《1 Engineering research fronts》
1 Engineering research fronts
《1.1 Development trends in the top 9 engineering research fronts》
1.1 Development trends in the top 9 engineering research fronts
The top 9 engineering research fronts related to the Field of Medicine & Health are summarized in Table 1.1.1. These fronts cover a variety of disciplines, including basic medicine, clinical medicine, medical informatics and biomedical engineering, pharmacy, public health and preventive medicine. These 9 research fronts also involve “new taxonomy based on aberrant molecules and targeted therapy,” “stem cell and cell therapies,” “precision medicine research based on biomedical big data,” “prevention and intervention of aging,” “safety evaluation, risk control, and quality standards of traditional Chinese medicine,” “regenerative medicine and regeneration microenvironment,” “discovery of emerging highly pathogenic viruses and their epidemic warning and control,” “neurodegenerative disorders,” and “gut microbiota and development of tumor.” All core papers on these fronts published between the years 2012−2017 are listed in Table 1.1.2.
(1) New taxonomy based on aberrant molecules and targeted therapy
New taxonomy based on aberrant molecule is the cornerstone of precision medicine. It is a new classification method of cancer based on the molecular variation of cells that could drive occurrence and development of tumor, and can be used as a target for effective treatment. The first step in creating new taxonomy of tumors is information sharing. Data from a large number of cancer patients are available for extensive research, and the internal connections between these data are explored. These data are combined with the continuously developing new knowledge on basic biological process— form new knowledge network to serve all stakeholders. Further, the subclassification of particular diseases into those with different molecular mechanisms, prognoses, and/or treatments could be predicted by such hypotheses. Finally, all these ideas could be tested in an attempt to establish their validity, reproducibility, and robustness. Targeted therapy is a drug treatment approach based on molecular taxonomy of cancer, which aims at the specific molecular abnormality. It designs specific targeted drugs and selects the appropriate population for specific treatment so as to improve the efficacy and reduce side effects. Molecular taxonomy and targeted therapy are indivisibly whole. Molecular typing is the basis of targeted therapy, which, in turn, is the key to the establishment and validation of molecular taxonomy. In 2001, Imatinib was the first Food and Drug Administration (FDA)- approved targeted therapy drug for chronic myeloid leukemia
《Table 1.1.1》
Table 1.1.1 Top 9 engineering research fronts in medicine and health
| No. | Engineering research front | Core papers | Citations | Citations per paper | Mean year |
| 1 | New taxonomy based on aberrant molecules and targeted therapy | 847 | 189790 | 224.07 | 2014.69 |
| 2 | Stem cell and cell therapies | 189 | 31559 | 166.98 | 2014.4 |
| 3 | Precision medical research based on biomedical big data | 768 | 165076 | 214.94 | 2014.5 |
| 4 | Prevention and intervention of aging | 13 | 2054 | 158 | 2014.31 |
| 5 | Safety evaluation, risk control, and quality standards of traditional Chinese medicine | 131 | 790 | 6.03 | 2014.88 |
| 6 | Regenerative medicine and regeneration microenvironment | 11 | 1492 | 135.64 | 2015 |
| 7 | Discovery of emerging highly pathogenic viruses and their epidemic warning and control | 34 | 6277 | 184.62 | 2014.32 |
| 8 | Neurodegenerative disorders | 68 | 9590 | 141.03 | 2014.56 |
| 9 | Gut microbiota and development of tumor | 55 | 9241 | 168.02 | 2014.76 |
《Table 1.1.2》
Table 1.1.2 Annual number of core papers published for each of the top 9 engineering research fronts in medicine and health
| No. | Engineering research front | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| 1 | New taxonomy based on aberrant molecules and targeted therapy | 108 | 138 | 134 | 143 | 178 | 146 |
| 2 | Stem cell and cell therapies | 28 | 42 | 29 | 36 | 25 | 29 |
| 3 | Precision medical research based on biomedical big data | 127 | 121 | 131 | 146 | 116 | 127 |
| 4 | Prevention and intervention of aging | 1 | 4 | 3 | 1 | 3 | 1 |
| 5 | Safety evaluation, risk control, and quality standards of traditional Chinese medicine | 15 | 13 | 22 | 25 | 35 | 21 |
| 6 | Regenerative medicine and regeneration microenvironment | 1 | 1 | 3 | 1 | 2 | 3 |
| 7 | Discovery of emerging highly pathogenic viruses and their epidemic warning and control | 4 | 9 | 7 | 4 | 6 | 4 |
| 8 | Neurodegenerative disorders | 11 | 12 | 11 | 10 | 10 | 14 |
| 9 | Gut microbiota and development of tumor | 3 | 13 | 8 | 11 | 10 | 10 |
patients harboring the BCR-ABL fusion. Following this, the research based on targeted therapies for lung and associated cancers having molecular aberrations rapidly expanded: Gefitinib, an epidermal growth factor (EGF) receptor tyrosine kinase inhibitor (EGFR TKIs) is used to treat lung cancer with EGFR mutation; Crizotinib, an anaplastic lymphoma kinase (ALK) and c-ros oncogene 1(ROS1) inhibitor is applied in the treatment of patients harboring ALK/ROS1 fusion. Soon after, the ALK fusion gene was found to be associated with a series of cancers with specific clinical characteristics, and a new word, ALKoma, was coined to describe a new subtype of cancer. Targeted therapy has prolonged the median overall survival (OS) from 10 months to 50 months for advanced lung cancer with mutated ALK in past 10 years. The fields involved in cancer molecular taxonomy and targeted therapy encompass molecular biology research, bioinformatics, and gene analysis of the characteristics of cancer, discovery of drug targets and development of corresponding drugs, establishment of convenient molecular diagnostic methods, and design and implementation of innovative clinical trials. All these fields are at the forefront of current life sciences, guiding the research direction of medical science. By the next 20 years, research on targeted drugs will be a constant area of work.
(2) Stem cell and cell therapies
A stem cell possesses the potential for self-renewal and multi- directional differentiation. It is capable of differentiating into multiple functional cells depending upon different conditions. Stem cells can be roughly classified into embryonic stem cells (ESCs) and adult stem cells (ASCs) according to their developmental stages. The ESCs derived from the reprogramming of differentiated cells are called induced pluripotent stem cells (iPSCs). ESCs and iPSCs are highly proliferative and able to differentiate into any cell type in the body. ASCs are a type of undifferentiated cells existing in different body tissues, having limited proliferative ability, and can only differentiate into specific types of tissues. Stem cells are often referred to as “seed cells” in the medical community because of their potential for regenerating various tissues and organs within the human body. The stem cell technology utilizes this potential to repair, replace, or otherwise interfere with damaged tissues and organs. Therapeutic stem cell research has made meaningful attempts towards the treatment of a number of severe systemic diseases, such as nervous system diseases, and cardiovascular, cerebrovascular, hepatic, and renal diseases. It has brought new hopes for effective treatment, thus becoming a highly important field of investment for various national governments. Some developed countries and regions such as Europe, the USA, and Japan have prioritized stem cell research as a strategy for national science and technological development, and made enormous efforts to promote its clinical applications. A number of pre-clinical and clinical trials have been carried out around the world, including the treatment of Parkinson’s disease with dopaminergic neurons differentiated from neural stem cells, the treatment of chronic spinal cord injury with spinal cord neural stem cells, and the application of autologous interstitial precursor cells for the repair and regeneration of damaged tissues. These trials and findings have helped the development and emergence of several stem cell products into the market. Being the main supporters of the stem cell research industry, many major pharmaceutical companies have made huge investments and even adjusted the core of their businesses to seize the opportunities brought about by stem cell development and translational medicine. Globally, more than 700 companies are conducting research in this emerging field, and the competition is becoming increasingly fierce. In recent years, the stem cell industry has made rapid progress, and according to the latest prediction, the global stem cell research market will reach 400 billion USD by 2020.
(3) Precision medical research based on biomedical big data
Precision medicine is a new medical model based on personalized medicine, generated with the rapid development of genome sequencing technology and the cross-application of bioinformatics and big data science. In 2011, a long- form report by the American Academy of Sciences entitled “Towards Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease” first proposed precision medicine. Its main content/role is to form a knowledge network revealing the molecular mechanism and genetic susceptibility of an individual’s disease by interactively analyzing the clinical information and omics data at one- or multi-dimensional levels in large-scale populations. The knowledge network generates disease taxonomy and provides disease prevention and treatments for patients based on their genome and other individual characteristics. The aim of precision medicine is to provide accurate and timely warning and treatment based on an individual’s genomic information and the molecular mechanisms of disease. This in turn can reduce the incidence of the disease, solve treatment inefficiencies, and reduce medical costs. As the future medical model and national health protection measure, precision medicine will bring about implementation of personalized medical services in many aspects. The disease risk of healthy or sub-healthy individuals will be predicted, especially for major chronic diseases, thereby reducing the incidence rate due to prior warnings. For the patient population, early diagnosis and precise treatment, like selection of the most appropriate drug, its dosage or duration of treatment for a given patient’s genotype, could be provided to further improve the efficacy and cure rate. For infants with various birth defects, pre-implantation genetic screening or diagnosis could be performed to reduce the disease risk in newborns. After the Precision Medicine Initiative was launched by President Obama, several precision medicine projects have been developed around the world, including the 100 000 Genomes Projects in the United Kingdom (UK), the Genomic Medicine Plan 2025 in France, the Genome South Korea in Ulsan, and the Zero Childhood Cancer Program in Australia. In 2016, China also released a special project focused on precision medicine research. The project aimed at common dangerous diseases with high prevalence, as well as rare diseases with relatively high prevalence in China, and conducting research from the perspective of cohort, platform, technology, system, and demonstration application. The ultimate mission is to improve population health and make precision medicine the new rise point in economic growth and social development.
(4) Prevention and intervention of aging
Aging is related to a progressive decline of physical, psychological, and social functions and a great risk of many disabilities, diseases, and even death. The pursuit of longevity is the common aspiration of human beings. Human bodies age at different rates, which are determined by both genetic and environmental factors. The omics analyses (genomics, metabolomics, metagenomics, epigenetics, etc.) and multi- dimensional/time point big data information of large sample sizes or multi-center cohort data can be used to build highly accurate methods to assess aging and its feasible intervention schemes. This would definitely provide strong scientific evidence to clarify the mechanisms of aging, in addition to providing the technical means and prevention strategies for aging intervention. This research front contributes to food, lifestyle, medical, and pharmaceutical development, in addition to precise health management, personalization and utility evaluation of public health policies, and improvement of the human aging processes, associated with geriatric and neurodegenerative diseases. The accurate diagnosis and medical guidance based on cancer genetic testing has begun to show its effectiveness. However, the assessment and prevention of aging based on the individual’s health status is still lacking evidence and feasible measures. Aging-related diseases and rates require more research and investments in order to improve people’s yearning for a better life and enhance the industrial structure, including ecological civilization. The integration of multi-omics, environment, high throughput data, and validation with prospective cohorts will enable finding precise and simpler aging assessment methods apart from more effective and feasible control of aging. Food, medicine, lifestyle, and psychological interventions, combined with machine learning application, artificial intelligence, and deep biostatistical inference are the trend and hotspot of aging prevention and intervention research.
(5) Safety evaluation, risk control, and quality standards of traditional Chinese medicine
The uncertain safety of traditional Chinese medicine (TCM) has been a key factor restricting the development of the TCM industry and public health. However, the complexity of TCM ingredients and the unpredictability of their nonlinear interactions with the human body; the integrity of multiple components, pathways, and target points; and the role reversal and inter-restriction of TCM effective substances; have become the main challenges for TCM safety evaluation. In view of the above hurdles, TCM safety evaluation should focus on elucidating the toxic characteristics, especially the “toxic” TCMs with curative effects, on the basis of the good laboratory practice (GLP) evaluation system. It should strengthen studies on toxicity control and detoxification instead of disabling TCM; it should enhance the risk control management of TCM research and pay more attention on its correlation and systematization. This is mainly performed by identification, processing, compatibility, and syndrome/symptom differentiation. With the new changes brought about by the intensified risk consciousness, quality improvement, and progress in science, TCM risk control has gradually extended to the early prediction and real-time dynamic monitoring of toxicity, adverse reaction reports, and lifelong follow-through. TCM theories are fusions of traditional and modern medicine enabling us to study its toxicity and prescription of toxicity. Further, TCM helps to effectively characterize the material basis of formula for individual drugs and their ingredients in order to provide sufficient guarantee of the safety and efficacy of TCM. The relationships between toxicity, effectiveness, compatibility, and pattern of toxic TCM needs to be elucidated. Moreover, much clarity is needed to see the toxic characteristics, like its occurrence, mechanism of toxic reactions, and its in vivo metabolism. In order to promote scientific and rational use of drugs in clinical practice, better understand the risk of “toxic TCMs,” and effectively establish quality control standards, we need to establish toxicity control methods, scientific assessment pattern, and technical system for the early prediction of toxicity associated risk of “toxic TCMs.”
(6) Regenerative medicine and regeneration microenvironment
Regenerative medicine refers to the recovery of impaired tissues and organs to rescue normal functions, using biological engineering principles. The key barrier for regenerative medicine is how to establish the best regeneration microenvironment, i.e., the local microenvironment required for regeneration, to promote tissue regeneration process. Since the last century, studies indicate a very intricate relationship between regeneration microenvironment and regenerative medicine. The mechanism of regeneration involves the participation of cells having regeneration potential, and the microenvironment containing soluble factors and extracellular matrix. Amongst other physical or chemical factors secreted by the cells in the regeneration microenvironment, the trophic factors, adhesion molecules, and extracellular matrix molecules play important roles in regeneration. Studies on the regeneration microenvironment have provided key theories and technical guidance for regenerative medicine. The establishment of new theories, technology, and products based on these investigations will create new areas for the development of regenerative medicine and benefit mankind.
(7) Discovery of emerging highly pathogenic viruses and their epidemic warning and control
Identifying emerging highly pathogenic (HP) viruses is a measure for their isolation through real-time monitoring and control of their epidemics. This aids to achieve timely detection of the epidemic causing pathogens, their effective prevention, and treatment of the viral infection (conceptual elaboration). The key scientific issues include pathogen identification, studying their transmission routes, epidemic monitoring, and development of control strategies such as vaccines and therapeutics. Owing to their potential threat to human health and socio-economic stability, the discovery of emerging HP viruses, early epidemic warning, and corresponding epidemic control will pave the way for combating emerging infectious disease worldwide. At present, the well-established gene sequencing technologies and bioinformatics can be applied to quickly identify the species and genetic information of the emerging highly pathogenic viruses in order to monitor their epidemics. Moreover, through the isolation of viruses, the related viral strains can be timely obtained and their transmission and pathogenicity can be promptly studied. In recent years, great progress has been made in the development of protein-based therapeutics (e.g., antibodies), and vaccines can be developed in a short period of time for urgent treatment of infected patients and prevention of epidemics Recently, the international community has once again joined hands to launch the “Global Virus Group Project.” With the advancing development of novel technologies, discovery and characterization of pathogens, the monitoring and early indications of epidemics, and development of prevention and treatment strategies can be completed more precisely, promptly, securely and effectively. At present, China has established advanced comprehensive systems for virus identification and public health surveillance, providing international early warning programs and setting up platforms to swiftly develop prophylactic and therapeutic programs.
(8) Neurodegenerative disorders
Neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis are characterized by chronic and progressive neuronal loss, which is accompanied by neuroinflammation in the selected central nervous system (CNS) region. Aging is the most significant risk factor for induction of neurodegeneration. However, despite many years of research, early diagnosis and treatment of these diseases remain highly challenging. Studies on the molecular foundation underlying these diseases will ultimately provide some solutions for resolving the dilemma. The key scientific questions in this field relate to the elucidation of pathogenic factors, as well as the critical molecular mechanisms of pathogenesis that would facilitate early diagnosis and interventions. In the past decades, various disease-causing genes coding for familiar neurodegenerative disorders have been identified. Research in this field has transitioned from the description of pathological changes in the past to the functional studying a specific disease-causing gene. It is worth noting that current research has undergone significant changes at various levels: ① At the molecular level, studies have now extended from gene regulation to epigenetic regulation. ② At the cellular level, the interactions between the neuronal and non-neuronal cells (e.g., neuroglia) have received increasing attention in the past several years. ③ At the neural circuitry level, the hidden roles of the interactions between the diseased CNS regions and remote connecting CNS regions are being explored. ④ At the system level, the cross-talks between the CNS and peripheral tissue and organs (e.g. brain-gut axis and immune system) and their influence on the pathogenesis of neurodegenerative diseases have recently received much attention. ⑤ Technically, sequence-based techniques are used to redefine the categorization of various sub-types of neural cells. Innovative animal models are urgently needed to deeply promote neurodegenerative disease research. Brain imaging techniques with higher spatiotemporal resolutions are under development. Taken together, studies on the molecular basis of neurodegenerative disorders will lay a foundation for further understanding the neurodegenerative disease pathogenesis while simultaneously promoting the development of a new approach for the early diagnosis and treatment of these diseases.
(9) Gut microbiota and development of tumor
The human gut microbiota has become an extremely complex community after long-term co-evolution. The bacteria and their hosts have a close symbiosis, and maintaining a well- balanced intestinal flora is vitally important to human health. The imbalance in gut microbiota may influence the occurrence and development of tumors. The key scientific problems include the mechanisms of gut microbiota in influencing the occurrence and development of tumors, and drug resistance of tumors and the detailed microbiota involved. Since the USA first proposed the Human Microbiome Project in 2007, the study of intestinal flora has become a hot topic. The gut microbiota can not only regulate the innate immunity of the body, but can also stimulate the immune response either through the bacteria themselves, or through their metabolites. The imbalance of gut microbiota may lead to abnormal immune mechanisms, leading to tumor formation, especially colorectal cancer. Specific bacteria can also exert chemotherapeutic effect on tumors and even confer drug resistance. However, there are still many problems that remain unsolved. Therefore, we need to systematically clarify the mechanism of intestinal flora imbalance involved in the tumor development and put forward novel ideas and directions for the early stage prevention and control of tumorigenesis.
《1.2 Interpretations for three key engineering research fronts》
1.2 Interpretations for three key engineering research fronts
1.2.1 New taxonomy based on aberrant molecules and targeted therapy
New taxonomy based on aberrant molecule is the cornerstone of precision medicine. It is a new classification method of cancer based on the molecular variation of cells that could drive occurrence and development of tumor, and can be used as a target for effective treatment. The first step in creating new taxonomy of tumors is information sharing. Data from a large number of cancer patients are available for extensive research, and the internal connections between these data are explored. These data are combined with the continuously developing new knowledge on basic biological process— form new knowledge network to serve all stakeholders. Further, the subclassification of particular diseases into those with different molecular mechanisms, prognoses, and/or treatments could be predicted by such hypotheses. Finally, all these ideas could be tested in an attempt to establish their validity, reproducibility, and robustness. Targeted therapy is a drug treatment approach based on molecular taxonomy of cancer, which aims at the specific molecular abnormality. It designs specific targeted drugs and selects the appropriate population for specific treatment so as to improve the efficacy and reduce side effects. Molecular taxonomy and targeted therapy are indivisibly whole. Molecular typing is the basis of targeted therapy, which, in turn, is the key to the establishment and validation of molecular taxonomy. In 2001, Imatinib was the first Food and Drug Administration (FDA)- approved targeted therapy drug for chronic myeloid leukemia patients harboring the BCR-ABL fusion. Following this, the research based on targeted therapies for lung and associated cancers having molecular aberrations rapidly expanded: Gefitinib, an epidermal growth factor (EGF) receptor tyrosine kinase inhibitor (EGFR TKIs) is used to treat lung cancer with EGFR mutation; Crizotinib¬, an anaplastic lymphoma kinase (ALK) and c-ros oncogene 1(ROS1) inhibitor is applied in the treatment of patients harboring ALK/ROS1 fusion. Soon after, the ALK fusion gene was found to be associated with a series of cancers with specific clinical characteristics, and a new word, ALKoma, was coined to describe a new subtype of cancer. Targeted therapy has prolonged the median overall survival (OS) from 10 months to 50 months for advanced lung cancer with mutated ALK in past 10 years. The fields involved in cancer molecular taxonomy and targeted therapy encompass molecular biology research, bioinformatics, and gene analysis of the characteristics of cancer, discovery of drug targets and development of corresponding drugs, establishment of convenient molecular diagnostic methods, and design and implementation of innovative clinical trials. All these fields are at the forefront of current life sciences, guiding the research direction of medical science. By the next 20 years, research on targeted drugs will be a constant area of work.
At present, some key scientific issues in research on cancer molecular taxonomy and targeted therapy are as follows: development of new detection technologies and simultaneous innovation and application of bioinformatics methods to capture the molecular variation of cancer cells in the microenvironment; construction of the holographic map at the molecular level of cancer and visualization of the molecular tumor typing; integration and sharing of large-scale data of cancer patients and the rapidly expanding knowledge of tumor molecular evolution to build a new and shareable network of cancer knowledge; identification of key active targets and drug-susceptible compounds; and innovative clinical trials and companion diagnostic methods. Overall development trend in this field is the extension by cancer molecular variation research to the microenvironment of molecular information research, including targeted therapy and immunotherapy, comprehensive integration to build a new cancer molecular taxonomy, seamless connection with the drug development process simultaneously, the speed of targeted drugs reaching the market, and earlier clinical benefits to the patients.
The research fronts include: (1) new techniques used for cancer molecular taxonomy, such as single-cell sequencing, multi-dimensional tissue mass spectrometry, second- and even third-generation sequencing, liquid biopsy, etc.; (2) location of new targeted aberrant genes and determining their functions; (3) optimized design and screening of compounds for drug production; (4) discovering biomarkers for immune checkpoints application; (5) big data analysis for real-world clinical molecular evolution data calculation and deep learning technology; (6) organization and implementation of innovative clinical trials, such as basket tests, umbrella tests for seamless development, etc.
Papers concerning fronts of “new taxonomy based on aberrant molecules and targeted therapy” are mostly published by researchers from the USA, accounting for 77.59% of all studies and ranking first within 15 countries. France and Germany rank second and third, respectively. Among the three Asian countries, i.e., South Korea, Japan, and China, papers published in this area from China ranked 14th. Citations per paper on this research front “new taxonomy based on aberrant molecules and targeted therapy” are high, ranging from 225.05 to 294.63 (Table 1.2.1).
Among the top 10 organizations with the greatest output of core papers on the “new taxonomy based on aberrant molecules and targeted therapy,” nine are from the United States, and the remaining one is from the Institute Gustave Roussy in France. The top three organizations are the UT MD Anderson Cancer Center, Memorial Sloan-Kettering Cancer Center, and Dana-Farber Cancer Institute (Table 1.2.2). More importantly, the newly listed targeted drugs, discovery of original targets, and global research and development (R&D) of drugs in recent years are almost monopolized by Unite States, Europe, and Japan, indicating that these countries have extremely strong innovation capacity and are leading the development direction of the global front, leaving China far behind. At the same time, the collaboration network among the top 10 countries or regions and among institutions are close in the engineering research front of “new taxonomy based on aberrant molecules and targeted therapy” (Figure 1.2.1 and Figure 1.2.2). It further demonstrates that cooperation and collaborations between institutes are very important and urgent.
From the perspective of technology and development trend, almost all of the most important core papers from China are based on large-scale clinical trials, and most of the top- ranking research institutions are good for clinical trials. This is precisely because of the huge patient resources in China. This advantage would not exist even if Western countries combined with global resources for clinical trials. This trend needs more attention by our policy makers.
Suggestions for the development of “Cancer molecular taxonomy and targeted therapy”: (1) strengthening the research and transformation of key technologies of tumor molecular typing and targeted therapy, such as single-cell sequencing, multi-mass spectrometry and new knowledge framework, especially the substantive cooperation between
《Table1.2.1》
Table1.2.1 Countries or regions with the greatest output of core papers on the “new taxonomy based on aberrant molecules and targeted therapy”
| No. | Country/Region | Core papers | Percentage of core papers | Citations | Percentage of citations | Citations per paper |
| 1 | USA | 658 | 77.59% | 159 929 | 84.24% | 243.05 |
| 2 | France | 298 | 35.14% | 83 149 | 43.80% | 279.02 |
| 3 | Germany | 286 | 33.73% | 73 802 | 38.88% | 258.05 |
| 4 | UK | 261 | 30.78% | 65 664 | 34.59% | 251.59 |
| 5 | Italy | 239 | 28.18% | 64 624 | 34.04% | 270.39 |
| 6 | Spain | 203 | 23.94% | 55 926 | 29.46% | 275.5 |
| 7 | Canada | 184 | 21.70% | 49 067 | 25.85% | 266.67 |
| 8 | Australia | 176 | 20.75% | 51 855 | 27.31% | 294.63 |
| 9 | Belgium | 144 | 16.98% | 32 407 | 17.07% | 225.05 |
| 10 | South Korea | 135 | 15.92% | 35 398 | 18.65% | 262.21 |
| 11 | Switzerland | 115 | 13.56% | 23 635 | 12.45% | 205.52 |
| 12 | Japan | 109 | 12.85% | 26 706 | 14.07% | 245.01 |
| 13 | Poland | 103 | 12.15% | 30 047 | 15.83% | 291.72 |
| 14 | China | 102 | 12.03% | 21 767 | 11.47% | 213.4 |
| 15 | Netherlands | 93 | 10.97% | 23 672 | 12.47% | 254.54 |
《Table1.2.2》
Table 1.2.2 Institutions with the greatest output of core papers on the “new taxonomy based on aberrant molecules and targeted therapy”
| No. | Institution | Core papers | Percentage of core papers | Citations | Percentage of citations | Citations per paper |
| 1 | Univ Texas MD Anderson Cancer Center | 167 | 19.69% | 44 188 | 23.28% | 264.6 |
| 2 | Mem Sloan Kettering Cancer Center | 161 | 18.99% | 50 392 | 26.54% | 312.99 |
| 3 | Dana Farber Canc Inst | 153 | 18.04% | 42 927 | 22.61% | 280.57 |
| 4 | Massachusetts Gen Hosp | 91 | 10.73% | 29 236 | 15.40% | 321.27 |
| 5 | Univ Calif Los Angeles | 80 | 9.43% | 26 751 | 14.09% | 334.39 |
| 6 | Mayo Clin | 77 | 9.08% | 22 088 | 11.63% | 286.86 |
| 7 | Univ Calif San Francisco | 64 | 7.55% | 16 326 | 8.60% | 255.09 |
| 8 | Inst Gustave Roussy | 61 | 7.19% | 21 581 | 11.37% | 353.79 |
| 9 | Univ Penn | 59 | 6.96% | 17 214 | 9.07% | 291.76 |
| 10 | Weill Cornell Med Coll | 55 | 6.49% | 16 523 | 8.70% | 300.42 |
《Figure 1.2.1》
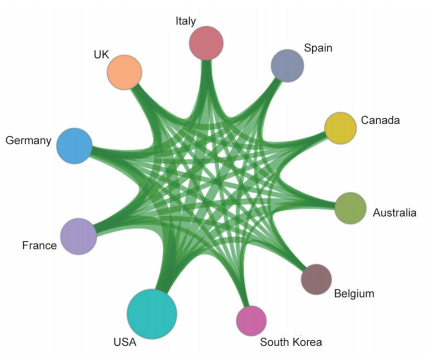
Figure 1.2.1 Collaboration network among major countries or regions in the engineering research front of “new taxonomy based on aberrant molecules and targeted therapy”
research institutions and clinical centers; (2) strengthening the cultivation of bioinformatics skills, which may be the biggest bottleneck restricting the development of this field in future; (3) accelerating the development of new drugs, especially the originally targeted drugs, and interconnecting the diagnosis and new drug development together to remove all the institutional obstacles hindering the development of new drugs; (4) incentive implementation of information sharing and global cooperation; (5) selection of 1–2 projects to explore new research system (these projects should be mandatorily conducted by the highly skilled researchers in leading institutes and guided by clinical outcomes).
1.2.2 Stem cell and cell therapies
A stem cell is a type of cell with the potential for self- renewal and multi-directional differentiation. It is capable of differentiating into multiple functional cells under certain conditions. Stem cells can be roughly classified into embryonic stem cells (ESCs) and ASCs according to their developmental stages. The ESCs derived from the reprogramming of differentiated cells are called induced pluripotent stem cells (iPSCs). ESCs and iPSCs are highly proliferative and are able to differentiate themselves into any cell type of the body; ASC, a type of undifferentiated cell existing in different tissues of the body, has limited proliferative ability and can only differentiate into specific types of tissue in the body.
Stem cells are often referred to as “seed cells” in the medical community because of their potential for regenerating various tissues and organs within the human body. The stem cell technology utilizes this potential to repair, replace, or otherwise interfere with damaged tissues and organs. Therapeutic stem cell research has made meaningful attempts towards the treatment of a number of severe systemic diseases, such as nervous system diseases, and cardiovascular, cerebrovascular, hepatic, and renal diseases. It has brought new hopes for effective treatment, thus becoming a highly important field of investment for various national governments. Some developed countries and regions such as Europe, the USA, and Japan have prioritized stem cell research as a strategy for national science and technological development, and made enormous efforts to promote its
《Figure 1.2.2》
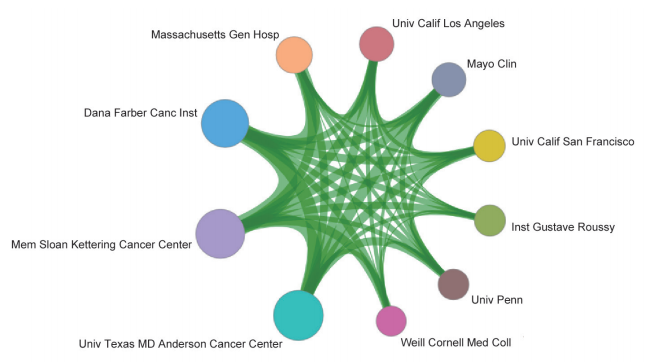
Figure 1.2.2 Collaboration network among major institutions in the engineering research front of “new taxonomy based on aberrant molecules and targeted therapy”
clinical applications. A number of pre-clinical and clinical trials have been carried out around the world, including the treatment of Parkinson’s disease with dopaminergic neurons differentiated from neural stem cells, the treatment of chronic spinal cord injury with spinal cord neural stem cells, and the application of autologous interstitial precursor cells for the repair and regeneration of damaged tissues. These trials and findings have helped the development and emergence of several stem cell products into the market. Being the main supporters of the stem cell research industry, many major pharmaceutical companies have made huge investments and even adjusted the core of their businesses to seize the opportunities brought about by stem cell development and translational medicine. Globally, more than 700 companies are conducting research in this emerging field, and the competition is becoming increasingly fierce. In recent years, the stem cell industry has made rapid progress, and according to the latest prediction, the global stem cell research market will reach 400 billion USD by 2020.
Throughout developments at home and abroad, the key technical problems to be solved in stem cell research and development include: (1) Establishment and culturing of pluripotent stem cells at the clinical level: Maintenance mechanisms of the pluripotency of pluripotent stem cells, especially the ground state; optimization of in vitro reprogramming methods; characterizing the surface molecular antigens and transcription factors of pluripotent stem cells; and revealing pluripotency withdrawal, lineage differentiation mechanisms, and tumorigenicity of pluripotent stem cells. (2) Isolation and culturing methods of tissue stem cells at the clinical level: The heterogeneity of tissue stem cells and the unidentified surface markers are impeding the maintenance of long-term growth and expansion of stem cells cultured by current in vitro methods. (3) Directional differentiation of pluripotent stem cells: Using multi- lineage differentiation potential of pluripotent stem cells, as well as growth factors and drug interventions in vitro, and establishing methods for pluripotent stem cells to directionally differentiate into specific tissues or cells, such as islet β cells, endothelial cells, and hematopoietic stem cells. (4) Trans-differentiation: transforming into a new cell from the body’s own mature somatic cells as a starting cell by expressing some specific genes or by drug induction. Recently, it has been reported to be useful in in situ trans-differentiation by allowing other cells from the damaged tissue to transform into the cells of interest for therapeutic purposes. (5) Forming complex structures in vitro: Stem cells can be spontaneously formed into micro-organs in vitro using 3-dimensional (3D) culture technology with the support of some special materials. They have certain functions and structures similar to normal body tissues and organs, laying the foundation of artificial tissue and organ transplantation in the future. (6) Stem cell genetic and epigenetic manipulation techniques: Given the special biological characteristics of stem cells, it is important to know how to use the emerging genome editing technology to manipulate stem cells and realize gene therapy potential for patient treatment.
These key questions are also the focus of the current academia. In the field of “stem cell and cell therapy,” the top three core research publishing countries were the USA, China, and UK. The core papers of the “stem cell and cell therapy” research are all cited frequently (122.71–253.82), indicating that many countries attach great value to the research in this field. Also, the top three cited countries/regions are Italy, Australia, and the Netherlands (Table 1.2.3). As far as cooperation goes, there are collaborations between the top 10 core research papers-publishing countries (Figure 1.2.3). Among the 10 organizations that have published the highest number of core papers on stem cell and cell therapy, the top three are from the USA and China: Harvard University, Stanford University, and Shanghai Jiao Tong University (Table 1.2.4). As shown in the collaboration network of the top 10 core paper producing/yielding agencies, there is cooperation between different parts of the agencies (Figure 1.2.4).
Based on the statistical analysis results, China is currently at a level similar to that for foreign developed countries regarding the front of “stem cell and cell therapy” research. During the 12th Five-Year Plan and 13th Five-Year Plan periods, the state further increased its investment in stem cell research and specifically established major scientific research
《Table1.2.3》
Table 1.2.3 Countries or regions with the greatest output of core papers on the “stem cell and cell therapies”
| No. | Country/Region | Core papers | Percentage of core papers | Citations | Percentage of citations | Citations per paper |
| 1 | USA | 115 | 60.53% | 19 537 | 61.88% | 169.89 |
| 2 | China | 28 | 14.74% | 3 436 | 10.88% | 122.71 |
| 3 | UK | 23 | 12.11% | 3 855 | 12.21% | 167.61 |
| 4 | Germany | 22 | 11.58% | 3 282 | 10.40% | 149.18 |
| 5 | Japan | 22 | 11.58% | 3 920 | 12.42% | 178.18 |
| 6 | Italy | 17 | 8.95% | 4 315 | 13.67% | 253.82 |
| 7 | Netherlands | 16 | 8.42% | 2 986 | 9.46% | 186.63 |
| 8 | France | 15 | 7.89% | 2 382 | 7.54% | 158.8 |
| 9 | Canada | 14 | 7.37% | 1 831 | 5.80% | 130.79 |
| 10 | Australia | 13 | 6.84% | 2 521 | 7.98% | 193.92 |
《Table1.2.4》
Table 1.2.4 Institutions with the greatest output of core papers on the “stem cell and cell therapies”
| No. | Institution | Core papers | Percentage of core papers | Citations | Percentage of citations | Citations per paper |
| 1 | Harvard Univ | 23 | 12.11% | 4 010 | 12.70% | 174.35 |
| 2 | Stanford Univ | 14 | 7.37% | 2 252 | 7.13% | 160.86 |
| 3 | Shanghai Jiao Tong Univ | 8 | 4.21% | 801 | 2.54% | 100.13 |
| 4 | Howard Hughes Med Inst | 7 | 3.68% | 1 094 | 3.47% | 156.29 |
| 5 | Univ Hlth Network | 7 | 3.68% | 604 | 1.91% | 86.29 |
| 6 | Univ Toronto | 7 | 3.68% | 708 | 2.24% | 101.14 |
| 7 | Johns Hopkins Univ | 6 | 3.16% | 1 578 | 5.00% | 263 |
| 8 | Univ Med Ctr Utrecht | 6 | 3.16% | 1 512 | 4.79% | 252 |
| 9 | Univ Miami | 6 | 3.16% | 1 084 | 3.43% | 180.67 |
| 10 | Boston Childrens Hosp | 6 | 3.16% | 956 | 3.03% | 159.33 |
《Figure 1.2.3》
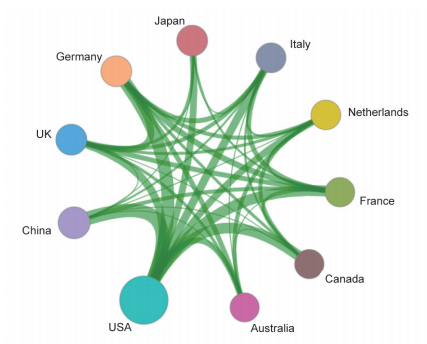
Figure 1.2.3 Collaboration network among major countries in the engineering research front of “stem cell and cell therapies”
projects, such as the “Scientific Research Program for Stem Cell Research” and “Strategic Science and Technology Pilot Project for Stem Cell and Regenerative Medicine,” enabling some progress in basic stem cell research, key technologies, and resource platform construction. However, compared with developed countries such as the USA, Europe, and Japan, there still remains a large gap. To be more specific, China’s R&D investment is lower than that of other countries. As such, the basic research output is lagging behind, and the core technologies and original results of stem cell translational research are insufficient. Most of the domestic stem cell transformation applications and the industrial layout are concentrated in the field of tissue stem cell storage, relying on resource collection and expansion in
《Figure 1.2.4》
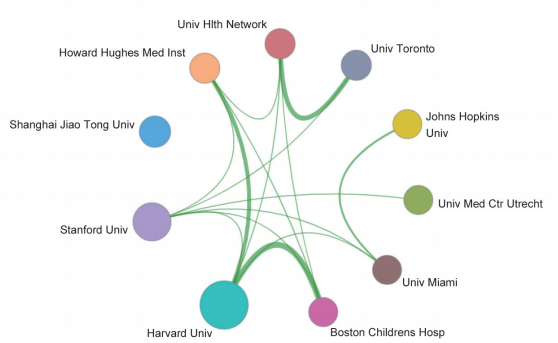
Figure 1.2.4 Collaboration network among major institutions in the engineering research front of “stem cell and cell therapies”
scale for profitability. However, no standardized stem cell transformation application or products are coming into the market. Registered stem cell-related clinical trials in China are mainly based on mature ASCs, notably lacking in pluripotent stem cell transformation applications that are quite highly trending in stem cell and regenerative medicine. In addition, the technical specifications, standards, and ethics related to stem cell therapy in China are relatively backward with respect to other countries. The lack of research groups and academic institutions specializing in stem cell quality control and standard research has resulted in some stem cell related products illegitimately evading government supervision and lacking preclinical research data. This has provoked severe international criticism and negatively affected the development of stem cell research and translational medicine in China.
For the past few years, the stem cell industry has maintained a momentum of rapid development. Developed countries such as the USA occupy the main market of the stem cell industry. China’s stem cell biotechnology industry is mainly an upstream industry until now, with stem cell storage as the mainstay. To accelerate the process of turning stem cell basic research results into clinical applications, China has introduced a series of creative regulations and policies. Nevertheless, the pace of clinical transition of stem cells needs to be further expedited. One of the main issues is the lack of strict industrial and national standards for various stem cell products, which can be resolved by advocating the research on quality control standards for stem cell products and strengthening the training of relevant professionals.
1.2.3 Precision medical research based on biomedical big data
Precision medicine is a new medical model based on personalized medicine, generated with the rapid development of genome sequencing technology and the cross-application of bioinformatics and big data science. In 2011, a long- form report by the American Academy of Sciences entitled “Towards Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease” first proposed precision medicine. Its main content/role is to form a knowledge network revealing the molecular mechanism and genetic susceptibility of an individual’s disease by interactively analyzing the clinical information and omics data at one- or multi-dimensional levels in large-scale populations. The knowledge network generates disease taxonomy and provides disease prevention and treatments for patients based on their genome and other individual characteristics. The aim of precision medicine is to provide accurate and timely warning and treatment based on an individual’s genomic information and the molecular mechanisms of disease. This in turn can reduce the incidence of the disease, solve treatment inefficiencies, and reduce medical costs. As the future medical model and national health protection measure, precision medicine will bring about implementation of personalized medical services in many aspects. The disease risk of healthy or sub-healthy individuals will be predicted, especially for major chronic diseases, thereby reducing the incidence rate due to prior warnings. For the patient population, early diagnosis and precise treatment, like selection of the most appropriate drug, its dosage or duration of treatment for a given patient’s genotype, could be provided to further improve the efficacy and cure rate. For infants with various birth defects, pre-implantation genetic screening or diagnosis could be performed to reduce the disease risk in newborns. After the Precision Medicine Initiative was launched by President Obama, several precision medicine projects have been developed around the world, including the 100 000 Genomes Projects in the United Kingdom (UK), the Genomic Medicine Plan 2025 in France, the Genome South Korea in Ulsan, and the Zero Childhood Cancer Program in Australia. In 2016, China also released a special project focused on precision medicine research. The project aimed at common dangerous diseases with high prevalence, as well as rare diseases with relatively high prevalence in China, and conducting research from the perspective of cohort, platform, technology, system, and demonstration application. The ultimate mission is to improve population health and make precision medicine the new rise point in economic growth and social development.
At present, the biomedical big data resources are maintained at different organizations and lack standardization. They are shared and utilized at low efficiency, and the omic data are not integrated with medical information, leading to the underutilization and non-processing of big biomedical data. The annual data generation capability in the life sciences has jumped by nine orders of magnitude from the gigabyte (GB) to the exabyte (EB) level in just over a decade (1 EB = 1 billion GBs). Therefore, big data centers that collect, manage, integrate, and utilize medical data have become indispensable basic support platforms for various precision medical programs. In the field of biological data collection and sharing, the USA, Europe, and Japan have established three major biological data centers (NCBI, EBI, and DDBJ) since the 1980s. These centers control most of the world’s biological data. In the field of clinical medical data collection and sharing, the National Institute of Health (NIH) funded the establishment of the clinical data warehouse i2b2 as early as 2004 to manage health information coming from various sources (https://i2b2.cchmc.org/). At present, the extended framework developed by i2b2 can integrate genomic data to assist in personalized treatment. In addition, the European Union (EU) is currently actively building a biomedical big data center named BioMedBridges. In the field of bioscience data integration with medical information, the Health Level-7 (HL-7) Version 3 standard is expanding from the medical field to the health and omics fields. Mainstream medical information integration engines such as Ensemble are developing semantics-based integrated configuration technology. The “Big Data to Knowledge” (BD2K) plan of the USA involves studying relevant methods and software tools for data integration, management, analysis, and sharing. In 2014, the UK Medical Research Council established the “Medical Bioinformatics Program” to study the integration of omics data and health records. Currently, China has established several data centers for different research purposes, including: the Big Data Center of the Beijing Institute of Genomics, Chinese Academy of Sciences (CAS); the NONCODE database of the Institute of Biophysics, CAS; the Bioinformatics Center of Peking University; the Shanghai Center for Bioinformatics Technology; and the Bioinformatics Comprehensive Analysis Platform and the Basic Medical Science Data Center of the Beijing Institute of Genomics in Shenzhen. China promotes the standardization and sharing of medical data through a series of standards such as the Metadata Specification of Health Information Dataset, the Classification and Coding Rules of Health Information Dataset, the Rules for Data Element Standardization of Health Information, and the Basic Structure and Data Standards for the Electronic Medical Record. An integration engine based on interface technologies such as HL-7 and Digital Imaging and Communications in Medicine (DICOM) was also created to integrate disparate medical data. There are several important tasks that need to be performed in the field of precision medicine based on biomedical big data: (1) the basis is the accumulation of large-scale phenotype and omics data, including a large number of clinical population and prospective cohorts of natural populations; (2) the premise is the establishment of a big data platform with the management and sharing service or communication interfaces between the existing data centers; (3) the critical process is the establishment of a standardization technology system that can manage, analyze, and integrate the biomedical information efficiently based on heterogeneous computing, cloud computing, artificial intelligence, and blockchain technologies is the critical process; and (4) the establishment of a disease-centered medical knowledge base and a clinical decision support system is the starting point and ultimate goal of rapid translation from basic research to clinical application. The storage and integration, deep mining, and transformation applications of biomedical big data will play huge roles in future health science research and in achieving the goal of national health.
Papers regarding the fronts of “precision medicine research based on biomedical big data” are mostly published by researchers from USA, accounting for 74.74% of all studies and ranking first within 10 countries. the UK and Canada rank second and third, respectively. China ranks fourth in terms of papers published in this front, indicating that China is catching up fast. Citations per paper in this topic are high, and range between 122.52 and 285.03 (Table 1.2.5). The top three organization are Harvard University, Dana-Farber Cancer Institute, and Memorial Sloan-Kettering Cancer Center (Table 1.2.6). In parallel, the cooperation among the top 10 countries or regions and among institutions are closely promoted regarding “precision medicine research based on biomedical big data” (Figures 1.2.5 and 1.2.6). It further demonstrates that mutual cooperation between institutes is very essential and urgent.
《Table 1.2.5》
Table 1.2.5 Countries or regions with the greatest output of core papers on the “precision medicine research based on biomedical big data”
| No. | Country/Region | Core papers | Percentage of core papers | Citations | Percentage of citations | Citations per paper |
| 1 | USA | 577 | 74.74% | 136 758 | 82.84% | 237.02 |
| 2 | UK | 158 | 20.47% | 44 160 | 26.75% | 279.49 |
| 3 | Canada | 116 | 15.03% | 31 964 | 19.36% | 275.55 |
| 4 | China | 107 | 13.86% | 13 110 | 7.94% | 122.52 |
| 5 | Germany | 104 | 13.47% | 25 253 | 15.30% | 242.82 |
| 6 | Italy | 81 | 10.49% | 22 652 | 13.72% | 279.65 |
| 7 | Australia | 70 | 9.07% | 17 717 | 10.73% | 253.1 |
| 8 | Netherlands | 68 | 8.81% | 19 007 | 11.51% | 279.51 |
| 9 | France | 68 | 8.81% | 18 494 | 11.20% | 271.97 |
| 10 | Spain | 63 | 8.16% | 17 957 | 10.88% | 285.03 |
《Table 1.2.6》
Table 1.2.6 Institutions with the greatest output of core papers on the “precision medicine research based on biomedical big data”
| No. | Institution | Core papers | Percentage of core papers | Citations | Percentage of citations | Citations per paper |
| 1 | Harvard Univ | 142 | 18.39% | 50 610 | 30.66% | 356.41 |
| 2 | Dana Farber Canc Inst | 123 | 15.93% | 38 814 | 23.51% | 315.56 |
| 3 | Mem Sloan Kettering Canc Ctr | 96 | 12.44% | 28 722 | 17.40% | 299.19 |
| 4 | Univ Texas MD Anderson Canc Ctr | 93 | 12.05% | 22 483 | 13.62% | 241.75 |
| 5 | Massachusetts Gen Hosp | 71 | 9.20% | 20 971 | 12.70% | 295.37 |
| 6 | Brigham & Womens Hosp | 66 | 8.55% | 20 917 | 12.67% | 316.92 |
| 7 | Univ Calif San Francisco | 59 | 7.64% | 16 021 | 9.70% | 271.54 |
| 8 | NCI | 52 | 6.74% | 14 251 | 8.63% | 274.06 |
| 9 | Univ Cambridge | 52 | 6.74% | 16 478 | 9.98% | 316.88 |
| 10 | MIT | 51 | 6.61% | 20 359 | 12.33% | 399.2 |
《Figure 1.2.5》
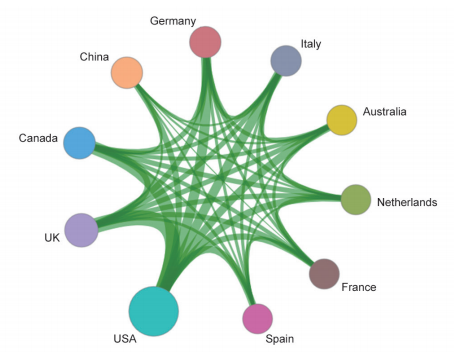
Figure 1.2.5 Collaboration network among major countries in the engineering research front of “precision medicine research based on biomedical big data”
《2 Engineering development fronts》
2 Engineering development fronts
《2.1 Development trends in the top 10 engineering development fronts》
2.1 Development trends in the top 10 engineering development fronts
The top 10 engineering development fronts related to the field of Medicine & Health are summarized in Table 2.1.1. These fronts cover a variety of disciples, including basic medicine, clinical medicine, pharmacy, medical informatics and biomedical engineering, public health and preventive medicine. Artificial intelligence (AI) and disease diagnosis, and AI health management are emerging fronts. Traditional research has focused on stem cell technologies, biomedical materials, tumor immunotherapy, genome editing, robotic surgery system, telemedicine, personalized therapeutic cancer vaccine, and medical 3D printing technology. All patents involved in these fronts published between the years 2012–2017 are listed in Table 2.1.2.
(1) Stem cell technologies
Stem cell technology refers to the repair, improvement, or regeneration of human tissues and organs and treatment of diseases based on the study of separation, subculturing, induction, and differentiation of stem cells. Stem cells, defined
《Figure 1.2.6》
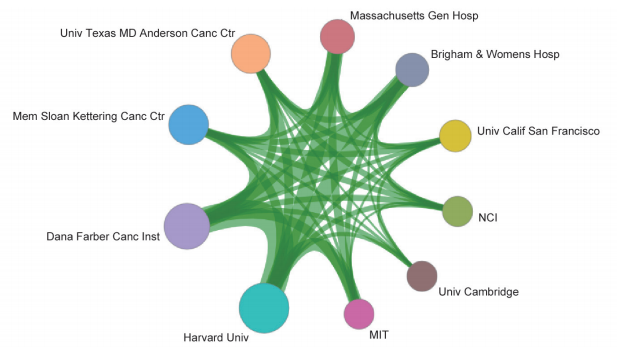
Figure 1.2.6 Collaboration network among major institutions in the engineering research front of “precision medicine research based on biomedical big data”
《Table 2.1.1》
Table 2.1.1 Top 10 engineering development fronts in medicine and health
| No. | Engineering development front | Published patents | Citations | Citations per patent | Mean year |
| 1 | Stem cell technologies | 8 458 | 8 943 | 1.06 | 2014.88 |
| 2 | AI and disease diagnosis | 747 | 2 064 | 2.76 | 2015.48 |
| 3 | Biomedical materials | 2 326 | 2 696 | 1.16 | 2015.19 |
| 4 | Tumor immunotherapy | 8 508 | 23 004 | 2.7 | 2014.89 |
| 5 | AI health management | 745 | 2 053 | 2.76 | 2015.48 |
| 6 | Genome editing | 2 529 | 12 042 | 4.76 | 2015.89 |
| 7 | Robotic surgery system | 2 976 | 35 337 | 11.87 | 2014.96 |
| 8 | Telemedicine | 2 195 | 5 102 | 2.32 | 2014.91 |
| 9 | Personalized therapeutic cancer vaccine | 460 | 844 | 1.83 | 2015.06 |
| 10 | Medical 3D printing technology | 1 896 | 2 121 | 1.12 | 2016.05 |
《Table 2.1.2》
Table 2.1.2 Annual number of patents published for the top 10 engineering development fronts in medicine and health
| No. | Engineering development front | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| 1 | Stem cell technologies | 1 145 | 1 117 | 1 122 | 1 433 | 1 537 | 2 104 |
| 2 | AI and disease diagnosis | 58 | 69 | 68 | 95 | 175 | 282 |
| 3 | Biomedical materials | 208 | 266 | 276 | 398 | 481 | 697 |
| 4 | Tumor immunotherapy | 1 088 | 1 108 | 1 239 | 1 353 | 1 630 | 2 090 |
| 5 | AI health management | 58 | 68 | 68 | 94 | 175 | 282 |
| 6 | Genome editing | 55 | 90 | 227 | 400 | 679 | 1 078 |
| 7 | Robotic surgery system | 298 | 374 | 507 | 483 | 593 | 721 |
| 8 | Telemedicine | 229 | 285 | 307 | 432 | 522 | 420 |
| 9 | Personalized therapeutic cancer vaccine | 50 | 51 | 73 | 59 | 101 | 126 |
| 10 | Medical 3D printing technology | 22 | 50 | 109 | 289 | 591 | 835 |
by the features of self-renewal and differentiation potential, can differentiate into all cell types and tissues of the body. Their unique features have offered great opportunities for treating devastating diseases such as blood system diseases (e.g., leukemia), nervous system diseases (e.g., Parkinson’s disease), cardiovascular diseases (e.g., myocardial infarction), and endocrine diseases (e.g., diabetes). In addition to cell therapy, stem cells have broad applications in the field of tissue and organ repair, disease modeling, drug screening, and precision medicine. With the continuous improvement of stem cell technology, its related treatment is expected to become the third choice for disease treatment, after drug treatment and surgery, leading to profound changes in the existing clinical treatment model. The huge potential of stem cell research in medicine has gathered support from all countries in the world, which promote the development of stem cell technology. Significant advances have been made in many key stem cell technologies, such as stem cell isolation, mechanism of cell fate conversion, and functional cell acquisition. Great achievements of basic research have accelerated the use of stem cells in clinical trials. Hematopoietic stem cell transplantation has become a mature and widely used treatment. A series of stem cell clinical trials have shown good therapeutic effects. Stem cell therapy was reported to halt a female patient’s macular degeneration and brightened her vision. In addition, the number of stem cell-related drugs has reached 350.
(2) AI and disease diagnosis
AI and disease diagnosis refers to the use of AI technology to carry out drug screening and early tumor warning predictions, pathological detection and analysis, along with disease diagnosis, classification, surgery planning, and treatment. AI also encompasses postoperative evaluation and rehabilitation in order to achieve accurate and intelligent disease diagnosis. The application of AI technology in the medical field has significantly improved physician efficiency. It is expected to alleviate the shortage of physicians, improve the accuracy of diagnosis and treatment, promote the optimal allocation of quality medical resources, and the entry of medical treatment into new heights of quantitative analysis.
(3) Biomedical materials
Biomedical materials are used to diagnose, treat, repair, or replace damaged tissues, organs, or functions of living organisms. The concept of biomedical materials originated in the mid-1940s, and the industry was formed in the 1980s. The application of biomedical materials not only saves the lives of a large number of critically ill patients but also significantly reduces the mortality of major diseases such as cardiovascular disease, cancer, and trauma. It also substantially improves the health and quality of human life. In parallel, reforms in the health care system play a guiding role in considerably reducing medical costs. They are an important material basis for solving current issues of difficult and expensive medical treatments so as to build a stable unified society. The development of biomedical materials has experienced the following stages: the use of partially oxide ceramics, medical carbon materials, medical metal and polymer materials, generally bio-inert materials stage, mainly with highly biologically active and controllable degradable bioactive glass, bioceramics, and composites thereof. Until now, research and development on biomaterials has focused on improving and developing traditional biomedical materials in addition to producing third-generation biomedical materials based on cellular and molecular level requirements.
(4) Tumor immunotherapy
Tumor immunotherapy is one of the most effective treatments based on the application of immunological theories and techniques to improve the immunogenicity of tumor cells, the killing sensitivity of effector cells, and the enhancement of the body’s immune response. The infusion of immune cells or effector molecules into the patient’s body could kill the tumor cells, thus inhibiting the growth of the tumor. Tumor immunotherapy includes the applications of monoclonal antibodies against immune checkpoints, therapeutic antibodies, immunological cell therapy, tumor vaccines, and small molecule inhibitors. It has attracted much attention in recent years due to its remarkable clinical efficacy, and has become a front in both the scientific and pharmaceutical enterprises. In December 2013, the Science journal ranked tumor immunotherapy as the first of 10 scientific breakthroughs of the year. In April 2016, cancer immunotherapy was put up on the cover of American Weekly magazine. So far, three programmed death-1 (PD-1) inhibitor types, blinatumomab and talimogene laherparepvec (T-VEC) oncolytic viral therapy have been approved by the American Food and Drug Administration or the European Medicines Agency. Identifying new immune checkpoints, chimeric antigen receptor therapy, and small molecule inhibitor screening are the focus of competition in this field. Compared with the traditional cancer treatment, tumor immunotherapy has the advantages of quick reaction, fewer side effects, and durable curative effects. However, there are also some drawbacks in tumor immunotherapy, such as the excessive immune response of the body, immunosuppressive microenvironment of tumor tissue, and heterogeneity of tumor cells. Future topics of research in this field include the improvement of the efficacy and specificity of treatment, control of adverse reactions, and expansion of indications. Specifically, we should focus on the engineering of precise immune cell therapies, screening of more biomarkers, and combining traditional tumor therapy, immunotherapy, or different immunotherapies. In conclusion, tumor immunotherapy is considered to be the “fourth treatment” after surgery, chemotherapy, and radiotherapy. In the future, radical treatment of patients with advanced, recurrent, and refractory tumors can be achieved using tumor immunotherapy.
(5) AI health management
AI health management refers to the process of comprehensively managing individual or population health risk factors in a digital and intelligent way using AI, big data, and similarly advanced technologies. With the continuous development of computer technologies, health management has experienced the era of health management v1.0 based on the Internet technology, the era of health management v2.0 based on mobile internet, and finally, the new era of health management v3.0 based on AI and big data. With further development of technologies such as big data, AI, and blockchain, AI health management is being placed into the market by laboratory research and is gradually being applied to sub-health groups, elderly groups, and chronic disease and high-risk groups, effectively reducing the risk of an individual's illness and medical expenses at the same time. It is estimated that by 2020, the health service industry will reach a market scale of 8 trillion CNY, an average annual growth rate of 26%. In particular, third-party medical examination organizations, which have healthy big data resources, are accelerating their layout and exploration. With the development of information technology such as AI, the improvement of modern medical disease spectrum, and the emergence of new ideas in medical management is in rapid progress. The future of AI health management will be vigorously developed while significantly reducing medical costs and improving the health quality of individuals. This will eventually achieve the primary goal of disease prevention, thus achieving great health.
(6) Genome editing
Genome engineering is a type of genetic engineering in which deoxyribonucleic acid (DNA) is inserted, deleted, and replaced in the genome of a living organism. From meganucleases that emerged in 1994 to the recently developed CRISPR- Cas9 technology in 2012, genetic engineering is progressing at a rapid pace. The CRISPR-Cas9 technology can easily and efficiently induce mutations at single or multiple loci in living cells. Beyond genome editing, many CRISPR-Cas9-based technologies have been developed, including gene activation, gene silencing, RNA editing, epigenetic modification, and base editing, which all provide powerful tools to unlock the mysteries of life, uncover disease mechanisms, and cure genetic diseases. The technology is already widely used in research and development to engineer better suited and clinically relevant disease models, screen for new therapeutic targets, and generate CRISPR-engineered plants resistant against diseases or neutralizing the effects of climate change in agriculture. The CRISPR-Cas9 technology also seems very promising for clinical application when it comes to human papillomavirus (HPV) infection, hemoglobinopathies, and chimeric antigen receptor (CAR) T-cell therapy on treatment of cancers. The first therapeutic application of the CRISPR technology will likely target cells with established delivery systems, such as ex vivo genome editing, to modify and return cells of an individual patient to treat thalassemia or cancer. For in vivo applications, high efficiency of precise editing, high fidelity of genetic tools, and robust delivery systems are persistent challenges in the field. These improvements will facilitate in vivo therapy on opthalmic diseases, hearing loss, spinal muscular atrophy, and Duchenne muscular dystrophy. The clinical application of genome editing will be undoubtedly accelerated by appropriate safety and efficacy measures for any potential therapy involving patients, new genome editing tools with independent property rights, and good manufacturing practice (GMP) workshop for drug delivery with genome editing elements.
(7) Robotic surgery system
Robotic surgery system is a smart surgical system used to assist a surgeon in performing complex minimally invasive surgeries. Since the first robot-assisted surgery in 1984, robotic surgery systems have undergone multiple generations of innovations in different technological companies. It can now participate in cardiac surgery, cardiac electrophysiology, colorectal and gastrointestinal surgery, breast surgery, gynecology, pediatrics, plastic surgery, spine surgery, transplantation, and urology. The technology has currently advanced to such an extent that doctors can now precisely control the operation stick even while being physically away from the patient. The in-situ master-slave control mode of operation increases its accuracy and smoothness to enhance the field of surgery with 3D high-definition cameras. The technology helps reduce the complexity of traditional minimally invasive surgery and shorten the doctors’ surgical learning curve. Since the advent of robotic surgery systems, the confinement created by traditional, minimally invasive surgeries have now been diminished. Surgeons are capable of performing fine operations in a small surgical space in a manner similar to open surgery, implying that high-risk surgeries are no longer a great challenge and there is a greater possibility for carrying out complicated minimally invasive surgeries. Innovations of the robotic surgical system are continually happening. For example, the structure of the mechanical arm has been improved to decrease in size and weight, and the sensing system will now provide tactile feedback to surgeons. At the same time, with remote surgery consultation and professional teaching of the robotic surgery system, medical efficiency would be further improved so that the limited medical resources of the country can be rationally distributed.
(8) Telemedicine
Telemedicine refers to the use of telecommunication and information technology to interactively deliver information for long-distance medical services. It is a new type of medical service that integrates modern medicine, computer technology, and communication closely together. A series of advanced technologies needs to be specifically developed to ensure the high quality and efficiency of telemedicine, such as the Internet, wireless communication, Internet of Things, virtual reality, electronic medical records, cloud computing, medical big data, and AI. In addition, intelligent terminals (e.g., computers, mobile phones, personal digital assistants, virtual reality equipment) and smart medical devices (e.g., remote blood pressure monitors, electrocardiograph, fetal heart rate monitors) are also important to solidify the intelligent medical interactions between patients and healthcare providers, medical institutions, and medical equipment. Telemedicine has a wide range of applications, including diagnostic systems, consultation systems for discussions on treatment plans, tele-surgery systems for treatment, education systems for teaching and training, and remote bed monitoring systems for home care patients. The purpose of telemedicine is to optimize the allocation of medical resources and provide remote or low-resource areas with smart and high-quality healthcare services. It is believed that telemedicine started relatively late in China, around 1986. Nowadays, as various technologies are gradually maturing, the telemedicine sector is also developing quickly. China has made significant progresses in remote diagnosis and consultation, diagnostic imaging, AI-assisted diagnosis, registration, distance education, and information sharing. The future development of telemedicine has taken another leap by advancing from just the treatment to disease prevention too, thereby providing personalized and intelligent healthcare services to individuals and enabling daily preventative health care and medical monitoring services.
(9) Personalized therapeutic cancer vaccine
Personalized therapeutic cancer vaccine is tailored to a single patient. These custom-tailored vaccines are designed based on each patient’s particular tumor mutations (neoantigens), with the goal of inducing high-affinity immune T-cell response against cancer. In July 2017, Nature magazine reported that the new tumor antigen vaccine based on peptide and mRNA respectively, successfully reduced or delayed the recurrence or metastasis of melanoma surgery in a small number of patients, marking individualized cancer treatment vaccines have been clinically successful for the first time (Nature: 2017, 547: 217-21; Nature, 2017, 547: 222-6). As a brand new cancer therapy, this technology can theoretically be applied to any cancer, and has great potential in preventing recurrence and metastasis, which remain major problems in the current treatment of cancer. This technology is also expected to overcome the shortcomings of existing tumor immunotherapy, such as the complex mechanism of action of immune checkpoint inhibitors and the genetic engineering of CAR-T cell therapy. Personalized therapeutic cancer vaccines are becoming a new research front in tumor immunotherapy and reflect the future direction of precision medicine. Nonetheless, research in this field still faces many challenges, such as longer vaccine customization cycle, which takes three months on an average, thus limiting its application to slow- growing cancers. In addition, it is still necessary to determine which types of tumors can benefit from immunotherapy. The vaccines are most commonly used on tumors with several mutations, such as melanoma. The development of this technology still requires intensive research, including the rapid detection of individual tumor-specific T-cell epitopes, an efficient T-cell epitope delivery pathway, and the rapid detection of single-cell expression profiles of tumor cells. It also requires quick and efficient technology for analysis of individual T-cell epitopes combined with cloud computing and AI, as well as a highly efficient intracellular introduction pathway of T-cell epitope vaccine. Finally, our purpose is to accelerate the development and clinical transformation of personalized therapeutic cancer vaccines.
(10) Medical 3D printing technology
Medical 3D printing technolog y is defined as the material forming or processing technology aimed at making applicable, personalized 3D medical products based on the patients’ medical imaging data. The research front of medical 3D printing focuses on of 3D printer optimization, development of new printable materials and precise modeling software, and the multicellular precise 3D printing technology. With the progress in precision medicine and personalized medicine, as well as novel 3D-printable biomaterials and printers, this technology has become a leading manufacturing process in healthcare and medicine for a wide range of applications, including anatomical models, medical devices, surgical guides, 3D-printed implants, artificial tissues/ organs, and drug screening. The 3D-printed products, such as orthopedic metal implants, ceramic dentures, and dural patches, have obtained FDA or China Food and Drug Administration (CFDA) certification, whereas 3D bioprinted tissues/organs are still in the experimental stage. Compared with foreign countries, the current 3D printing industry in China has shown high enthusiasm in the research and development of equipment as well as the application related services. Until now, the performance scale of these equipment is close to the advanced international level. However, several problems still need to be solved, such as the shortage and poor quality of printable materials, insufficient competition due to small scale of enterprises and meager investments, incompatibility between payment system and medical insurance, and lack of corresponding standards for 3D printing products. With the development of 3D printing technology, personalized medical products can not only achieve “adjustable scale and size” but also satisfy diverse needs such as anatomical structures, mechanical properties, and biological functions. Thus, it is evident that the process has evolved from medical models to bioprinted artificial organs. These technological advancements are of great significance for the development of regenerative medicine, which may be revolutionized fundamentally in the next 20 years.
《2.2 Interpretations for three key engineering development fronts》
2.2 Interpretations for three key engineering development fronts
2.2.1 Stem cell technologies
Stem cell technology refers to the repair, improvement, or regeneration of human tissues and organs and treatment of diseases based on the study of separation, subculturing, induction, and differentiation of stem cells. Stem cells, defined by the features of self-renewal and differentiation potential, can differentiate into all cell types and tissues of the body. Their unique features have offered great opportunities for treating devastating diseases such as blood system diseases (e.g., leukemia), nervous system diseases (e.g., Parkinson’s disease), cardiovascular diseases (e.g., myocardial infarction), and endocrine diseases (e.g., diabetes). In addition to cell therapy, stem cells have broad applications in the field of tissue and organ repair, disease modeling, drug screening, and precision medicine. With the continuous improvement of stem cell technology, its related treatment is expected to become the third choice for disease treatment, after drug treatment and surgery, leading to profound changes in the existing clinical treatment model. The huge potential of stem cell research in medicine has gathered support from all countries in the world, which promote the development of stem cell technology. Significant advances have been made in many key stem cell technologies, such as stem cell isolation, mechanism of cell fate conversion, and functional cell acquisition. Great achievements of basic research have accelerated the use of stem cells in clinical trials. Hematopoietic stem cell transplantation has become a mature and widely used treatment. A series of stem cell clinical trials have shown good therapeutic effects. Stem cell therapy was reported to halt a female patient’s macular degeneration and brightened her vision. In addition, the number of stem cell-related drugs has reached 350.
Presently, the development of stem cell technology must solve the following key technical problems: improvement and optimization of methods for stem cell isolation and pluripotency maintenance; understanding the mechanism of stem cell differentiation and regulation, acquisition, and functional studies on ASC; investigation of the machinery of tissue and organ formation; construction of artificial tissue and organs; establishment and regulation of in vivo functions after stem cell transplantation; and creation of evaluation standards for safety and effectiveness.
The current hot spots in the field of international stem cell technology include: (1) Pluripotent stem cell induction method that optimizes the reprogramming recipe using transcription factors, chemicals, and CRISPR-based methods for efficiently inducing a somatic cell to pluripotent stem cell. This will ensure a safe method of human pluripotent stem cell induction for clinical application. (2) Maintenance of pluripotency through the identification of additional transcription factors and epigenetic enzymes necessary for stem cell self-renewal (e.g., mesenchymal, hematopoietic, and neural stem cell acquisition, maintenance, and large-scale expansion in adult stem cells). (3) Committed differentiation, wherein, the stem cells can be differentiated into specific lineages and functional cells by improving culture methods or optimizing induction strategies. This involves proliferation of differentiated cells by the optimization of the physical and biological conditions for large-scale stem cell expansion to gradually yield quick differentiation and functional cell acquisition. (4) Establishment and regulation of in vivo functions after stem cell transplantation, i.e., establishment of cell tracking techniques to evaluate the therapeutic effect of specific cells types on diseases. (5) Stem cell-based therapy for tissue and organ repair, which uses micro-organ culturing or biological materials to construct functional brain, pancreas, liver, and teeth. There is a huge potential market of stem cell research in medicine due to its rapid development in the past 10 years. It has gradually moved from the laboratory to clinic and toward industrialization. The global stem cell industry has had a potential market of approximately 80 billion USD over the past two years, estimated to reach 400 billion USD by 2020. In China, the stem cell industry is also quite promising. According to a research report, the Chinese stem cell industry has formed a complete upstream to downstream industrial chain covering all its clinical applications. The income of the stem cell industry is expected to increase from 2 billion CNY to 30 billion CNY in the next five years, with an annual growth rate of 170%.
In total, the stem cell technology has been applied on 8458 patents in the past six years. China, the USA, and South Korea are ranked among top three countries with the highest number of patents in force (Table 2.2.1). The patents filed by Chinese authors/researchers account for 41.55% of the total global patents. China has become one of the key countries conducting research on this aspect of engineering development, with an average cited frequency of 0.63 (Table 2.2.1). This finding provides evidence that the quality of patents needs improvement. As shown in the cooperation network of patent-producing countries (Figure 2.2.1), the USA, Japan, Switzerland, and South Korea cooperate more closely than other countries. The top three institutions with the maximum proportion of core patent inventors are the Guangzhou Salia Stem Cell Technology Co., Kyoto University (KYOU), and University of Southern California (REGC) (Table 2.2.2). In addition, the collaboration network among international institutions shows the cooperation of Seoul National University Industry Foundation (USEO) and Konkuk University Industrial Cooperation Corp (UKUK) (Figure 2.2.2).
2.2.2 AI and disease diagnosis
AI and disease diagnosis refers to the use of AI technology to carry out drug screening and early tumor warning predictions, pathological detection and analysis, along with disease diagnosis, classification, surgery planning, and treatment. AI also encompasses postoperative evaluation and rehabilitation in order to achieve accurate and intelligent disease diagnosis. The application of AI technology in the medical field has significantly improved physician efficiency. It is expected to alleviate the shortage of physicians, improve the accuracy of diagnosis and treatment, promote the optimal allocation of quality medical resources, and the entry of medical treatment into new heights of quantitative analysis.
《Table 2.2.1》
Table 2.2.1 Countries or regions with the greatest output of patents on the “stem cell technology”
| No. | Country/Region | Published patents | Percentage of published patents | Citations | Percentage of citations | Citations per patent |
| 1 | China | 3 514 | 41.55% | 2 203 | 24.63% | 0.63 |
| 2 | USA | 1 694 | 20.03% | 3 123 | 34.92% | 1.84 |
| 3 | South Korea | 1 181 | 13.96% | 824 | 9.21% | 0.7 |
| 4 | Japan | 863 | 10.20% | 1 094 | 12.23% | 1.27 |
| 5 | Taiwan of China | 169 | 2.00% | 179 | 2.00% | 1.06 |
| 6 | Germany | 111 | 1.31% | 176 | 1.97% | 1.59 |
| 7 | France | 90 | 1.06% | 118 | 1.32% | 1.31 |
| 8 | UK | 85 | 1.00% | 110 | 1.23% | 1.29 |
| 9 | Russia | 82 | 0.97% | 15 | 0.17% | 0.18 |
| 10 | Switzerland | 70 | 0.83% | 156 | 1.74% | 2.23 |
《Table 2.2.2》
Table 2.2.2 Institutions with the greatest output of patents on the “stem cell technology”
| No. | Institution | Published patents | Percentage of published patents | Citations | Percentage of citations | Citations per patent |
| 1 | Guangzhou Saliai Stemcell Science and Technology Co Ltd | 209 | 2.47% | 72 | 0.81% | 0.34 |
| 2 | Kyoto University | 103 | 1.22% | 247 | 2.76% | 2.4 |
| 3 | University of Southern California | 87 | 1.03% | 156 | 1.74% | 1.79 |
| 4 | Seoul National University Industry Foundation | 81 | 0.96% | 29 | 0.32% | 0.36 |
| 5 | LG Chem Co Ltd | 66 | 0.78% | 76 | 0.85% | 1.15 |
| 6 | The Catholic University of Korea Industry-Academic Cooperation Foundation | 55 | 0.65% | 4 | 0.04% | 0.07 |
| 7 | Industry-Academic Cooperation Foundation Yonsei University | 52 | 0.61% | 20 | 0.22% | 0.38 |
| 8 | Konkuk University Industrial Cooperation Corp | 49 | 0.58% | 1 | 0.01% | 0.02 |
| 9 | Zhejiang University | 47 | 0.56% | 22 | 0.25% | 0.47 |
| 10 | Agency for Science Technology and Research, | 44 | 0.52% | 100 | 1.12% | 2.27 |
The key technical problems to be solved in AI and disease diagnosis research include accuracy and related problems of medical data annotation, machine learning problems of limited or incomplete medical data, and mixed learning problems of multi-source medical data. Additional areas of technical hurdles include use of AI in different feature selection cases in disease applications, privacy protection in medical data, and application of AI in surgical intervention and rehabilitation. At present, the fronts of AI research include: (1) Eye diseases: Deep network learning techniques are used to study retinal fundus images, which can be used for the diagnosis of glaucoma, macular degeneration, and diabetic retinopathy. (2) Tumor treatment: Individualized and authoritative treatment programs are developed for cancer patients by integrating pathological sample feature extraction and genome sequencing data combined with clinical guidelines and evidence-based medicine. They have been used in lung and esophageal cancer. (3) Pathological diagnosis: The feature extraction and deep learning have been used for quantitative diagnosis and disease prognosis assessment. They have been used for treatment of cancers of lung, cervix, breast, stomach, and intestines. (4) Medical
《Figure 2.2.1》
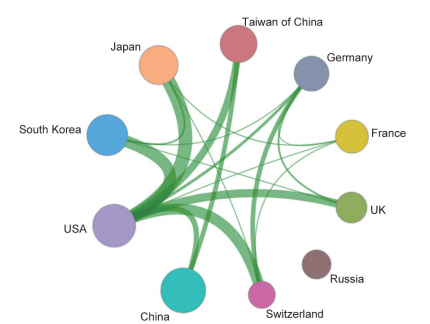
Figure 2.2.1 Collaboration network among major countries in the engineering development front of “stem cell technology”
《Figure 2.2.2》
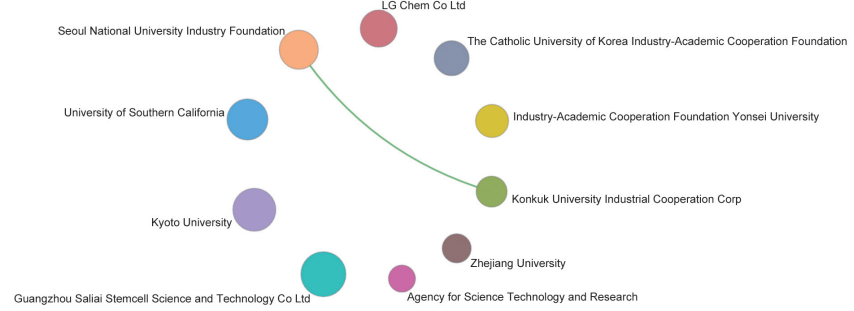
Figure 2.2.2 Collaboration network among major institutions in the engineering development front of “stem cell technology”
imaging: Through large databases and deep learning, many AI algorithms have been successfully used in a variety of medical imaging applications. For example, Alzheimer’s disease classification accuracy using brain MRI has reached 91.67% by using AI techniques and AI-assisted heart MRI imaging system (American Aeterys) has been certified by the FDA. (5) Skin diseases: AI technology carries out migration learning through the corresponding model and can diagnose skin cancer and melanoma with an accuracy rate of over 90%, greatly reducing the cost of medical testing. (6) Surgical robot: A series of surgical robots has been applied in urology, cardiology, orthopedics, and extra-sacral surgery. A typical example is the Da Vinci surgical robot system, which was developed by Intuitive Surgical Inc., USA. By using these surgical robots, the operation becomes more efficient and safe. The medical market demand for AI is very large and has been growing at a rate of 40% annually. However, the high-end market is still dominated by the USA and other western countries.
AI and disease diagnosis has been applied on 747 patents in the past six years. China, USA, and South Korea are ranked as the top three countries with the most patents in force. The patents applied by Chinese and US authors account for 41.37% and 33.07% of the total patents, respectively. The USA and China have become key countries researching on this aspect of engineering development. However the average cited frequency of China is only 1.66 (ranked ninth among the top 10 countries), which was significantly lower than the index in the Israel (13), USA (4.73), and Switzerland (4.2) (Table 2.2.3). As shown in the cooperation network of patent-producing countries (Figure 2.2.3), the USA is cooperating closely with Germany and India.
The top three institutions with maximum number of patent inventors are Siemens Healthcare Gmbh (SIEI), State Grid Corporation, China (SGCC), and International Business Machines Corporation, US (IBMC) (Table 2.2.4). Moreover, the collaboration networks among international institutions show low cooperation with regard to AI and disease diagnosis (Figure 2.2.4).
2.2.3 Biomedical materials
Biomedical materials are used to diagnose, treat, repair, or replace damaged tissues, organs, or functions of living organisms. The concept of biomedical materials originated in the mid-1940s, and the industry was formed in the 1980s. The application of biomedical materials not only saves the lives of a large number of critically ill patients but also significantly reduces the mortality of major diseases such as cardiovascular disease, cancer, and trauma. It also substantially improves the health and quality of human life. In parallel, reforms in the health care system play a guiding role in considerably reducing medical costs. They are an important material basis for solving current issues of difficult and expensive medical treatments
《Table 2.2.3》
Table 2.2.3 Countries or regions with the greatest output of patents on the “AI and disease diagnosis”
| No. | Country/Region | Published patents | Percentage of published patents | Citations | Percentage of citations | Citations per patent |
| 1 | China | 309 | 41.37% | 513 | 24.85% | 1.66 |
| 2 | USA | 247 | 33.07% | 1 168 | 56.59% | 4.73 |
| 3 | South Korea | 46 | 6.16% | 33 | 1.60% | 0.72 |
| 4 | Germany | 32 | 4.28% | 95 | 4.60% | 2.97 |
| 5 | Japan | 22 | 2.95% | 58 | 2.81% | 2.64 |
| 6 | India | 21 | 2.81% | 48 | 2.33% | 2.29 |
| 7 | Canada | 16 | 2.14% | 51 | 2.47% | 3.19 |
| 8 | Israel | 7 | 0.94% | 91 | 4.41% | 13 |
| 9 | Switzerland | 5 | 0.67% | 21 | 1.02% | 4.2 |
| 10 | France | 5 | 0.67% | 16 | 0.78% | 3.2 |
《Table 2.2.4》
Table 2.2.4 Institutions with the greatest output of patents on the “AI and disease diagnosis”
| No. | Institution | Published patents | Percentage of published patents | Citations | Percentage of citations | Citations per patent |
| 1 | Siemens Healthcare GmbH | 33 | 4.42% | 81 | 3.92% | 2.45 |
| 2 | State Grid Corporation | 19 | 2.54% | 11 | 0.53% | 0.58 |
| 3 | International Business Machines | 15 | 2.01% | 34 | 1.65% | 2.27 |
| 4 | HeartFlow Inc | 11 | 1.47% | 57 | 2.76% | 5.18 |
| 5 | Iteris Inc | 10 | 1.34% | 19 | 0.92% | 1.9 |
| 6 | General Electric Co | 7 | 0.94% | 33 | 1.60% | 4.71 |
| 7 | Merge Healthcare Inc | 7 | 0.94% | 3 | 0.15% | 0.43 |
| 8 | Beihang University | 7 | 0.94% | 8 | 0.39% | 1.14 |
| 9 | Microsoft Corporation | 6 | 0.80% | 26 | 1.26% | 4.33 |
| 10 | Jilin Agriculture Science & Technology College | 5 | 0.67% | 0 | 0.00% | 0 |
《Figure 2.2.3》
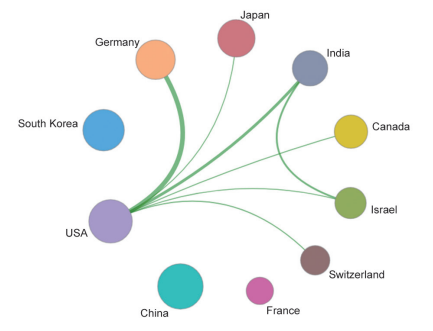
Figure 2.2.3 Collaboration network among major countries in the engineering development front of “AI and disease diagnosis”
needs to solve the following key technical problems:
(1) improvement and development of methods for evaluating the biocompatibility of biomedical materials; (2) design and synthesis of novel biodegradable materials; (3) development of artificial organs and tissue materials with comprehensive physiological function; (4) development of new drug delivery systems and drug carrier materials; (5) surface modification of materials; and (6) development of nanomedical materials.
The global medical device market is growing rapidly. As a new industry with low raw material and energy consumption and high technology added value, the biomedical material industry has shown a good development trend in recent years, and the market demand is very huge. In the past decade, the industry has been growing at an annual rate of 8%. It has
《Figure 2.2.4》
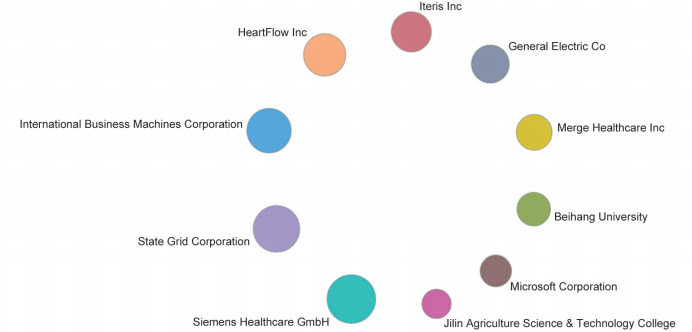
Figure 2.2.4 Collaboration network among major institutions in the engineering development front of “AI and disease diagnosis”
so as to build a stable unified society. The development of biomedical materials has experienced the following stages: the use of partially oxide ceramics, medical carbon materials, medical metal and polymer materials, generally bio-inert materials stage, mainly with highly biologically active and controllable degradable bioactive glass, bioceramics, and composites thereof. Until now, research and development on biomaterials has focused on improving and developing traditional biomedical materials in addition to producing third-generation biomedical materials based on cellular and molecular level requirements.
Presently, the development of biomedical materials reached 130 billion and 220 billion USD in 2013 and 2016, respectively. Western countries such as the USA occupy the high-end market of biomedical materials, and share of China in the domestic market of biomedical materials in is only 3%, mainly in the low-end market of products. However, in terms of R&D, China has the highest number of patent applications in the forefront of biomedical material development in 2012–2017, and the proportion of patents applied has reached 69.91%. However, the quality of patents still needs to be further improved. Moreover, the total number of patents cited and average number of citations of the patents are still far from those of the USA.
The current fronts in the field of international medical material research include: (1) Biodegradable materials that can be gradually degraded or metabolized with time after being implanted in the human body. The foreign body implanted will automatically degrade into non-toxic, harmless substances that can be expelled from the body soon after they are used. (2) Tissue engineering materials and artificial organs to construct biological devices by using engineering principles and methods. These would replace the damaged tissues or organs, involving the use of biological scaffolds, seed cells, and growth factors to establish complex 3D biological structures composed of cells and biological materials. It is imperative to develop biological materials with good biocompatibility and ability to gradually degrade and be assimilated in the human body. These materials are used mainly to develop artificial blood, liver, heart, kidney, pancreas, blood vessel, cornea, and cerebrovascular stents. (3) Materials like bones and cartilages to repair defective bones and joints using tissue engineering principles. Electrospinning technology is used on bones, cartilages, and adipose tissue stem cells to develop artificial bones, cartilage knee joints, hip joints, meniscus, ligament, and muscles. (4) Dental restorative materials for developing biomaterials for the restoration of maxillofacial, mandibular, and tooth defects. (5) Controlled-release materials for the delivery of drugs at a constant rate over a certain period of time. These are divided into natural and synthetic polymers.
(6) Bionic intelligent materials; these materials are designed
on the principle of synergistic interaction among biological macromolecules to produce intelligent/smart materials with the desired host response. They imitate the cooperative behavior of biomedical materials. (7) Antibacterial membrane biomaterials; a biofilm is often formed on the surface of implant materials to protect bacterial growth and results in postoperative infections. As postoperative clinical bacterial film infections occur in 2%–3% of the total cases, therefore, there is a huge market for developing antibacterial materials to solve postoperative bacterial film infection. (8) Nano- biomedical materials is a new interdisciplinary field regarding the structure and function of genes and proteins, including their identification, integration, transformation, special factor release, bioelectrochemical signal generation and conduction, and biomechanical and thermodynamic properties. They also involve the development of new technical tools using multidisciplinary research.
With the improved economic development and severe forms of population aging, the demand for the medical market worldwide is increasing. In China, the demand for the development of biomedical materials has dramatically increased, and China has now become the second largest biomedical material market in the world. The development of this industry is important to improve the quality of life and delay the social problems brought about by aging. At the same time, biomedical materials, as a high-tech industry with low energy consumption and high added value, also have a great development potential and economic value. China has made a series of advances in the development of traditional biomedical materials. In some high-end areas, localization has also been replaced by imports, but there is still a big gap when compared with the actual needs of clinical applications. However, with the development of new technologies such as material science and molecular biology, especially stem cell engineering and 3D printing, numerous scientific attempts are being made towards success of biomedical materials especially in China. There are also new challenges and opportunities for the promotion of biomedical materials in China.
In total, 2326 patents on biomedical materials have been applied within the past six years. China, USA, and Japan are ranked as the top three countries with the most patents in force. The patents applied by Chinese authors account for 69.91% (Table 2.2.5). The average cited frequency of China is 0.78 (Table 2.2.5). The number of patents granted in the USA is fewer than that in China, but the average cited frequency in USA is 3.27. This suggests that the patent quality of our country needs improvement. As shown in the cooperation network of patent-producing countries (Figure 2.2.5), USA, Japan, and China are closely cooperating with each other.
The top three institutions with the maximum number of inventors of patents in force are West China Hospital Sichuan University (USCU), Tokuyama Dental Corporation (TDNT), and Hefei Chuangwo Technology Co., Ltd. (Table 2.2.6). In addition, the collaboration network among international institutions shows the cooperation of Shanghai Institute of Ceramics Chinese Academy of Sciences (CAGU) and Shanghai Jiao Tong University (USJT) (Figure 2.2.6).
《Table 2.2.5》
Table 2.2.5 Countries or regions with the greatest output of patents on the “biomedical materials”
| No. | Country/Region | Published patents | Percentage of published patents | Citations | Percentage of citations | Citations per patent |
| 1 | China | 1 626 | 69.91% | 1 273 | 47.22% | 0.78 |
| 2 | USA | 223 | 9.59% | 730 | 27.08% | 3.27 |
| 3 | Japan | 169 | 7.27% | 196 | 7.27% | 1.16 |
| 4 | South Korea | 107 | 4.60% | 51 | 1.89% | 0.48 |
| 5 | Taiwan of China | 28 | 1.20% | 45 | 1.67% | 1.61 |
| 6 | Germany | 25 | 1.07% | 54 | 2.00% | 2.16 |
| 7 | UK | 25 | 1.07% | 88 | 3.26% | 3.52 |
| 8 | Switzerland | 19 | 0.82% | 30 | 1.11% | 1.58 |
| 9 | France | 17 | 0.73% | 14 | 0.52% | 0.82 |
| 10 | India | 16 | 0.69% | 1 | 0.04% | 0.06 |
《Table 2.2.6》
Table 2.2.6 Institutions with the greatest output of patents on the “biomedical materials”
| No. | Institution | Published patents | Percentage of published patents | Citations | Percentage of citations | Citations per patent |
| 1 | West China Hospital Sichuan University | 39 | 1.68% | 48 | 1.78% | 1.23 |
| 2 | Tokuyama Dental Corporation | 36 | 1.55% | 36 | 1.34% | 1 |
| 3 | Hefei Chuangwo Technology Co Ltd | 30 | 1.29% | 0 | 0.00% | 0 |
| 4 | South China University of Technology | 25 | 1.07% | 32 | 1.19% | 1.28 |
| 5 | Donghua University | 23 | 0.99% | 40 | 1.48% | 1.74 |
| 6 | Wuhu Yangzhan New Material Technology | 21 | 0.90% | 0 | 0.00% | 0 |
| 7 | Zhejiang University | 20 | 0.86% | 1 | 0.04% | 0.05 |
| 8 | Soochow University | 18 | 0.77% | 28 | 1.04% | 1.56 |
| 9 | Shanghai Institute of Ceramics Chinese Academy of Sciences | 17 | 0.73% | 4 | 0.15% | 0.24 |
| 10 | Shanghai Jiao Tong University | 17 | 0.73% | 15 | 0.56% | 0.88 |
《Figure 2.2.5》
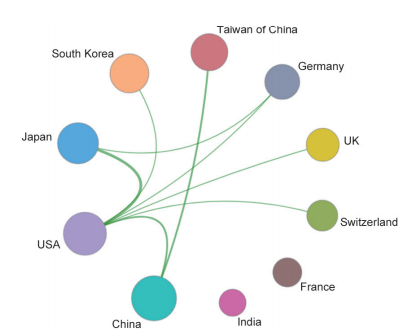
Figure 2.2.5 Collaboration network among major countries in the engineering development front of “biomedical materials”
《Figure 2.2.6》
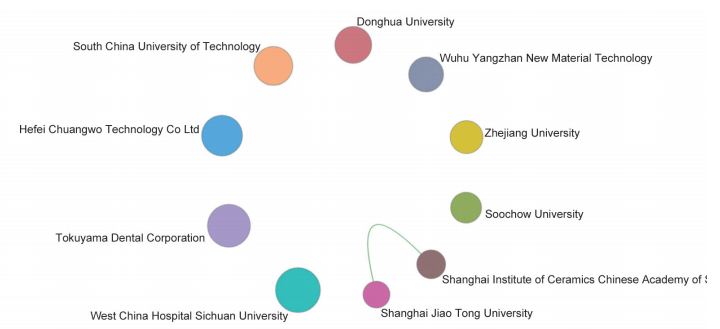
Figure 2.2.6 Collaboration network among major institutions in the engineering development front of “biomedical materials”
Participants of the Field Group
Leaders
CHEN Saijuan, YANG Baofeng
Academicians
ZHANG Boli, XU Jianguo, DING Jian, FU Xiaobing, HAO Xishan, LIU Zhihong, WANG Hongyang, CHEN Xiangmei, CHENG Jing,
ZHAN Qimin, XIA Zhaofan, WANG Guangji, ZHANG Zhiyuan, NING Guang, LI Song, GU Xiaosong, SUN Yinghao, HUANG Luqi,
TIAN Zhigang, LI Zhaoshen, DONG Jiahong
Other Experts
ZHOU Rongbin, GUO Deyin, YI Fan, LIU Bing, LIAO Zhuan, ZOU Wenbin, WANG Yueying, SHI Jian, YING Songmin, ZHANG Cheng,
XIE Weifen, WANG Linhui, XIAO Liang, JIANG Jianxin, LI Qingfeng, WU Hao, XU Qin, BAI Guo, SHEN Hongbing, QI Zhongtian,
SHAO Zhongjun, WU Tangchun, SONG Hongbing, ZHONG Wu, XIA Ningshao, HUANG Bin, JIANG Jiandong, LI Bing, ZHOU Xinbo,
ZHENG Hairong, LI Gang, OUYANG Hongwei, WANG Xiumei, XIE Huiqi, ZHANG Junhua, XIAO Xiaohe, GUO Lanping, GAO Yue,
YANG Hongjun, ZHANG Weidong, DUAN Jinao, YU Shishan, XIAO Wei, LI Haiyan, Guo Dean
The Division Medical and Health Care at CAE:
LI Dongmei, ZHANG Wentao, ZHAO Xilu
Editorial Office Frontiers of Medicine: XI Xiaodong, YAN Xiaoyu
Library of Shanghai Jiao Tong University School of Medicine: QIU Xiaochun, DENG Peiwen, WU Hui, FAN Rong, KOU Jiande, LIU Jie,
Shanghai Innovation Evaluation and Consulting Center for Science and Technology: JIANG Hongbo, LU Jiao
Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine: HUANG Jinyan, CHEN Yinyin, DAI Yuting, QIAO Niu
Report Writers
Engineering Research Front
WU Yilong, CHENG Tao, FANG Xiangdong, HUANG Jinyan, WU Tangchun, GAO Yue, GU Xiaosong, JIANG Shibo, LU Lu, ZHOU Jiawei,
LI Zhaoshen, SHI Jian, XIE Weifeng
Engineering Development Front
PEI Duanqin, WANG Bo, TIAN Wei, HAN Xiaoguang, LIU Wenyong, CUI Daxiang, LI Zhaoshen, LIAO Zhuan, ZOU Wenbin, MING Dong,
YANG Hui, SUN Yinghao, WANG Linhui, WU Zhenjie, XIAO Liang, DONG Jiahong, ZHANG Xu, JI Jiafu, LUO Qingquan, TENG Gaojun,
JIAO Yun, LI Song, XIA Ningshao, DAI Kerong, WANG Jinwu














 京公网安备 11010502051620号
京公网安备 11010502051620号




