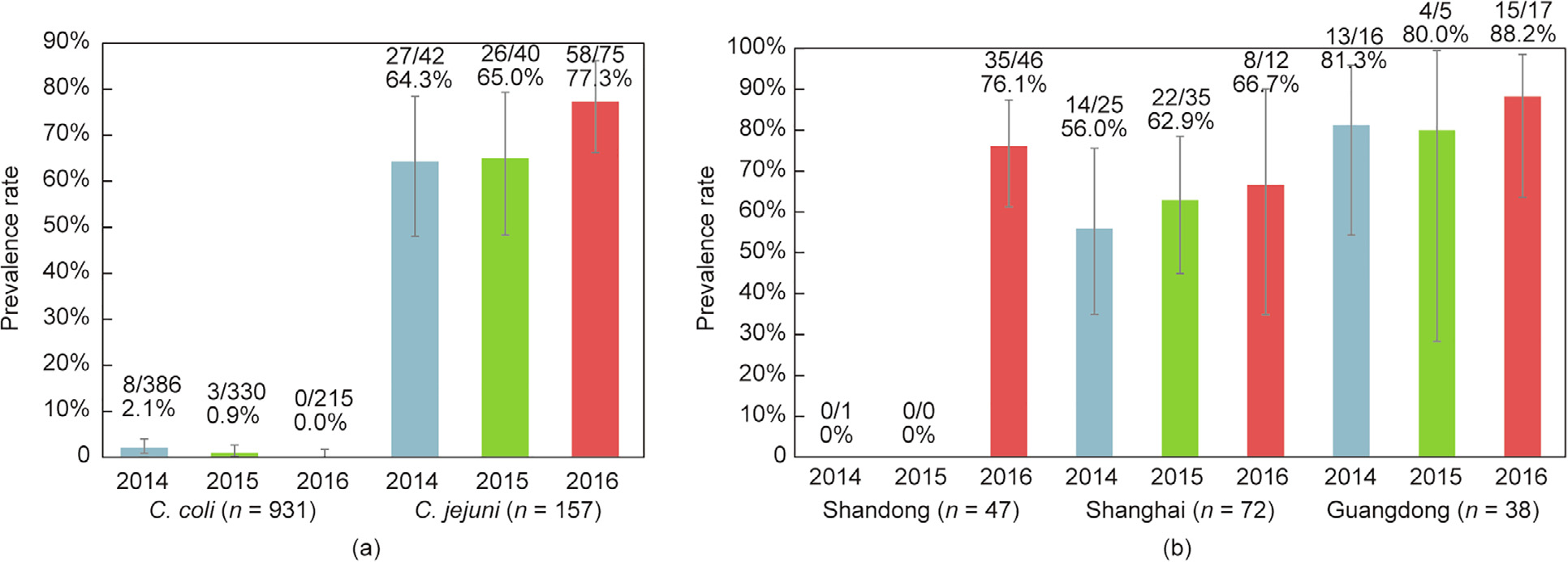
Fig. 1. Prevalence rate of RE-cmeABC-positive isolates from three regions by year. (a) Comparison of RE-cmeABC-positive isolates in C. coli and C. jejuni from the three regions by year; (b) percentage of RE-cmeABC-positive C. jejuni isolates from three regions by year. The error bar represents 95% CI.
The percentages of RE-cmeABC-positive C. jejuni isolates for each year are shown in Fig. 1(a). The positive isolates maintained a high level and did not significantly increase during the three years of the study (P > 0.05). Similarly, the prevalence of RE-cmeABC-positive C. jejuni from Guangdong and Shanghai slightly increased over the three years of sampling (Fig. 1(b)). However, the rate from Shandong remained at a low level during 2014 and 2015, while a rapid emergence of high prevalence (76.1%, 35/46, 95% CI: 61.2–87.4) occurred in 2016 (Fig. 1(b)). These data are of particular concern with regards to public health, as RE-cmeABCpositive C. jejuni now presents at a high rate in these major livestock and poultry production areas of China.
《3.2. Antimicrobial susceptibility profiles》
3.2. Antimicrobial susceptibility profiles
Antimicrobial susceptibility testing (AST) showed that all RE-cmeABC-positive C. jejuni were fully resistant to CIP, followed by their resistance to TET, florfenicol (FFC), GEN, CLI, and ERY, with the resistance rates of 99.1%, 74.8%, 45.9%, 13.5%, and 11.7%, respectively (Table 1). To assess the contribution of RE-cmeABC toward antimicrobial susceptibility to C. jejuni, we supplemented the AST of RE-cmeABC-negative C. jejuni for analysis. The resistance rates were significantly different in the RE-cmeABC-negative versus RE-cmeABC-positive C. jejuni isolates for FFC, CLI, and ERY (P < 0.05), but were not significantly different for CIP, TET, and GEN (P > 0.05) (Table 1). The resistance of RE-cmeABC-positive isolates to FFC was significantly higher than that of RE-cmeABC-negative isolates (74.8% vs. 39.1%, P < 0.0001). The OR of FFC for RE-cmeABC-positive isolates (OR = 4.61) were significantly higher than those for RE-cmeABC-negative isolates (Table 1). FFC, which belongs to the phenicols, is unapproved for clinical use, but has been used extensively for the treatment of respiratory diseases in food-producing animals [27]. Therefore, this antimicrobial agent may play an important role in the selection of RE-cmeABC-positive Campylobacter in animal production. Unlike FFC resistance, the resistance levels to CLI and ERY in RE-cmeABC-positive were significantly lower than those of RE-cmeABC-negative (OR = 0.32 and 0.34, respectively) (Table 1). Although RE-cmeABC could contribute a four-fold increase in the MIC of ERY, it was insufficient to cross the breakpoint on its own, unless the target-gene mutations of 23S rRNA were present as well [28]. These data suggest that RE-cmeABC may not be the major resistance mechanism of CLI and ERY in C. jejuni.
《Table 1》
Table 1 MIC profiles of C. jejuni with or without RE-cmeABC.

RE-cmeABC has been shown to have an enhanced exporter function compared with wide-type cmeABC, the latter of which can pump drugs out of cells; this is true for fluoroquinolones, phenicols, macrolides, and TETs [21]. Although there is no significant difference between RE-cmeABC-positive and -negative isolates in their resistance to CIP and TET, the isolates harboring RE-cmeABC shifted the MIC distribution to a higher range for both CIP and TET (Fig. 2), which was consistent with previous results [21]. Interestingly, the high-level resistant (≥ 16-fold of MIC breakpoint) isolates of CIP and TET were significantly higher in RE-cmeABC-positive isolates than in RE-cmeABC-negative isolates (P < 0.0001 and P = 0.0004, respectively). These results indicate that RE-CmeABC increases the antibiotic MICs at the population level, which is consistent with previous results [21]. As a verification of the mechanism of high-level resistance to CIP, we detected the single nucleotide mutation (C-257 to T) that is frequently observed in the gyrA gene. However, all RE-cmeABC-positive isolates harbored the C257T mutation, which further confirmed that the combination of the GyrA mutation and RE-CmeABC contributes to the high-level resistance to CIP. It is unknown whether the MIC values are related to the expression level of CmeABC. CmeABC is primarily regulated by CmeR, which binds to the promoter region of the cmeABC operon and functions as a transcriptional repressor [29].
《Fig. 2》
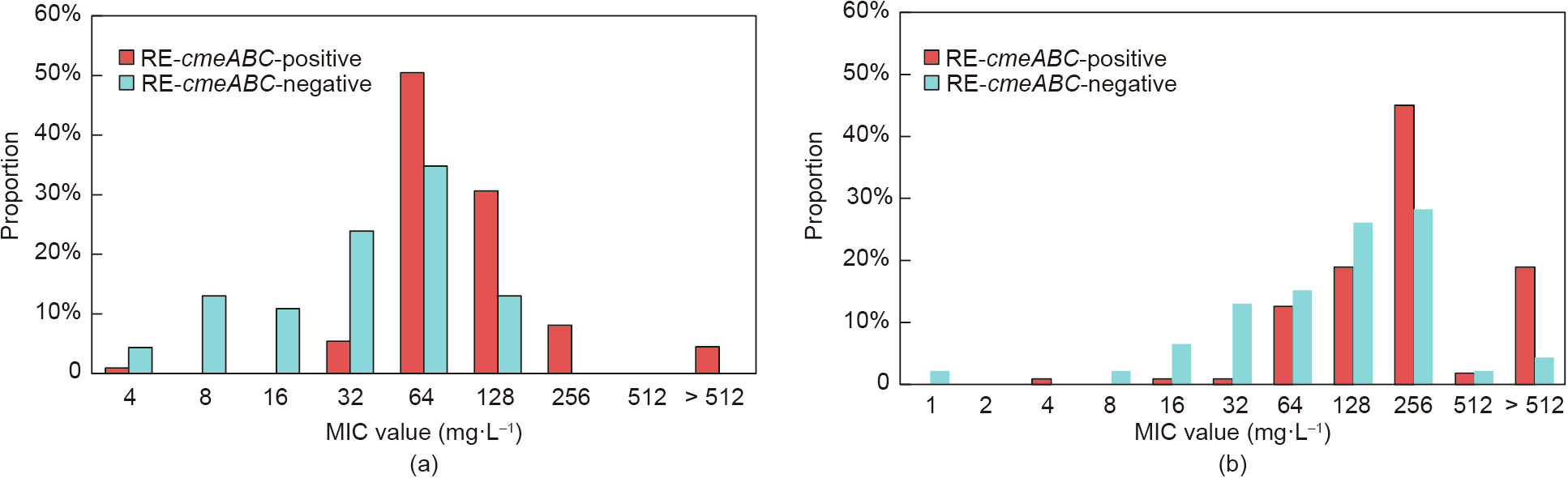
Fig. 2. Distribution of CIP and TET MICs for RE-cmeABC-positive isolates and -negative isolates. (a) Distribution of CIP MICs for C. jejuni; (b) distribution of TET MICs for C. jejuni.
《3.3. Genotyping》
3.3. Genotyping
The 97 representative RE-cmeABC-positive C. jejuni (37 from Shanghai, 25 from Guangdong, and 35 from Shandong) were analyzed by PFGE. Using a cutoff of 80% pattern similarity, the RE-cmeABC C. jejuni isolates were clustered into 30 PFGE patterns, including 13 unique patterns and 17 clusters (Fig. 3). The isolates from Shanghai showed the most patterns (eight clusters and seven unique patterns), followed by the Guangdong isolates (11 clusters and six unique patterns), and the Shandong isolates (five clusters). No predominant clones existed in the Shanghai and Guangdong isolates, except for pattern A (27.0%, 10/37) and pattern W (20%, 5/25). These findings suggested that horizontal transmission may be involved in the dissemination of RE-cmeABC-positive C. jejuni in Shanghai and Guangdong. In contrast, pattern B (65.7%, 23/35) was the predominant clone in Shandong, which indicates that regional expansion of a particular clone accounts for a larger proportion of the dissemination. Pattern R was shared by three isolates from Shanghai and Guangdong, while pattern U was shared by three isolates from Guangdong and Shandong. Moreover, strain 16SHKX48-1C, which was isolated from Shanghai, showed pattern J and had 100% homology to the isolates collected from Guangdong in 2016. Meanwhile, four isolates of pattern L, which were collected from Shanghai and Shandong, also shared 100% homology. These findings suggest that some RE-cmeABC-positive C. jejuni with the same PFGE patterns have spread into various regions, and that these isolates are stably transferrable and appear to be highly adaptable.
《Fig. 3》

Fig. 3. PFGE typing of 97 representative RE-cmeABC-positive C. jejuni collected from 2014 to 2016. SmaI was used for PFGE analysis. The regions included Shandong (SD), Shanghai (SH), and Guangdong (GD).
《4. Conclusion》
4. Conclusion
This study investigated the presence and antimicrobial susceptibility of RE-cmeABC-positive Campylobacter from food-producing animals in three regions of China during the three successive years of 2014–2016. By comparing our results with previously collected RE-cmeABC-positive Campylobacter from 2012 to 2014, we observed a shift of this multidrug efflux pump from C. coli to C. jejuni in chickens. The FFC resistance rate of RE-cmeABC-positive isolates was significantly higher than that of RE-cmeABC-negative isolates, while the CIP and TET resistance levels were not significantly different between these isolates. However, RE-cmeABC was found to increase the MIC distribution of the antibiotics. Moreover, several PFGE-indiscriminate RE-cmeABC-positive C. jejuni isolates were identified in both Shanghai and Guangdong, as well as in both Guangdong and Shandong. This finding suggests the possibility of a wide dissemination of RE-cmeABC in Campylobacter, which would poses a significant threat to human health.
《Acknowledgements》
Acknowledgements
This work was in part supported by the National Key Research and Development Program of China (2017YFC1601501), the China Postdoctoral Science Foundation (2018M631638), and by the National Natural Science Foundation of China (31802247).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Dejun Liu, Weiwen Liu, Xing Li, Hong Yao, Zhangqi Shen, Yang Wang, and Jianzhong Shen declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




