《1 Engineering research fronts》
1 Engineering research fronts
《1.1 Trends in Top 10 engineering research fronts》
1.1 Trends in Top 10 engineering research fronts
The Top 10 engineering research fronts in the field of medicine and health include basic medicine, clinical medicine, medical informatics and biomedical engineering, and traditional Chinese medicine (Table 1.1.1). These 10 fronts also involve “immune heterogeneity and intervention strategies of solid tumors”, “mechanisms in tumor heterogeneity and evolutionary dynamics”, “stem cell aging”, “design and precise prediction of protein folding structure”, “artificial intelligence in drug design”, “biomacromolecular phase separation and phase transition”, “mechanisms of genome regulation”, “mechanism of neoantigen generation and its role in tumor immunity” “3D printing and organ regeneration”, and “AI- assisted disease diagnosis and treatment system”. All core papers on these fronts published between 2016 and 2021 are listed in Table 1.1.2.
(1) Immune heterogeneity and intervention strategies of solid tumors
The heterogeneity of the immune microenvironment is prevalent in solid tumors, and changes during tumor progression and upon therapeutic intervention in a spatial or temporal manner. This immune heterogeneity is closely related to disease progression and treatment responsiveness. A comprehensive understanding of immune heterogeneity and the development of corresponding intervention strategies are crucial for the appropriate assessment of immune heterogeneity in clinical practice and promoting the development of effective personalized management. By using multi-site biopsy sampling, multi-omics sequencing, single- cell sequencing, and longitudinal liquid biopsy methods, several studies have shown the complexity of tumor immune heterogeneity and its potential value in guiding clinical diagnosis and treatment strategies. Current research mainly focused on establishing and improving of the diagnostic techniques for immune heterogeneity, developing new tools for the research of immune heterogeneity, and developing therapeutic strategies that target immune heterogeneity.
The immune heterogeneity of solid tumors is an important frontier research field in tumor immunology and immunot- herapy, and many countries have large research investment and extensive cooperation. China is currently in a trend of following the similar studies abroad. Intensive effort should take advantage of China’s quantitatively clinical sample resources. Institutions should establish a research platform for investigating immune heterogeneity of solid tumors and a
《Table 1.1.1》
Table 1.1.1 Top 10 engineering research fronts in medicine and health
| No. | Engineering research front | Core papers | Citations | Citations per paper | Mean year |
| 1 | Immune heterogeneity and intervention strategies of solid tumors | 945 | 144 130 | 152.52 | 2017.9 |
| 2 | Mechanisms in tumor heterogeneity and evolutionary dynamics | 474 | 47 136 | 99.44 | 2018.3 |
| 3 | Stem cell aging | 610 | 63 316 | 103.8 | 2017.5 |
| 4 | Design and precise prediction of protein folding structure | 491 | 41 898 | 85.33 | 2018.2 |
| 5 | Artificial intelligence in drug design | 453 | 49 852 | 110.05 | 2018.1 |
| 6 | Bio-macromolecular phase separation and phase transition | 858 | 79 732 | 92.93 | 2017.6 |
| 7 | Mechanisms of genome regulation | 280 | 37 959 | 135.57 | 2017.3 |
| 8 | Mechanism of neoantigen generation and its role in tumor immunity | 211 | 56 200 | 266.35 | 2017.7 |
| 9 | 3D printing and organ regeneration | 700 | 79 548 | 113.64 | 2017.8 |
| 10 | AI-assisted disease diagnosis and treatment system | 975 | 202 804 | 208 | 2019.2 |
《Table 1.1.2》
Table 1.1.2 Annual number of core papers published for the Top 10 engineering research fronts in medicine and health
| No. | Engineering research front | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| 1 | Immune heterogeneity and intervention strategies of solid tumors | 201 | 208 | 211 | 188 | 114 | 23 |
| 2 | Mechanisms in tumor heterogeneity and evolutionary dynamics | 81 | 101 | 89 | 73 | 62 | 68 |
| 3 | Stem cell aging | 169 | 155 | 140 | 95 | 43 | 8 |
| 4 | Design and precise prediction of protein folding structure | 107 | 104 | 73 | 72 | 47 | 88 |
| 5 | Artificial intelligence in drug design | 79 | 81 | 109 | 96 | 74 | 14 |
| 6 | Bio-macromolecular phase separation and phase transition | 265 | 165 | 203 | 134 | 80 | 11 |
| 7 | Mechanisms of genome regulation | 93 | 70 | 63 | 32 | 16 | 3 |
| 8 | Mechanism of neoantigen generation and its role in tumor immunity | 51 | 50 | 53 | 37 | 16 | 4 |
| 9 | 3D printing and organ regeneration | 143 | 172 | 159 | 136 | 55 | 35 |
| 10 | AI-assisted disease diagnosis and treatment system | 51 | 104 | 155 | 198 | 214 | 253 |
systematic tumor sample bioresource library and information database, enhance the application of spatial omics technology in decoding immune heterogeneity, active carry out clinical trials and encourage multimodal combination therapy, and strengthen international cooperation to promote data sharing.
(2) Mechanisms in tumor heterogeneity and evolutionary dynamics
Tumorigenesis and progression are dynamic evolution processes. Somatic mutations, chromosomal rearrangements, copy number variations, and other alterations occur in healthy tissues, leading to genomic instability, tumor suppressor gene loss, and proto-oncogene activation and resulting in malignant characteristics. Early driving ancestor mutations gradually extended into multi-branch mutations, forming different subclones. Under the pressure of microenvironment and therapy, dominant clones continue to be selected, with progression, metastasis, and resistance. These molecular events are involved in every cancer stage. The understanding of the dynamic evolution mechanisms of tumor has long been limited to the genome itself, but with the advancement of technology and the deepening of research, mechanisms such as epigenetic aberrations and microenvironment interaction networks have promoted evolution. Cell identity can be epigenetically encoded during development similar to that in tumors. Epigenetic regulation such as DNA methylation, chromatin plasticity, and histone modifications determine the “on” or “off” of genes, induce transient changes in gene expression, and ultimately act on tumor evolution. The tumor microenvironment, including blood vessels, immune cells, fibroblasts, and extracellular matrix, exerts direct selection pressure on tumor cells, and adaptive changes made by tumor cells shape the microenvironment. Research on the dynamic evolution mechanisms of tumor is gradually moving towards systematization, integration, and deepening, in which the rapid development of high-throughput and single-cell sequencing has played a great add-on role. Understanding the dynamic evolution mechanisms of tumor is critical to cancer prevention, diagnosis, prognosis stratification, drug resistance identification, and the development of novel therapeutic strategies.
(3) Stem cell aging
Stem cells, a class of cells capable of self-renewal and differentiation, play a critical role in maintaining the structure and function of tissues and organs, and coping with damage repair. However, during aging, the number, function, or regenerative capacity of stem cells is undermined, leading to a faster degeneration than the regeneration in tissue dynamics. Eventually, the exhaustion of stem cells results in the loss of tissue homeostasis and the occurrence of a series of aging- related diseases, including neurodegeneration, hematopoietic and immune dysfunction, declined reproductive capacity, decreased muscle mass, rarefaction of bone, and pulmonary fibrosis. Therefore, unveiling the molecular mechanisms of stem cell aging and deciphering the critical intrinsic and extrinsic regulatory factors of stem cells will facilitate the development of new methods enhancing stem cell homeostasis and function, thus providing novel strategies to alleviate aging-related diseases and extend healthspan.
Over the past decades, a series of breakthroughs have been achieved in the field of stem cell research, including the identification of different types of adult stem cells, the elucidation of the molecular mechanisms of functional regulation, and the maintenance of certain stem cell states via small molecule-based strategies. However, many challenges remain in the field of stem cell aging research. For instance, researchers have yet to determine how to develop novel models of stem cell aging, how to systematically investigate the mechanisms of stem cell aging, and how to establish novel stem cell-based strategies for the interventions against aging and aging-related diseases. Adult stem cells in different tissues are heterogeneous with regard to molecular characteristics, regulatory networks, and microenvironments. Therefore, new models and technologies are demanded for stem cell aging research. In addition, the use of multidisciplinary approaches to systematically investigate the mechanisms of stem cell aging at multiple dimensions could facilitate the regenerative strategies for the reactivation of senescent stem cells and thus the reconstruction of the homeostasis and function of aged tissues and organs. These advancements can also provide a basis for the development of interventions for stem cell rejuvenation and treatments for aging-related diseases.
(4) Design and precise prediction of protein folding structure
The design and precise prediction of protein folding structure are based on artificial intelligence (AI) methods for predicting the 3D structure and designing skeleton and sequence of protein based on multiple sequence alignment and deep learning for a specific biological function. Protein plays a key role in life and its function is determined by the 3D structure based on the paradigm of sequence-structure- function. Researchers have used X-ray to measure the 3D structure of protein in the 1950s. Until now, X-ray, Neclear magnetic resonance (NMR), and cryo electron microscopy (cryoEM) remain as the main technologies for protein structure analysis. Protein Data Bank collected these 3D structures and constructed an integrate database, including approximately 194 000 released experimental protein 3D structures. However, a huge gap exists between released 3D protein structure and sequence. To improve this issue, scientists have constructed different types of computer models to predict the folding of protein in the 1970s. In 1994, John Moult began to organize the Critical Assessment of Protein Structure Prediction for once every 2 years. However, the GDT score of protein structure is less than 60 in the beginning. Until 2020, AlphaFold2 has a break for this competition, in which the average GDT score is higher than 92, and it can solve the protein folding for the 50-year-old grand challenge in biology. The rapid development of protein folding structure prediction also promotes the iterative optimization of protein design. The skeleton design based on hallucination and inpainting and the automatic generation of sequence have improved the precision of protein design. Currently, the main challenge of protein folding structure prediction and design lays in how to effectively improve the prediction precise for disordered regions, because the pLGDT score of AlphaFold2 is approximately 50 for these regions with low confidence. Therefore, AI combined with the simulation of precise force field might be used to improve the prediction precise and realize end-to-end information return. Moreover, researchers have yet to determine how to improve the performance of prediction for protein folding structure with less homologous sequences. Furthermore, the prediction of protein complex needs to be improved. For the protein design, dynamic conformation should be included the skeleton design of protein, because the dynamics conformation has strong relationship with the function. Finally, the coverage of sequence space should be increased for the automatic generation for sequence. Accordingly, iterative optimization can be used for the precise prediction and design of protein folding structure by using more effective AI architecture combined with sequence and structure big data with greater computational power. In recent years, many practitioners in China have already played instrumental roles in the field of protein structure prediction and design, especially in the area of the methodology for protein design. Nevertheless, most commonly used research platforms and solutions of protein structure prediction still follow the previous works. In these frontier fields of signal transduction, disease diagnosis, and virtual drug screening, the application of protein folding structure prediction and design for China remains at the initial stage.
(5) Artificial intelligence in drug design
Artificial intelligence (AI) has become one of the strategic frontiers in the field of pharmaceutical science and technology, and as an emerging technology, it has also been gradually applied to drug discovery and design. At present, AI-assisted drug design is still in its infancy, and many bottlenecks are encountered as follows:
1) The sources and types of drug research and development (R&D) data urgently need to be broadened, and the quality needs to be improved. AI-based drug design is based on data, from which trends are discovered, emphasizing the need for high-quality labeled data. Open access data of drug development are limited at this stage, and most of them involve in vitro test data of compounds in the drug discovery stage. Drug R&D data involves enterprise confidentiality and subject privacy with low degree of sharing and no standardization. In small-molecule targeted drug design, the amount of biological activity data for a given target is small, especially negative and novel target data, while the quality of artificial experimental data varies greatly.
2) AI models should fully consider the intracorporal process of drugs and the biological characteristics of their targets. Most of the existing AI models that are used in drug design only start from the perspective of compound structure, and they do not fully consider the metabolism and transformation of drugs in the body and the biological characteristics of the targets, including three-dimensional or stereoscopic characteristics of the drug-target interaction, induced fit effect, physiological environment, and off-target effect. The accuracy of model prediction is not ideal enough.
3) Molecular generative models lack standardized evaluation methods, and many aspects need to be improved in molecular collaborative optimization. Technically, the molecular generative model based on AI expands the chemical space of the designed molecule, considers the synthesizability of the molecule, and realizes collaborative optimization. However, most of the small molecules designed by existing AI models only target one or two properties of the molecule. The molecules designed to consider the biological activity, synthesizability, structural diversity, and druggability often have problems such as difficult synthesis, great toxicity, and poor druggability. The operability of the prediction results is poor, and the experimental verification is difficult. Therefore theoretical research and practice have no closed loop.
4) Solving several key scientific problems in the field of drug design is also key to the successful application of AI. At present, considering the limitation of model accuracy, AI- aided design of molecules has several possibilities, which require further prediction and screening by traditional computer-aided drug design techniques. Therefore, the prediction of protein-ligand interaction patterns and affinity is still the key point.
Technical advice on the above key scientific issues or difficulties are as follows. ① Strengthen the mining and modeling of data in the clinical research stage of drugs, realize the utilization and model sharing of confidential data of enterprises through federated learning (“data available and invisible”), and make full use of limited data through data enhancement, thereby establishing an AI model that predicts the multidimensional nature of drugs (including the fate of clinical research). ② Develop computational methods to characterize the biological characteristics of drug targets (e.g., three-dimensional stereoscopic and induced fit), study the fusion with compound structure information, and provide data-based AI models physical and biological significance. ③ Develop the evaluation system of molecular generative models, quantitatively compare the efficiency and performance of different models, select the optimal molecular generation framework or model, carry out molecular design by using high-precision activity/property prediction model as a scoring function, closely combine with organic synthesis and biological activity evaluation to achieve a closed loop between theory and practice, and improve model performance through experimental data feedback. ④ Develop the scoring function of high-precision protein-ligand affinity, improve the speed of combined free energy calculation, achieve a balance between precision and speed, and solve key scientific problems in the field of traditional drug design. Thus, new AI technologies and platforms specifically applied to drug design should be developed to improve the efficiency of new drug R&D.
(6) Bio-macromolecular phase separation and phase transition
Interactions between multivalent binding partners, where each partner has multiple binding sites of its counterpart, have intrinsic propensities to undergo liquid–liquid phase separation (LLPS), resulting in a dilute solution phase and a macromolecule-enriched condensed phase. The biological significance of LLPS is exemplified by the numerous membrane-less organelles in cells. The connection between LLPS and membrane-less organelles was revealed in seven seminal studies approximately 10 years ago. In 2009, Tony Hyman and coworkers from Max Planck Institute studied the physical state of P-granules, a class of membrane-less organelles in Caenorhabditis elegans embryos, and reported that P-granules were assembled via LLPS. In 2012, Michael Rosen and Steve McKnight from the University of Texas Southwestern Medical Center separately reconstituted condensates derived from phase separation by using simple biochemical systems. The Rosen study explicitly revealed that multivalent interactions drive LLPS, and the McKnight study mainly delt with a special type of condensates, hydrogels, which are often solidified from liquid condensates through a liquid-solid phase transition process. In the subsequent years, scientists worldwide have employed the concept of phase separation/transition to rationalize numerous biochemical phenomena.
As a newly appreciated organization principle in cells, phase separation actually plays essential roles in various biological processes and aggregate-associated diseases. Consequently, biomolecular condensates become a fronter biomedical research area. LLPS is a major driving force for the assembly of membrane-less organelles in cells, and this process is involved in important biological processes, such as gene expression regulation, signal transduction, maintenance of cellular structures, cellular homeostasis, stress-response, cell-fate determination, and cell proliferation. Notably, phase separation is an integral aspect of cellular functions. The relationships between aberrant phase separation and diseases are a cutting-edge question in the field of biomolecular phase separation. Generally, aberrant phase separation may play essential roles in neurodegenerative diseases and many types of cancers. Hence, phase separation provides a new angle that provides a better understanding of pathological mechanism and novel therapies. For instance, the field is actively engaged in screening for small molecule drugs that can reverse aberrant phase separation and evaluating their therapeutical potentials. In parallel, new LLPS-based techniques are actively being devised for biomedical research.
Nowadays, the concept of phase separation enhances various biomedical research fields. Chinese scientists have been a series of breakthroughs in the field, and many are highly recognized worldwide. However, while scientists are enthusiastic in studying phase separation, many research efforts are limited to simply categorize more phase separation phenomena or employ phase separation to explain some unexplained biochemical phenomena. Studies have not fully determined the underlying mechanisms, such as the mechanism in which cells precisely control the assembly and disassembly of biomolecular condensates, how multiple types of membrane-less organelles maintain their independent identity while fulfilling functional cooperativity, the mechanism in which membrane-bound membrane organelles and membrane-less organelles interact and the specific biological functions that the two kinds of organelles jointly control, to the method to carry out phase separation studies in vivo, and whether small molecule drugs precisely and specifically control (aberrant) phase separation. While studying bio-macromolecular phase separation is challenging, it offers opportunities in advancing many biomedical research fields. The improved understanding of phase separation in biology will allow its full utilization in substantiating both basic research and translational studies and enabling novel modalities in precise medicine.
(7) Mechanisms of genome regulation
The fine-tuned spatiotemporal regulation of gene expression forms the molecular basis of cellular structural and functional diversity. With the rapid development of genome sequencing technology, the focus of genomics research has shifted from the analysis of the linear sequence of DNA to the study of genome structure, function, and regulatory mechanism. The mechanistic research on genome regulation, which focuses on the non-coding genomic DNA, studies the composition and structure of gene regulatory elements from the perspectives of epigenetic modifications, chromosome state and three- dimensional conformation, and non-coding RNA, analyzes their dynamics in three-dimensional nuclear space, construct the regulatory network between regulatory elements and genes, and dissect the mechanisms of gene regulatory elements on cell-specific gene expression. The current strategies used in genome regulation research include the construction of chromatin epigenetic modification and three- dimensional structure map through multi-omics methods under different physiological and pathological conditions, the use of multi-omics data and computational biology to predict cell type-specific gene regulatory elements and their targets genes, and the use of gene editing technologies such as CRISPR to verify the function of gene regulatory elements in cells and model organisms. The mechanistic study of genome regulation is the foundation for understanding the complexity of cell diversity and phenotype, and it is mainly used to interpret the pathogenesis of non-coding variants of human diseases from the perspective of gene expression regulation.
Several critical scientific questions need to be addressed in future as follows: ① cell-type-specific gene regulation mechanisms and models, which could be mainly addressed using single-cell multi-omics and spatial-omics to establish the cellular holographic atlas of chromatin epigenomes, transcriptomes, and cellular phenotype information, and identify gene regulatory elements and network prediction models by integrating multi-omics data; ② functional annotation of genomic regulatory elements, which could be mainly addressed by developing high-throughput, multi-scale new technologies and systems to determine the functions of gene regulatory elements, and systematically building a functional database with spatiotemporal and cell-type information; ③ the key regulatory mechanism of the genome, which could be mainly addressed using genetic perturbation, high-resolution live-cell imaging, and phase separation to analyze the interactions between non-coding regulatory elements and genes, analyze the regulatory factors and complexes involved in the interaction, and establish several general models of genome regulation; ④ the pathogenic mechanism of non-coding variants, which could be mainly addressed by integrating GWAS and other population genetic data and the above-mentioned gene regulation databases to establish disease-type-specific non-coding biomarkers and reveal the initiation and development mechanisms of complex diseases.
In recent years, many large international cooperative organizations, such as DNA Elements Encyclopedia (ENCODE) and 4D Nucleome, have achieved substantial groundbreaking work in the study of genome regulation mechanisms by systematically producing massive multi-omics data and developing new technologies and data analysis pipelines. Chinese scientists have also made many important achievements in the field of genome regulation research, especially in analyzing the dynamics of the genome regulation process in early mammalian embryonic development and establishing large-scale disease biobanks and databases. In the future, Chinese scientists need more effort to develop original and cutting-edge genomics technologies and systems, establish cooperative and multi-institutional platforms and organizations, and build large-scale genomic databases and standards for the Chinese population.
(8) Mechanism of neoantigen generation and its role in tumor immunity
Neoantigens originate from somatic mutations in tumor cells, which, if occurring in the protein encoding region, will cause the protein to undergo corresponding amino acid mutations. If the mutated amino acid is located on a peptide fragment that can be presented by MHC, it will be presented to the cell surface, and then recognized as “non-self” by T cells, triggering T cells to attack. It is the natural target for T cells to recognize tumor cells. Neoantigens are different from tumor-associated antigens. The amino acid sequence of tumor-associated antigens is not mutated, but the expression level is increased. They are not considered “non-self” by immune cells for the principle of central immune tolerance, and they do not produce an immune response. Considering that somatic mutations occur randomly, neoantigens have individual characteristics, and neoantigens are difficult to analyze with the use of previous experimental techniques at the individual level. Until the cost of gene sequencing has been greatly reduced, humans have the opportunity to perform comparative gene sequencing on cells from different sources of individuals, analyze the somatic mutations of tumor cells at the omic level, and determine corresponding neoantigens. In 2010, Nature first reported the whole genome comparative sequencing results of individual tumor tissues and control tissues of patients. The detected somatic mutations were more than 20 000 for lung cancer cells and more than 30 000 for melanoma cells. In 2017, Nature took the lead in reporting the first individualized cancer vaccine, which was designed from the profile of neoantigens analyzed from the somatic mutation detected from a melanoma patient. Specific immune response and actual efficacy were observed, resulting in a new research field.
Tens of thousands of sequencing results are available in the international tumor gene sequencing databases, such as The Cancer Genome Atlas (TCGA) and ICGC. Statistics show that the vast majority of somatic mutations are individual characteristics. Besides, only a few tumor-driven mutations, such as Kras G12V, BRAF V600E, IDH1 R132H, and PIK3CA H1047R, have a certain distribution in patients. Different individuals have different tumor somatic mutation profiles and different tumor neoantigen profiles. Therefore, therapeutic drugs need to be customized for each patient. This kind of precision medical technology, which is specially designed to customize a drug for a patient, has begun to take shape.
The somatic mutations of tumor cells can be detected using genomic analysis software and the gene sequencing results of the patient’s tumor tissue and self-control sample. The mutation of amino acid in the corresponding protein can be analyzed according to the Central Dogma of Biology. This part of bioinformatics technology is relatively easy, and the analysis software is quite mature. Whether the mutated peptide will be presented by the MHC is the key to determining whether the somatic mutation has a corresponding neoantigen. Many MHC gene polymorphisms occur in the general population, causing individuals to have different MHC genotypes, which will present different peptides. Only those mutant peptides resulted from somatic mutations have sufficient affinity with their own MHC molecules can become a neoantigen. Furthermore, whether the neoantigen can activate T cell immunity and cause enough tumor cell killing effect results in the difficulty in further designing neoantigen vaccine. The establishment and maturity of prediction technology not only depends on the accumulation of a large number of experimental data, but also requires the progress of artificial intelligence technology, especially the understanding of the interaction of MHC, neoantigens and TCR in the spatial structure. Considering that neoantigens important for tumor treatment and participate in the occurrence and development of refractory diseases such as diabetes, atherosclerosis, and Alzheimer’s disease, the AI prediction of neoantigens has become a new race track for researchers at home and abroad. Bioinformatics and artificial intelligence technology have shown unprecedented importance in medicine.
Chinese researchers and foreign scholars only have a little gap in this field. In particular, it has many advantages in AI analysis technology in China. Many achievements have been obtained in transformation research, and the neoantigen- loaded T cell therapy technology and DC vaccine have been approved by the national CDE for clinical trials. Neoantigen peptide vaccine, neoantigen mRNA vaccine, and neoantigen DNA vaccine are all under rapid research. It can be expected that neoantigens will change the passive situation of tumor and other refractory diseases.
(9) 3D printing and organ regeneration
The reconstruction of artificial organs with normal physiological functions in vitro, which is the cutting-edge technology in the biological manufacturing field, is critically important for the repair or transplantation of diseased organs. Biological 3D printing technologies are developed to control the three-dimensional controllable assembly of cells in space and time to construct multicellular functional bodies with biological activities. During this process, a diverse of biological ink containing cells and biological materials as the basic building units has been developed as the printing raw material. Thus, biological 3D printing is a new discipline that emerged from the cross-interaction of many fields, such as life science, materials science, engineering, and informatics, which can provide new technical means and opportunities for the development of regenerative medicine, advanced medical devices, and other biotechnological industries in the 21st century.
Human artificial organs have multi-type cell composition in multi-scale and multi-dimension assembly, and the complex interactions among inter-cells and between cells and the extracellular matrix microenvironment finally endow the artificial organs with certain physiological functions. How to simulate the three-dimensional spatial composition of cells and the complex multicellular interactions is the key to determine whether artificial organs can be accurately constructed. This research gap poses of the following challenges to bio-3D printing: ① diverse type of cells are available; ② bio-inks with excellent biocompatibility, printability, cultivability, and matching the physicochemical properties of extracellular matrix need to be synthesized; ③ it must have the ability to print multiple types of cells with high precision at high survival rate and accurately control their spatial distribution at both macro and micro scales; ④ the nutrient supply and metabolism circulation of organs need to be ensured at different scales for long-term culture; ⑤ the printed artificial organs must be able to produce part or all of the functions of real human organs under external induction or spontaneous action.
Currently, many advances have been made in 3D printing of artificial tissues and organs. For some tissues with simple composition, including skin, cartilage, and bones, their manufacture and clinical translation applications have been achieved. For complex organs such as heart, liver, and kidney, although artificial organs can be printed with similar shapes, their functions are still far from those of real human organs because of the limited cell types, low printing accuracy, and mismatched macro and microstructures. The artificial organ printing in the future will focus on the transformation from hypotaxis (shape-like) to parataxis (function-like). Specifically, 3D printing technology will be developed from low precision to high precision and even to the single-cell precision of biological composition. The composition and function of organs will vary from a single cell with a single function to multi-cells with a multi-functional synergy. The organ scale will range from micro-sized and shaped-like organs to macro- sized organs with real physiological function.
Many domestic universities and research institutions have begun the research on bio-3D printing and artificial organ manufacturing for a long time. Some achievements are in the forefront of the world, but a big gap remains from the world’s advanced level. Core bio-3D printing technologies and equipment mainly mimic those of US and Europe counterparts. Artificial organs are mainly based on follow-up research. The advanced level of bio-3D printing technology as a key factor determines the functionality of the manufactured artificial organs. Thus, to catch up with the world’s advanced levels, institutions should focus on the R&D of advanced biological 3D printing technology and functional printing materials, study the structure and function of organs, encourage the cross-cooperation of clinical, basic, and engineering sciences. The fabrication a few of the important artificial organs should be focused on to promote the development of organ printing and regeneration in China.
(10) AI-assisted disease diagnosis and treatment system
The AI-assisted disease diagnosis and treatment system often uses novel AI technologies such as deep learning to mine high-dimensional quantitative information from medical big data such as medical images, pathologic images, and multi- omics data. The extracted information could reflect changes at the molecular and cellular levels of diseases and could thus be used for disease screening, diagnosis, and prediction of treatment response. The common AI-assisted disease diagnosis and treatment methods include feature engineering- based radiomics and end-to-end deep learning. Radiomics extracts many handcrafted features from multimodal medical imaging data, selects key features related to clinical tasks, and builds a machine learning model to assist in the diagnosis and treatment. Deep learning uses deep neural networks for feature extraction, model construction, and prediction in an end-to-end manner. Its representative methods include convolutional neural network and transformer. Methods that integrate radiomics and deep learning have also received attention.
Many review papers published in the top medical journals CA: A Cancer Journal for Clinicians and Nature Medicine show that AI has formed some typical clinical application cases in assisting with disease diagnosis and treatment. In some clinical tasks, the performance of AI can reach or even exceed the judgment of clinicians. Some AI technologies have also been adopted into domestic and foreign clinical guidelines. AI is mainly applied in tumors, cardiovascular and cerebrovascular diseases, and other diseases. In terms of tumors, a series of studies have used AI technology to mine high-dimensional quantitative features in tumor macroscopic images, correlate microscopic pathological and genetic information, and then assist in tasks such as early screening, classification and staging, and prediction of treatment response. AI has achieved remarkable results in lung nodule screening, prediction of EGFR mutation in lung cancer, prediction of occult peritoneal metastasis in gastric cancer, diagnosis of microvascular invasion in liver cancer, pathological classification of brain tumor, and evaluation of immunotherapy response in lung cancer. In addition, the combination of medical image and pathological image has been applied to predict the treatment response of neoadjuvant chemotherapy in colorectal cancer and the outcome of radio-chemotherapy in nasopharyngeal cancer. In terms of cardiovascular and cerebrovascular diseases, researchers focus on the vascular plaque, such as the diagnosis of coronary plaque composition, prediction of fractional flow reserve, and judgment of carotid artery stenosis. AI has also been applied in liver fibrosis staging, prediction of bone age in children, first-trimester screening for trisomy 21, and diagnosis of COVID-19. The above-mentioned applications show that AI could provide an effective auxiliary means for reducing the workload of clinicians and improving the efficiency and effectiveness of disease diagnosis and treatment.
The development of AI-assisted disease diagnosis and treatment products has received great attention. The USA, the European Union, and other countries and regions have approved several AI-based medical products. In 2022, the Medical Device Technology Evaluation Center of the China National Medical Products Administration (NMPA) issued the Guidelines for the Registration and Review of Artificial Intelligence Medical Devices and actively promoted the approval of AI medical products. As of August 2022, nearly 50
AI medical products have been issued Class III registration certificates in China, promoting the clinical applications of AI.
In summary, AI-assisted disease diagnosis and treatment systems have shown a flourishing development trend in the world and have received extensive clinical attention. In the future, the AI-assisted disease diagnosis and treatment system will be developed in the direction of standardization. By overcoming the influence of different equipment and acquisition parameters, the accuracy and generalization of AI systems will be greatly improved, and AI will ultimately benefit patients.
《1.2 Interpretations for three key engineering research fronts》
1.2 Interpretations for three key engineering research fronts
1.2.1 Immune heterogeneity and intervention strategies of solid tumors
Tumor immunotherapy has marked a major milestone in the history of cancer clinical treatment, but its efficacy remains considerably limited because of various factors. For instance, the tumor immune heterogeneity (TIH) has received the most attention of scientists. In TIH, in the process of tumorigenesis, with the continuous progression, evolution, and selection of tumor cells, the anti-tumor immunity develops from immune clearance and balance to immune escape, which is manifested as the heterogeneity of the constitution, phenotype, and function of immune cell subsets involved in anti-tumor immunity. In the process of tumor metastasis, considering the metastatic selectivity of the tumor cells (such as epithelial- mesenchymal cells and tumor stem cells) and their tendency to metastasize to organs, as well as the immune heterogeneity (IH) of the metastatic organs, the IH of primary tumor and its metastases and even different metastatic organs is formed. During tumor treatment, considering the selective killing of tumor cells, the effect of therapeutic agents on immune cells, and the remodeling of tumor microenvironment, IH manifests as temporal heterogeneity. Thus, IH is not only manifested in different tumor types, different populations of the same tumor type, and patients with different molecular typing, but also in different metastases of the same patient and different regions within the same tumor and different stages of tumor progression and treatment processes. Therefore, the temporal and spatial heterogeneity of tumor immunity should be fully determined for the development of new immunotherapeutic strategies, the discovery of novel biomarkers, the realization of precision immunotherapy, and the development of strategies to overcome immunotherapy resistance.
TIH originates mainly from genetic instability, differences in epigenetic modifications, fitness of the microenvironmental perturbations, and response to antitumoral therapies. Under the influence of these factors, tumor immunity exhibits two dimensions of heterogeneity, namely, spatial heterogeneity (SH) and temporal heterogeneity (TH). Both SH and TH are primarily determined by tumor cells and tumor microenvironment. Spatial and temporal differences in their localization, abundance, or activity, including the expression of immune checkpoints, secretion of immunosuppressive and proinflammatory cytokines, infiltration of immunosuppressive or effector cells, state of the vasculature, and distribution of metabolic nutrients, collectively determine TIH, thus influencing the clinical prognosis and treatment response of patients.
IH remarkably affect the diagnosis and treatment of cancer. In terms of its effect on biomarkers for tumor immunotherapy, the expression level of PD-1L has been widely used as a clinical indicator for the prediction of treatment response to the immune checkpoint inhibitors (ICIs) in various types of solid tumors. However, PD-1L expression displays distinct heterogeneity at intra- and intertumoral levels in both spatial and temporal dimensions, resulting in limited efficacy of PD-1L as a biomarker to predict the effectiveness of immunotherapy. Tumor mutation burden (TMB), a reasonable approximate substitute for neoantigen burden, has been used to identify populations with potential benefit from ICIs immunotherapy in various solid tumors. Nevertheless, the response of patients with high TMB to ICI immunotherapy is highly heterogeneous, and a large proportion of patients with low TMB levels can benefit from ICI immunotherapy and vice versa. In terms of cancer treatment effect, different metastatic sites of the same tumor have different responses to immunotherapy. Bone and liver metastases are resistant, while lymph node metastases are sensitive to immunotherapy. On the other hand, different conventional cancer treatment approaches can cause dynamic changes in the tumor immune microenvironment (TIME), leading to favorable or unfavorable effects on tumor therapy. Hence, TIH is a key bottleneck for the development of immunotherapeutic biomarkers and the realization of precision immunotherapy.
At present, the diagnosis and treatment strategies for IH are mainly established from the following aspects:
1) Establishment and improvement of the diagnostic techniques for IH. Currently, non-invasive diagnostic techniques such as liquid biopsy can be used to dynamically assess TIH, including the detection of circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and interstitial fluid and the evaluation of tumor progression and ICI efficacy. Considering the spatiotemporal characteristics of IH, the application of existing technologies such as single- cell sequencing and spatial transcriptome sequencing will obtain huge data. These data need to be combined with the advantages of artificial intelligence machine learning, data computing, and image recognition to rapidly and systematically evaluate the TIME of patients and assist in pre- clinical research and clinical treatment.
2) Development of new models for IH research. Given that the IH of solid tumors is mainly exhibited as TH and SH, its research model selection is important. At present, the research is mainly carried out in mice with high homology to humans, and the changes of TIME in humans can be analogized by studying the TIME of mouse models, including transgenic mice, drug-induced tumor model, and cell line- derived xenograft. The use of organoid model has been able to screen drugs that are sensitive to tumor cells and achieve individualized treatment to a certain extent. However, the maintenance of IME in vivo on organoids still faces challenges caused by the close connection between the whole body and the cells/molecules of TIME. The dilemma of using humanized animal models to study TIH can be attributed in the donor immune system and the immune rejection of the transplanted tumor, which largely limit the use of this model in TIME study. In the future, in vitro organoid models, which can reflect the TIME in vivo, need to be developed.
3) Exploration of therapeutic strategies targeting immune heterogeneity. ① Development of adoptive cell therapy targeting neoantigens. Under the guidance of precision medicine concept, the discovery of individualized tumor neoantigens and design of corresponding antigen-specific T cells for adoptive therapy are among the practical solutions for targeting TIH. The discovery of neoantigens relies on the clinical application and individualized analysis of transcriptomics, proteomics, and metabolomics. Considering the discovery of specific neoantigens of individual patient and the high-cost and long-cycle of the autologous neoantigen- specific T cell preparation, the adoptive T cell therapy (ACT), which is established using neoantigens, is still in a case-by- case stage. Alternately, ACT targeting shared neoantigens have been tested in clinical trials, but the adoptive T cell depletion remains unsolved. In the future, clinical trials of ACT with multiple antigen targets and individualized neoantigen-specific ACT will be carried out. ② Enhancement of immunogenicity by the induction of epitope spreading and antigenic drift. TIH occurs, because the immune surveillance of the body eliminates the tumor cells with dominant antigenic epitopes, while the tumor cells with cryptic epitopes survive and progress. Cytotoxic agents, photodynamic therapy, irradiation, thermal ablation or freezing, and oncolytic viruses can be used to induce the immunogenic death of tumor cells, promote epitope spreading, especially the cryptic epitopes, and increase antigenic drift for strengthening the immune response against tumors for the moment and years ahead. ③ Overcoming IH by use of combination therapy. The application of ICIs is a breakthrough in cancer treatment. Although some patients can benefit from the therapy for their survival, the emergence of drug resistance promotes the occurrence of IH to a certain extent. Immunotherapy combined with strategies targeting other causes of IH, as a multi-pronged approach, is helpful to overcome IH. Numerous clinical trials of combination therapy are being carried out, including conventional treatment such as combined chemotherapy, radiotherapy, and targeted therapy. The combination of metabolic target drugs, costimulatory molecular agonists, tumor vaccines, ACT, and dual ICIs can also be used. In the future, breakthroughs will be made in the integration of multi-target therapeutic agents, such as immune checkpoint antibody-drug conjugates, bispecific T cell engagers (BiTEs), and immunotherapy combined with intestinal microbiota transplantation.
The analysis of the formation mechanism of TIH and development of strategies for cancer diagnosis and treatment are the challenges existed in pre-clinical research and clinical treatment on tumor immunity. The precise treatment of cancer coincides with the specific characteristic of immunology, and the design of individualized immunotherapy regimens for patients based on TIH features may become a reality. This research depends on the development of new technologies. First, the technology for the rapid and systematic monitoring of TIME variations needs to be further improved to help in the early monitoring of the efficacy of basic research and clinical trials. Second, design concepts for new therapeutic agents also need to be updated, such as the design of prodrugs of immunotoxic molecules and development of dual-target antibodies and cells by applying the characteristics of IH. Lastly, the identification and application of individualized tumor neoantigens and cell populations and the use of novel individualized tumor vaccines and oncolytic drugs are expected to improve the efficacy of immunotherapy.
Currently, in the front of “immune heterogeneity and intervention strategies of solid tumors”, the top three countries with core papers published are the USA, the UK, and China. Among them, China accounts for 14.50% of the published papers, making it one of the major countries in research of this front (Table 1.2.1). From the perspective of the cooperation network among main countries (Figure 1.2.1), the Top 10 countries have highly close cooperation relations.
The Top 10 institutions with core papers published in “immune heterogeneity and intervention strategies of solid tumors” were from the USA, France, and China. The top three institutions, including Harvard University, Dana-Farber Cancer Institute, and University of Texas MD Anderson Cancer Center (Table 1.2.2), were from the USA, while Chinese Academy of Sciences ranks ninth. According to the cooperation network among the main institutions (Figure 1.2.2), scientific research institutions in the USA have strong cooperation, and some cooperation is perceived among other institutions.
《Table 1.2.1》
Table 1.2.1 Countries with the greatest output of core papers on “immune heterogeneity and intervention strategies of solid tumors”
| No. | Country | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | USA | 489 | 51.75 | 84 966 | 173.75 | 2017.8 |
| 2 | UK | 142 | 15.03 | 23 480 | 165.35 | 2018 |
| 3 | China | 137 | 14.5 | 17 443 | 127.32 | 2018.6 |
| 4 | Germany | 99 | 10.48 | 19 621 | 198.19 | 2017.8 |
| 5 | France | 90 | 9.52 | 17 400 | 193.33 | 2018 |
| 6 | Italy | 89 | 9.42 | 16 431 | 184.62 | 2017.8 |
| 7 | Spain | 57 | 6.03 | 13 972 | 245.12 | 2017.7 |
| 8 | Netherlands | 51 | 5.4 | 10 578 | 207.41 | 2017.9 |
| 9 | Canada | 49 | 5.19 | 9 487 | 193.61 | 2017.7 |
| 10 | Australia | 44 | 4.66 | 8 867 | 201.52 | 2017.7 |
《Figure 1.2.1》
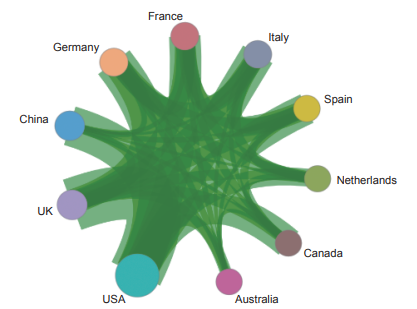
Figure 1.2.1 Collaboration network among major countries in the engineering research front of “immune heterogeneity and intervention strategies of solid tumors”
《Table 1.2.2》
Table 1.2.2 Institutions with the greatest output of core papers on “immune heterogeneity and intervention strategies of solid tumors”
| No. | Institution | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | Harvard University | 83 | 8.78 | 18002 | 216.89 | 2017.6 |
| 2 | Dana-Farber Cancer Institute | 51 | 5.4 | 11641 | 228.25 | 2017.6 |
| 3 | University of Texas MD Anderson Cancer Center | 48 | 5.08 | 11923 | 248.4 | 2018.1 |
| 4 | Memorial Sloan Kettering Cancer Center | 48 | 5.08 | 10668 | 222.25 | 2017.4 |
| 5 | Johns Hopkins University | 31 | 3.28 | 7090 | 228.71 | 2018.1 |
| 6 | Stanford University | 28 | 2.96 | 6144 | 219.43 | 2018.1 |
| 7 | Brigham and Womens Hospital | 27 | 2.86 | 5425 | 200.93 | 2017.1 |
| 8 | Institut National de la Santé et de la Recherche Médicale | 24 | 2.54 | 5876 | 244.83 | 2017.3 |
| 9 | Chinese Academy of Sciences | 22 | 2.33 | 2487 | 113.05 | 2018.6 |
| 10 | Weill Cornell Medical College | 21 | 2.22 | 5998 | 285.62 | 2017.7 |
《Figure 1.2.2》
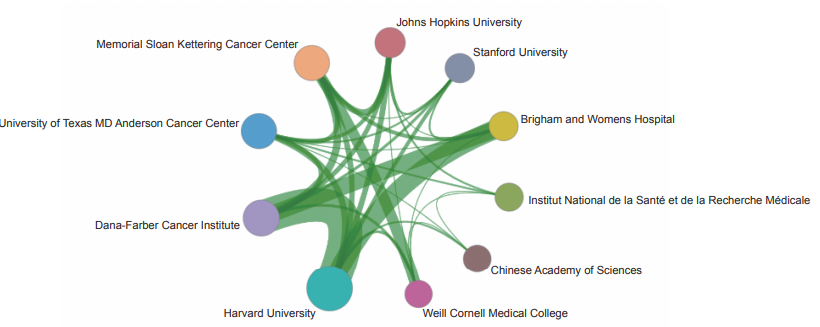
Figure 1.2.2 Collaboration network among major institutions in the engineering research front of “immune heterogeneity and intervention strategies of solid tumors”
The immunological heterogeneity is universally presented spatially or varies temporally along with tumor evolution or therapeutic intervention across almost all solid tumors. The heterogeneity of anti-tumor immunity shows a profound association with the progression of disease and responsiveness to treatment, particularly in the realm of immunotherapy. Therefore, an accurate understanding of tumor immunological heterogeneity is essential for the development of effective therapies. Facilitated by multi-regional and -omics sequencing, single cell sequencing, longitudinal liquid biopsy and organoid technologies, recent studies have demonstrated the potential to investigate the complexity of immunological heterogeneity of the tumors and its clinical relevance in immunotherapy. Single-cell omics profiling has been transformative for the fields of immunology and immuno-oncology. The single- cell sequencing technologies have progressed with rapid development. With both technological advances and cellular throughput increasing exponentially, multiple layers of information including epigenomic, genomic, transcriptomic, and proteomic characteristics of individual cells and their combinations can be obtained at unprecedented resolution. Such high resolution is ideally suited to studying the properties of immune cells, which are well-known for their diverse developmental lineages, antigen specificities, phenotypic plasticity, and adaptability to various microenvironments. Organoids provide a new and reliable model system for the interaction of the immune system with tumor cells. Organoids currently provide the most accurate in vitro system for the culture of human epithelial cells of almost any organ and show great promise for both fundamental and translational research in the future. New technologies have been developed to explore mechanisms of tumor immune heterogeneity and facilitate the development of more effective personalized therapies. For details see the development roadmap (Figure 1.2.3).
Based on the above statistical analysis results, for the research front of “immune heterogeneity and intervention strategies of solid tumors”, China is follows the trend of similar studies abroad. Some suggestions are proposed for this frontier field as follows:
1) By using China’s quantitative advantage in clinical sample resources, a research platform for investigating TIME heterogeneity of solid tumors and a systematic tumor sample bioresource library and information database can be established. These platforms can be helpful for the analysis of the constitutive characteristics and evolution rules of TIME in human solid tumors, which further assist in establishing effective clinical intervention strategies, providing essential support for improving the treatment prognosis of patients with solid tumors, and promoting the sustainable development of society and economy.
2) The application of spatial omics technology in decoding TIH can be enhanced. Emerging technologies including spatial transcriptome, spatial proteome, spatial metabolome, spatial epigenome, and spatial multi-omics should be developed to explore the temporal-spatial heterogeneity of immunity, upon which, the composition of immune environment can be depicted at multiple levels, and the transcriptional regulation and intercellular communication in tumors may be analyzed. In addition, the development of computational strategies for the data mining of novel spatial omics help in determining SH at the intratumoral and intertumoral levels and even between individuals. By using dynamic biopsy tissue samples, the dynamic evolution mechanism of the immune spatial environment could be explored. Moreover, the analysis of the relationship between the TIME function and the formation of heterogeneity and related regulatory mechanism is important. Based on this, intervention targets that overcome IH may be discovered. Accordingly, new therapeutic strategies, novel clinically relevant biomarkers, and immunotherapy regimens may be developed, ultimately facilitating the immune spatial atlas to become a key resource for impelling the intervention strategies development.
3) Research on new models and technologies of IH ought to be deepened. The full combination of gene editing, library screening, organoid culture, and radiation-induced mutation and the development of in vitro and in vivo models that mimic the establishment, composition, and evolution of IH can guide the discovery of key molecules and characteristics associated with IH.
4) Actively carrying out clinical trials and encouraging multimodal combination therapy are recommended. By focusing on the critical molecules, cells, and signaling pathways occurred in the composition and evolution of TIH, the original principle and technology can be transformed into one or more stages of clinical intervention strategies. By conducting prospective clinical trials, promoting potential clinical application, and proving its effectiveness, clinical benefits, and risks, new technologies for clinical diagnosis and treatment may generate.
5) International cooperation should be strengthened to promote data sharing. Currently, international research
《Figure 1.2.3》
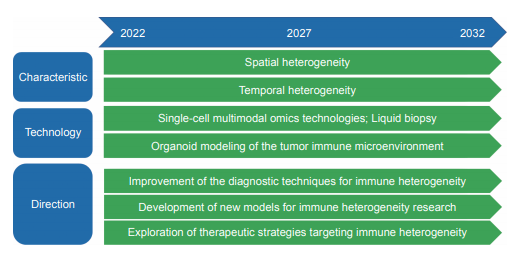
Figure 1.2.3 Roadmap of the engineering research front of “immune heterogeneity and intervention strategies of solid tumors”
institutions have certain advantages in TIH research theory, omics data production and accumulation, bioinformatics analysis methods, and clinical data integrity and system- atization. Exchanges and cooperation with leading academic institutions should be further strengthened to facilitate the establishment of an effective sharing mechanism for clinical and genetic data and promote the development of related research fields.
1.2.2 Mechanisms in tumor heterogeneity and evolutionary dynamics
In 1859, Charles Darwin first proposed the theory of evolution under natural selection in The Origin of Species, and in 1976, the American pathologist Peter Nowell introduced the theory of evolution into the field of cancer. Cancers evolve according to Darwinian rules, that is, mutation and selection of beneficial new mutations drive the expansion of subclones, and between and within selected clones, the cellular populations experience neutral evolution. From an evolutionary biology perspective, tumor is considered to be an evolving ecosystem.
For more than a decade, scientists have conducted research to reproduce the cellular structure, functional properties, and evolution in various cancer types. Tumors have complex ecosystems, in which they form and evolve under strong selective pressures from the microenvironment, including components such as nutrition, metabolism, immunity, and therapy. These pressures promote spatiotemporal diversity of malignant and non-malignant (i.e., endothelial, stromal, and immune) components in tumor niches, resulting in a specific degree of intra-tumoral heterogeneity (ITH) that can advance disease progression and confer resistance to therapy.
Multi-region genome-sequencing studies have revealed considerable variations in the genetic makeup of malignant cells across distinct anatomical locations and disease stages, as well as in distinct regions of the same tumor, and this process is known as spatial ITH. Longitudinal studies have also demonstrated that genetic features of the same lesion can substantially vary over time, which is known as temporal ITH. Importantly, ITH does not manifest exclusively at the genetic level, but it encompasses epigenetic, transcriptional, phenotypic, metabolic, and secretory components. Such components can vary independently from each other (e.g., genetically stable tumors with high epigenetic variability) or in a tightly interconnected manner (e.g., genetical and epigenetic alterations cooperating at defining transcriptomic and phenotypic profiles). Thus, the abundance, localization, and functional orientation of each cellular component of the TME evolves over space and time to dictate ITH. This spatiotemporal evolution is determined by the dynamic nature of its sources, including cancer-cell- intrinsic processes such as genetic instability and features of the TME. Mechanisms of clonal selection, cooperation, and competition operate in the context of multidirectional interactions between malignant cells and the other cellular compartments of the TME. However, the crucial features that define ITH and its spatiotemporal evolution are still largely uncharted.
The mechanisms of tumor dynamic heterogeneity are as follows:
1) Genetic heterogeneity. Genetic heterogeneity is crucial for cancer cell proliferation, invasion, and resistance to therapy, because it confers plasticity to evolving tumors. Thus, clonal diversity offers a fertile soil for tumor evolution, ultimately shaping genomic features such as oncogenic makeup and immunogenicity, and the tumor mutational burden (TMB) and karyotypic profile of the tumor.
2) Epigenetic heterogeneity. Cancer cells harness epigenetic aberrations to transition easily between cell states. Although these epigenetic changes generally reversible, they can be acquired by the cell progeny, thus influencing the clonal landscape of the tumor and its evolution.
3) Behavioral and immunological heterogeneity. Genetic and epigenetic processes determine the behavioral and immunological heterogeneity of cancer cells. Behavioral ITH influences rather ‘canonical’ processes involved in disease progression, such as proliferative properties and invasiveness, whereas immunological ITH revolves around antigenicity, adjuvanticity, and immuno-evasion.
4) Immune–stromal heterogeneity. As a multicellular ecosystem with complex cell composition, tumors such as immune and interstitial cells show considerable differences in species, number, state, and spatial distribution, which are important sources of tumor heterogeneity.
The key scientific issues that need to be addressed urgently in the research of tumor dynamic evolution mechanisms include: ① how to perform a more holistic analysis of tumors across time and space from isolated points to a complete timeline and overall picture; ② the role of epigenetics, transcriptomes, proteomes, metabolomes, and microenvironment in tumor evolution aside from genomics; the effect of tumor evolution on the tumor microenvironment and corresponding treatment; and the presence of other synergistic mechanisms; ③ differentiation methods for functional and nonfunctional ITH among the numerous variants detected; ④ identification method for different patterns and positive or negative selection events of the tumor evolution process; ⑤ optimization method for the sequencing and algorithm analysis and increasing the resolution and higher dimension data by using multi- omics technology; ⑥ classification technique for driver genes based on an in-depth understanding of evolutionary mechanisms; ⑦ the use of dynamic evolutionary mechanisms to serve the clinic, such as exploring more resistance mechanisms and novel treatment strategies; ⑧ how to improve the sensitivity of liquid biopsy for its application in the study of tumor dynamic evolution mechanisms, and to explore efficient body fluid biomarkers for different cancer types.
The development of high-throughput and single-cell sequencing technology has greatly promoted the study of tumor dynamic evolution mechanisms, and its main applications include:
1) Inferring clonal architecture. The integration of read depth and variant allele frequencies of somatic mutations in whole- genome sequencing data can be used to infer tumor purity, ploidy, and local copy number for each mutation and thus determine the cancer cell fractions (CCFs) that harbor the mutations and clonal architecture.
2) Depicting lineage landscape. Multi-sampling can define the dynamic evolutionary process of tumor clones. Considering the coordinated patterns of CCF fluctuations over time, multi-sampling at different time points can provide higher-resolution data. Analogous to temporal sampling, multiregional sampling in tumor helps in the evaluation of the spatial composition of clones within tumor and refining clonal relationships to improve clinical stratification.
3) Tracing genetic history in single cells. Despite the additional resolution that multi-sampling provides to the clonal deconstruction of cancer evolution, phylogeny needs to be resolved at single-cell resolution to derive the precise clonal dynamics and evolutionary history of a tumor.
4) Revealing the heterogeneity of cell states. Cell state plasticity, transcriptional state heterogeneity, and epigenetic plasticity can act as a mediator for cancer evolution and drive clonal evolution of tumors, and single-cell transcriptome and epigenetic sequencing as transformative technologies have an important applications in this field.
5) Defining the spatial dynamics of the tumor ecosystem. The spatial location of cancer cells and the resulting differential microenvironment interaction represent another dimension related to fitness. Spatial single-cell omics research has unique advantages in defining the spatial dynamics of tumor ecosystems and has become a rapidly developing frontier field.
Regarding the engineering research front related to “mechanisms in tumor heterogeneity and evolutionary dynamics”, the USA has the highest number of core papers, followed by China and the UK with average citations per paper ranging from 41.28 to 237.77 (Table 1.2.3). The citations per paper of China is 41.28, indicating a great room for improvement. Based on the cooperation network among main countries, close cooperation relationships are present between the Top 10 countries in terms of the number of core papers (Figure 1.2.4), indicating that “mechanisms in tumor heterogeneity and evolutionary dynamics” is the frontier direction of common concern in various countries.
The Top 10 institutions with the most published core papers on “mechanisms in tumor heterogeneity and evolutionary dynamics” are mainly from USA, China, and the UK. The institutions from the USA include Harvard University, Dana- Farber Cancer Institute, Memorial Sloan Kettering Cancer Center, Stanford University, and University of Texas MD Anderson Cancer Center. The institutions from China include Chinese Academy of Sciences and Peking University. The institutions from the UK include the University of Cambridge, Francis Crick Institute, and University College London (Table 1.2.4). According to the cooperation network among main institutions, a strong cooperation is present among scientific research institutions in the USA, and some cooperation is perceived among other institutions (Figure 1.2.5).
Tumor evolution encompasses a complex interplay of genetic, cell state, epigenetic, spatial and microenvironmental factors. Within the last decade, single-cell analysis has revolutionized our understanding of cellular processes and heterogeneity
《Table 1.2.3》
Table 1.2.3 Countries with the greatest output of core papers on “mechanisms in tumor heterogeneity and evolutionary dynamics”
| No. | Country | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | USA | 261 | 55.06 | 37 421 | 143.38 | 2018 |
| 2 | China | 148 | 31.22 | 6 109 | 41.28 | 2018.9 |
| 3 | UK | 78 | 16.46 | 15 234 | 195.31 | 2018.3 |
| 4 | Germany | 49 | 10.34 | 11 469 | 234.06 | 2018.1 |
| 5 | France | 42 | 8.86 | 3 228 | 76.86 | 2018 |
| 6 | Italy | 39 | 8.23 | 9 273 | 237.77 | 2017.6 |
| 7 | Australia | 39 | 8.23 | 5 685 | 145.77 | 2018.6 |
| 8 | Canada | 28 | 5.91 | 5 448 | 194.57 | 2018.3 |
| 9 | Spain | 25 | 5.27 | 4 468 | 178.72 | 2017.7 |
| 10 | Netherlands | 25 | 5.27 | 2 953 | 118.12 | 2018.5 |
《Figure 1.2.4》

Figure 1.2.4 Collaboration network among major countries in the engineering research front of “mechanisms in tumor heterogeneity and evolutionary dynamics”
《Table 1.2.4》
Table 1.2.4 Institutions with the greatest output of core papers on “mechanisms in tumor heterogeneity and evolutionary dynamics”
| No. | Institution | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | Harvard University | 54 | 11.39 | 11583 | 214.5 | 2017.8 |
| 2 | Chinese Academy of Sciences | 45 | 9.49 | 1629 | 36.2 | 2019 |
| 3 | Dana-Farber Cancer Institute | 33 | 6.96 | 8063 | 244.33 | 2017.6 |
| 4 | Memorial Sloan Kettering Cancer Center | 29 | 6.12 | 10341 | 356.59 | 2018.2 |
| 5 | University of Cambridge | 25 | 5.27 | 5391 | 215.64 | 2019 |
| 6 | Stanford University | 21 | 4.43 | 3474 | 165.43 | 2018.1 |
| 7 | Peking University | 21 | 4.43 | 1500 | 71.43 | 2018.8 |
| 8 | Francis Crick Institute | 20 | 4.22 | 6462 | 323.1 | 2018.5 |
| 9 | University of Texas MD Anderson Cancer Center | 18 | 3.8 | 6101 | 338.94 | 2018.2 |
| 10 | University College London | 18 | 3.8 | 5494 | 305.22 | 2018.6 |
across all disciplines of life science. Single-cell multimodal omics methods have greatly expanded our toolkit for delineating the complex molecular and cellular networks operating in diverse biological systems. High-throughput sequencing and novel multi-omics technologies have begun to integrate across these genetic and non-genetic determinants of tumor evolution at the critical resolution of the single cell— the fundamental evolutionary unit. These methods pave the way to address central questions on tumor evolutionary dynamics through the study of human tissue (Figure 1.2.6).
Based on the above statistical analysis results, for the research frontier on “mechanisms in tumor heterogeneity and evolutionary dynamics”, China follows the trend of similar studies abroad. Tumor evolution includes the complex interaction of genetic, cellular state, epigenetic, spatial, and microenvironmental factors. Considering the great individual differences in the population, the origin and evolutionary dynamics of tumors should be studied in a larger sample size cohort, and the corresponding theoretical and molecular mechanisms of tumor dynamic evolution need to be determined.
1.2.3 Stem cell aging
Aging is a complex biological process accompanied by systemic functional decline. Stem cell aging is a hallmark of organ degeneration and thus stem cell aging study is a basis for resolving aging and aging-related diseases, as well as a key starting point for developing aging-related interventions. Stem cells include totipotent stem cells (TSCs), pluripotent stem cells (PSCs), and adult stem cells (ASCs). ASCs, such as hematopoietic, skin, muscle, neural, and intestinal stem cells, are found in multiple tissues and have unidirectional or multidirectional differentiative potentials, playing indispensable roles in the homeostatic maintenance and functional restoration of given organs. These stem cells are
《Figure 1.2.5》
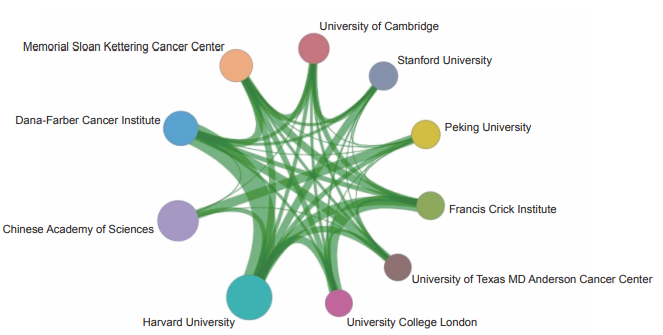
Figure 1.2.5 Collaboration network among major institutions in the engineering research front of “mechanisms in tumor heterogeneity and evolutionary dynamics”
《Figure 1.2.6》
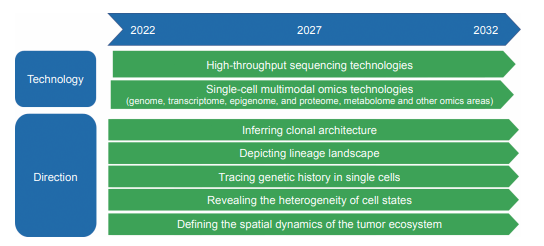
Figure 1.2.6 Roadmap of the engineering research front of “mechanisms in tumor heterogeneity and evolutionary dynamics”
tissue-specific and have profound interactions with immune, vascular and neural microenvironments, being vital to the maintenance of tissue regenerative ability and organ function.
Usually, various damages are accumulated in stem cells during the aging process, including genomic DNA damage, epigenetic changes, cell cycle abnormalities, excessive reactive oxygen species, mitochondrial dysfunction, proteostasis imbalance, altered microenvironment and metabolic dysregulation. In addition, systemic inflammation can also contribute to stem cell exhaustion. These intrinsic and extrinsic factors jointly lead to the gradual age-associated decline in the function and regenerative ability of stem cells. As aforementioned, stem cell aging is one of the most important drivers of organismal aging, leading to decreased tissue regenerative ability, impaired organ function and aging-related diseases. Therefore, it is imperative to systematically study the mechanisms of stem cell aging and develop intervention strategies for stem cell aging, thus improving the regenerative ability of tissues and organs in aging and aging-related diseases.
Currently, there are mainly two lines of stem cell-based interventive strategies, which are the activation of the regenerative activity of in situ stem cells and stem cell transplantation. To activate the regenerative activity of endogenous stem cells, we can use small molecules or gene intervention targeting stem cells to improve their self-renewal and differentiative capacities, or refine the microenvironment to increase the viability of stem cells and enhance their functions. For stem cell transplantation, adult stem cells can be expanded in vitro and re-introduced into the body, which can effectively replenish the reservoirs of stem cells in the tissue. It would further improve tissue homeostasis and function when combined with genetically enhanced stem cells with improved genetic characteristics. To date, stem cell-based therapies have shown great potentials for treating various types of diseases, including aging frailty, spinal cord injuries, type I diabetes, Parkinson’s disease, amyotrophic lateral sclerosis, Alzheimer’s disease, myocardial infarction, and osteoarthritis. In addition, the rapid progress in various cross-cutting fields has further advanced the mechanistic studies of stem cell aging, providing potential targets for the development of new intervention strategies. For example, organoid technology helps in building a research system for human organ aging and is being applied to observe the interactions between stem cells and their microenvironment in vitro. Gene editing and lineage tracing will help explore the effects of different genes on stem cell homeostasis and cellular senescence. Partial reprogramming technology helps in understanding the epigenetic landscape of stem cell aging and exploring to reset the epigenetic aging clock. Single-cell and spatial multi-omics have facilitated the discovery of the relationship between tissue spatiotemporal heterogeneity and the regulation of stem cell aging. Accordingly, the effective integration of the existing interdisciplinary technologies, the development of new perspectives and technologies, the exploration of novel mechanisms and potential targets in stem cell aging research will be critical for the development of new intervention strategies against aging and aging-related diseases so as to actively cope with population aging.
There are a few key scientific issues to be addressed in stem cell aging research as listed below.
1) Develop novel models of stem cell aging. Stem cell aging is a complex biological process accompanied with a high degree of heterogeneity and asynchronicity in different species and tissues. Thus, it is urgent to build up a multi-species model- based novel paradigm, along with various in vitro models of human stem cells, their derivatives, and organoids mimicking multiple diseases, to provide a prerequisite for the exploration of stem cell aging mechanisms.
2) Systematically investigate the mechanisms of stem cell aging. The exploration of the drivers for stem cell aging is the most advanced and active branch in the field of aging. However, the mechanism by which epigenetics, metabolism, immunity, inflammation, and rhythms under different circumstances influence stem cell aging lacks systematic investigations. Thus, the spatiotemporal, dynamic, and multi- dimensional landscape should be profiled, the common and specific mechanisms underlying stem cell aging await determination, complex issues such as the heterogeneity and spatiotemporal specificity during stem cell aging process ought to be solved, and the potential targets need to be explored using multi-dimensional technologies, such as single-cell multi-omics, spatial multi-omics, and high-resolution dynamic imaging combined with interdisciplinary research systems, including new materials, artificial intelligence, synthetic biology, regenerative medicine, optogenetics, biosensing, and gene editing. These studies will facilitate not only the mechanistic studies of stem cell aging but will also take the stem cell aging research from bench to bedside.
3) Establish novel stem cell-based strategies for the interventions against aging and aging-related diseases. As stem cell aging is one of the most important driving forces of organ aging, the exploration of stem cell aging based on new models and technologies will help in establishing novel intervention strategies against aging and aging-related diseases. Especially based on the in-depth understanding of stem cell aging, it is possible to confer stem cells with improved adaptability to the microenvironment of aging lesions and increased resistance to malignant transformation. High-throughput screening platforms can be used to explore rejuvenation factors that delay stem cell aging and promote stem cell regeneration. Targeted gene editing technologies such as CRISPR-Cas9 and efficient gene delivery systems can be used to facilitate the development of novel gene therapies to enhance stem cell performance. Bioengineering technologies, such as special biomimetic materials and nanorobots, can be employed to enhance stem cell homeostasis and resilience. By exploring the regulatory mechanisms of active and healthy lifestyles, such as exercise, diet control, and keeping regular schedules, to discover intervention targets of stem cell aging, which can be used to enhance stem cell activity and maintain organ homeostasis.
Highlights for future research:
1) Construct and utilize multi-species models, combined with multi-lineage human stem cells, their derivatives and organoids for aging and disease-mimicking, to generate a new paradigm for the systematic study of human stem cell aging.
2) Develop and optimize cross-hierarchical, multi-dimensional and high-resolution techniques to identify the specificity and heterogeneity for stem cell aging with spatiotemporal resolution.
3) Investigate the precise logic underlying the spatiotemporal regulation of cell-cell and cell-microenvironment crosslinks during stem cell aging, especially the interactions between stem cells and the immune, hematopoietic, metabolic, and neuroendocrine microenvironments.
4) Build up novel high-throughput screening platforms based on aging stem cells to identify novel small-molecule compounds and rejuvenation factors that can enhance the activity of stem cells.
5) Establish new strategies for cell transplantation using genetically enhanced stem cells and their derivatives, and evaluation of the long-term safety and efficacy in animal models and/or clinical trial systems.
6) Discover new drugs, vaccines or immune cell therapies specifically targeting senescent stem cell antigens to achieve safe and efficient removal of senescent stem cells.
7) Evaluate the effectiveness, safety and key regulatory mechanisms of healthy lifestyles, such as diet control, exercise, and rhythm regulation in delaying stem cell aging.
The roadmap of the engineering research front of “stem cell aging” is shown in Figure 1.2.7.
《Figure 1.2.7》
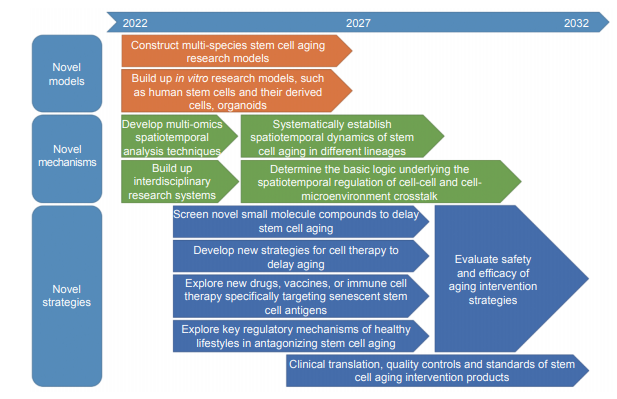
Figure 1.2.7 Roadmap of the engineering research front of “stem cell aging”
In the engineering research front of “stem cell aging”, the USA is leading in terms of the output of core papers, followed by China and the UK (Table 1.2.5). The citations per paper of China is 86.64. In terms of cooperation network among the main countries, the Top 10 countries have cooperative relationships, especially the Top 4 countries (the USA, China, the UK, and Germany), which have close cooperation in this field (Figure 1.2.8).
The Top 10 institutions with the most published core papers on “stem cell aging” are mainly from the USA, China, and the UK. The institutions from the USA include Harvard University, Stanford University, Mayo Clinic, Johns Hopkins University, University of California, Los Angeles (UCLA), University of California, San Francisco (UCSF), and Buck Institute for Research on Aging. The institution from China is Chinese Academy of Sciences. The institutions from the UK include University of Cambridge and University College London (Table 1.2.6). The cooperation network among the main institutions in this front is shown in Figure 1.2.9, and some institutions have cooperative relations.
According to the statistical analysis above, in the frontier study of “stem cell aging”, China currently keeps pace with the rest of the world. As such, the following suggestions are put forward.
1) Establish basic and translational innovation centers for stem cell aging research, and encourage the establishment of human stem cell resource banks, stem cell function
《Table 1.2.5》
Table 1.2.5 Countries with the greatest output of core papers on “stem cell aging”
| No. | Country | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | USA | 314 | 51.31 | 37 629 | 119.84 | 2017.6 |
| 2 | China | 100 | 16.34 | 8 664 | 86.64 | 2017.7 |
| 3 | UK | 85 | 13.89 | 9 725 | 114.41 | 2017.7 |
| 4 | Germany | 55 | 8.99 | 6 226 | 113.2 | 2017.4 |
| 5 | Italy | 44 | 7.19 | 4 888 | 111.09 | 2017.6 |
| 6 | Spain | 33 | 5.39 | 3 647 | 110.52 | 2017.6 |
| 7 | Canada | 31 | 5.07 | 3 801 | 122.61 | 2017.4 |
| 8 | France | 29 | 4.74 | 3 159 | 108.93 | 2017.7 |
| 9 | Netherlands | 28 | 4.58 | 3 788 | 135.29 | 2017.5 |
| 10 | Japan | 24 | 3.92 | 3 942 | 164.25 | 2017.8 |
《Figure 1.2.8》
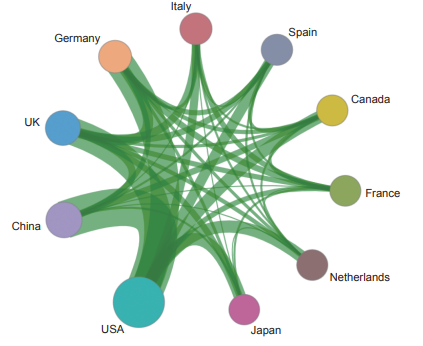
Figure 1.2.8 Collaboration network among major countries in the engineering research front of “stem cell aging”
《Table 1.2.6》
Table 1.2.6 Institutions with the greatest output of core papers on “stem cell aging”
| No. | Institution | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | Harvard University | 30 | 4.9 | 3 951 | 131.7 | 2017.9 |
| 2 | Stanford University | 27 | 4.41 | 2 558 | 94.74 | 2017.9 |
| 3 | Chinese Academy of Sciences | 25 | 4.08 | 3 220 | 128.8 | 2017.9 |
| 4 | Mayo Clinic | 23 | 3.76 | 3 741 | 162.65 | 2018.2 |
| 5 | Johns Hopkins University | 20 | 3.27 | 3 196 | 159.8 | 2017.7 |
| 6 | University of Cambridge | 17 | 2.78 | 1 590 | 93.53 | 2018.2 |
| 7 | University of California, Los Angeles | 16 | 2.61 | 2 508 | 156.75 | 2017.8 |
| 8 | University of California, San Francisco | 15 | 2.45 | 1 552 | 103.47 | 2018 |
| 9 | Buck Institute for Research on Aging | 14 | 2.29 | 2 916 | 208.29 | 2017.5 |
| 10 | University College London | 13 | 2.12 | 1 927 | 148.23 | 2016.8 |
《Figure 1.2.9》
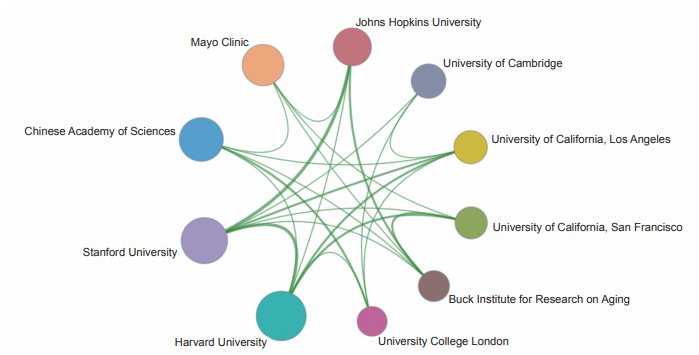
Figure 1.2.9 Collaboration network among major institutions in the engineering research front of “stem cell aging”
evaluation systems, and drug development systems for stem cell rejuvenation; facilitate the transition from research to the commercial promotion of stem cell therapeutic products against aging and aging-related diseases.
2) Accelerate the development of ethical standards, evaluation systems, and standards of stem cell aging research; strengthen the protection of intellectual property, and promote the commercialization of scientific and technological findings.
3) Launch and expand the national stem cell aging research programs and stem cell aging clinical translational research funds to increase the financial support for basic and translational studies as well as policy research in the field of stem cell aging.
4) Gather and cultivate the creative talents and world first- class research teams in the stem cell aging field; build up a scientific environment focusing on talents based on local research institutions of science and technology, innovative talent training center, medical establishments, and biomedical enterprises.
《2 Engineering development fronts》
2 Engineering development fronts
《2.1 Trends in Top 10 engineering development fronts》
2.1 Trends in Top 10 engineering development fronts
This section of the review describes the Top 10 engineering development fronts in the field of medicine and health, including the fields of basic medicine, clinical medicine, pharmacy, traditional Chinese medicine, medical informatics, and biomedical engineering. The emerging fronts are “in vivo gene editing technology”, “development and application of base editors in biomedicine”, “Proteolytic targeting chimera degrader”, and “lineage-traceable multiplexed single-cell sequencing technology”. Traditional research has focused on “organoid-based drug screening”, “AI + surgical robotics”, “a technical system for the efficient discovery of pharmacodynamic substances in traditional Chinese medicine”, “invasive brain–machine interface technology”, and “Stem cell expansion system and intelligent tumor risk assessment based on multimodal biomedical big data” (Table 2.1.1). All patents related to these 10 fronts published between 2016 and 2021 are listed in Table 2.1.2.
(1) Organoid-based drug screening
Organoids are established using human tissue stem cells or patient tumor tissues and are crucial in vitro models with stable phenotypic and genetic characteristics enabling long- term and stable subculture. Since the first mouse intestinal organoids was developed in 2009, organoid technology has sprung up as a powerful tool in precision medicine, regenerative medicine, drug screening, and disease modeling over the past 10 years and still enjoys limitless development
《Table 2.1.1》
Table 2.1.1 Top 10 engineering development fronts in medicine and health
| No. | Engineering research front | Core papers | Citations | Citations per paper | Mean year |
| 1 | Organoid-based drug screening | 185 | 473 | 2.56 | 2019.7 |
| 2 | In vivo gene editing technology | 726 | 5668 | 7.81 | 2019.1 |
| 3 | Development and application of base editors in biomedicine | 246 | 850 | 3.46 | 2019.9 |
| 4 | AI + surgical robotics | 1103 | 3363 | 3.05 | 2019.8 |
| 5 | Proteolytic targeting chimera degrader | 344 | 774 | 2.25 | 2018.5 |
| 6 | Lineage-traceable multiplexed single-cell sequencing technology | 24 | 80 | 3.33 | 2019.2 |
| 7 | A technical system for the efficient discovery of pharmacodynamic substances in traditional Chinese medicine | 2225 | 2136 | 0.96 | 2019 |
| 8 | Invasive brain–machine interface technology | 179 | 1492 | 8.34 | 2019.6 |
| 9 | Stem cell expansion system | 969 | 1394 | 1.44 | 2018.9 |
| 10 | Intelligent tumor risk assessment based on multimodal biomedical big data | 200 | 917 | 4.58 | 2019.8 |
《Table 2.1.2》
Table 2.1.2 Annual number of patents published for the Top 10 engineering development fronts in medicine and health
| No. | Engineering research front | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| 1 | Organoid-based drug screening | 6 | 13 | 21 | 29 | 42 | 74 |
| 2 | In vivo gene editing technology | 39 | 101 | 116 | 154 | 136 | 180 |
| 3 | Development and application of base editors in biomedicine | 1 | 3 | 23 | 51 | 77 | 91 |
| 4 | AI + surgical robotics | 44 | 45 | 73 | 195 | 281 | 465 |
| 5 | Proteolytic targeting chimera degrader | 61 | 55 | 79 | 31 | 48 | 70 |
| 6 | Lineage-traceable multiplexed single-cell sequencing technology | 1 | 3 | 4 | 5 | 4 | 7 |
| 7 | A technical system for the efficient discovery of pharmacodynamic substances in traditional Chinese medicine | 217 | 317 | 310 | 367 | 424 | 590 |
| 8 | Invasive brain–machine interface technology | 7 | 13 | 21 | 24 | 46 | 68 |
| 9 | Stem cell expansion system | 141 | 120 | 115 | 164 | 176 | 253 |
| 10 | Intelligent tumor risk assessment based on multimodal biomedical big data | 6 | 9 | 23 | 28 | 42 | 92 |
potential worldwide.
However, the following issues remain to be addressed for organoid-based drug screening: construction of various organoid and disease models, construction of complex organoid models, commercial production of organoid culture medium and basement membrane matrix, standardization and automation of organoid culture and drug screening, development of multi-dimensional analytical methods for organoids, high-content imaging and analysis techniques for organoids, tracking, supervision, and management specifications of human-derived organoids, and societal acceptance and ethical review system of organoids.
Several popular areas have undergone major progress, including the establishment of diversified organoid and disease models, the construction of vascularized, immune, and systematic organoid models, the optimization and industrialization of organoid culture systems, and the development of organoid high-content imaging systems and analytical methods. Organoid models are important for drug screening and drug R&D, and have been widely recognized and enthusiastically supported by regulatory authorities, organizations, and pharmaceutical companies globally.
China, is now the top owner of published core patents, followed by the USA and the Republic of Korea, in terms of organoid-based drug screening, accounting for 30.81% of the overall published patents (Table 2.2.1). Cross-border collaboration between research institutions is rarely seen. As a late starter in this field, China still manages to catch up with the advanced level by virtue of its prior knowledge of scientific research and policy and regulatory support. As the organoid industry enjoys high-speed development and broad prospects, accelerating the product transformation and clinical application of organoids in China will introduce breakthroughs in drug R&D and precision medicine.
(2) In vivo gene editing technology
The extensive development and optimization of CRISPR- Cas technologies have yielded robust tools for gene editing, including programmable nucleases, base editors, and prime editors. These gene editors have been widely used in various animal disease models to explore the efficacy and safety of gene editing. In recent years, several human clinical trials have demonstrated the potential of gene editing technologies represented by CRISPR. In comparison with the rapid development of gene editing tools, delivery remains a bottleneck for therapy development. Therefore, the efficient delivery of precision gene editing agents into tissues in mammals continue to play a critical role in advancing the field. Researchers have identified and engineered several classes of delivery vehicles that can overcome these complex molecular obstacles to intracellular delivery. Current state- of-the-art delivery systems, including viral vectors, LNPs, and VLPs, can satisfy these key criteria for efficient in vivo delivery vehicles, making them well-suited for the in vivo delivery of gene editing agents. In June 2021, the New England Journal of Medicine reported the first clinical research data that support the safety and efficacy of CRISPR gene editing in vivo. This is the first clinical result of CRISPR gene editing therapy in vivo, proving that the direct injection of CRISPR components can perform efficient gene editing in vivo, which further expands the application scope of CRISPR gene editing therapy and opens up new avenues for the treatment of many human diseases. In vivo gene-editing therapies are rapidly making their way to the clinic.
(3) Development and application of base editors in biomedicine
Base editor is a new generation of gene editing tool developed by combining the CRISPR-Cas system with an individual deaminase. Considering the capacity of base editors to achieve site-specific single-base substitution at in genomic DNA without introducing double-stranded DNA breaks, they offer more precise and secure genome editing effects than conventional artificial nuclease system such as CRISPR-Cas9. Thus, base editors are emerging as a research hotspot in precise gene editing and have been successfully applied in basic and applied research on various animals, plants, and microorganisms. In the field of biomedicine, base editors have demonstrated important applications in gene function investigation, directed protein evolution, genetic lineage tracing, disease modeling, and gene therapy. Since the two main base editors, namely, cytosine base editors (CBEs), which converts a C–G base pair into a T–A base pair, and adenine base editors (ABEs), which converts an A–T base pair into a G–C base pair, were developed, scientists have developed more than 100 optimized base editors with improved editing efficiency, precision, specificity, targeting scope, and capacity to be delivered in vivo to data. Subsequently, they have enriched the arsenal of base editors and expand their application potential in biomedicine. Currently, base editors have been successfully applied to therapeutic gene editing for the prevention or treatment of human diseases by correcting pathogenic mutations or introducing additional single nucleotide variants in genome in vitro or in vivo.
(4) AI + surgical robotics
AI + surgical robotics (AI + SR) is an intelligent diagnosis and treatment technology that studies the advanced learning algorithm of artificial intelligence for surgery and empowering the surgical robot. It allows the surgical robot to autonomously perceive the internal cavity environment of the patient, conduct real-time surgery decision-making, autonomously perform the surgical tasks without the operator’s intervention, and perform the surgery with increased precision, safety, automation and efficiency. The large-scale practical application of AI + SR in clinical practice can be realized by addressing the following key technical issues: ① intelligent instrument segmentation, motion tracking, and reliable perception of intracavitary surgical tools with environment; ② learning from the demonstration, recognition, and execution of complicated surgical operation tasks; ③ multifunctional and lightweight dexterous surgical robot hardware and intelligent human-machine interaction control systems. With the development and maturation of AI and surgical robotics, especially the breakthroughs in surgery-specific learning data sets and training models, new robot configuration design, and human-robot interaction technologies, the laboratory prototypes of AI + SR will realize actual clinical applications. They are also expected to be widely used in many fields such as general surgery, urology, cardiovascular surgery, thoracic surgery, gynecology, orthopedics, neurosurgery, and dentistry. Considering its broad prospects in the field of human health care, China and developed countries such as Europe, America, and Japan have prioritized the investment in AI and surgical robotics research, targeting “AI + SR” solutions for major diseases and key clinical technologies. Moreover, an “industry-academia- research-medical-inspection-application” collaborative innovation mechanism has been established. In recent years, a series of technology startups has emerged in China and continues to achieve breakthroughs in unit technology and launch some new products. China’s AI technology-based intelligent diagnosis and treatment equipment and surgical robot products have formed a certain scale, and its market size continues to grow at a high rate. The development of real-time intraoperative guidance in dynamic intracavity environment, multimodal data fusion and perception, human-robot intelligent interaction control in complicated surgical environment, multi-degree-of-freedom dexterous surgical robot configuration and drive technology, and limited surgical training data sets labeling and model training technology will be the key to promote AI + SR technology breakthrough. AI + SR is a high-end medical technology that integrates medicine, artificial intelligence, robotics, mechanics, control science, biomechanics, and computer science. The deep integration of multiple disciplines will aid in the diagnosis and treatment of many major and difficult diseases and generate huge social and economic benefits in the field of people’s health.
(5) Proteolytic targeting chimera degrader
Proteolytic targeting chimera (PROTAC) is a newly emerging technology, which draws target protein and E3 ligase close by heterobifunctional small molecules in cells, and induces the selective degradation of target protein through ubiquitin- protease system. PROTAC molecule is composed of three parts, namely, a target protein ligand, an E3 ligase ligand, and a linker that connects both ligands. By recruiting ligands at both ends, a ternary complex of “target protein-PROTAC-E3 ligase” is formed to induce the subsequent degradation of the target protein. PROTAC-induced degradation regulates the function of target protein in a catalytic manner. The quick and efficient discovery and optimization of PROTAC molecules is the key issue of this new technology. ① Ligand discovery of PROTAC molecules. The quick and effective screening of target protein ligands and the discovery of suitable E3 ubiquitin ligases and corresponding ligands that can be used in PROTAC technology are important scientific problems that restricts the design and development of PROTAC molecules, and they are limited by the lack of target protein ligands and E3 ligase ligands. The optimization of linkers between both ligands is also an important issue in PROTAC development. ② Selectivity and degradation activity. No rules have been set to improve the selectivity and degradation activity of PROTACs, and concise theoretical guidance is needed. The usual “hook effect” of PROTAC molecules will also affect the evaluation of degradation selectivity and activity. ③ Optimization of PROTAC druggability. Considering the unique mode of mechanism of PROTAC, the optimization of its drug-like properties also needs extensive exploration, and the optimization experiences learned from small molecule drugs cannot be simply copied. ④ Evaluation system for preclinical studies. Traditional methods cannot accurately evaluate the pharmacokinetics, pharmacodynamics, and toxicity of PROTAC molecules, and a mature preclinical evaluation system for PROTAC molecules has not been established. Therefore, how to rationally develop PROTAC drugs remains an urgent and unsolved problem. PROTAC can target the disease-related proteins that cannot be addressed by small molecule inhibitors or antibody technology, such as undruggable targets, drug-resistant targets to small molecule inhibitors, and proteins with scaffold functions. Therefore, PROTAC technology represents an innovative strategy for the development of new drug modality. In addition, PROTAC, as a novel chemical knock-down method, plays an important role in biomedical research. Currently, PROTAC technology has become a new frontier of drug discovery. More than 130 targets, including kinases, transcription factors, and receptors, can be degraded using the PROTAC technology. Several start- up and public biotech companies in Europe and America have focused on PROTAC drug development, and most international pharmaceutical giants have joined the PROTAC field. At present, more than 20 pharmaceutical companies in China have participated in the development of PROTAC pipelines. Currently, more than 10 PROTAC drugs have entered the clinical stage. Overall, PROTAC drug pipelines are still in the early stages of development. Among these tested drugs in clinical studies, most are used for the treatment of different types of cancer, and the first PROTAC drug has been advanced to clinical phase 2. In future, indications for treatment of PROTAC molecules will not only cover oncology, but also be expanded to other disease fields such as inflammation, immunology, neurodegeneration, stimulating immune response to fight cancer, and anti-virus. At the same time, new types of PROTAC molecules, molecular glues, and other innovative degradation technologies with similar mechanisms are constantly emerging. Targeted protein degradation agent is expected to become the fourth main drug modality independent of traditional small molecule inhibitor, antibody, and cell therapy. Considering that the PROTAC technology presents a great opportunity for China to realize cornering overtaking in the competition of drug discovery, China needs to accelerate the development of PROTAC drugs.
(6) Lineage-traceable multiplexed single-cell sequencing technology
Lineage-traceable multiplexed single-cell sequencing (SC- seq) technology integrates cutting-edge SC-seq technologies and computational analyses to re-construct the cell lineages in normal and disease tissues. It involves multiplexed cellular barcoding, multi-modal single-cell omics, and CRISPR-based cloning analysis, which enable the establishment of dynamic cell atlases at tissue-, organ- and organism-levels, and the underlying gene regulatory networks. With the increasing application of SC-seq technologies in life sciences and medicine, lineage-traceable multiplexed SC-seq technology has been used extensively to study the composition and developmental history of normal tissues and the etiology and transformation process of diseases. The inference of tumor clone evolution has promoted our understanding of the tumor progression and relapse after therapy, revealing useful biomarkers for precision oncology. Currently, laboratories around the world have come together to build the Human Cell Atlas and Tumor Cell Atlas. Lineage-traceable multiplexed SC-seq technology is among the most recent technological advancements of these projects that could revolutionize the study of human diseases. In the future, its maturation and further development will benefit from high-throughput long- read sequencing (DNA or RNA), custom-design of cellular and molecular barcoding for multiplexed sequencing, effective processing of clinical samples for SC-seq studies, and computational tools for multi-modal single-cell datasets. Lineage-traceable multiplexed SC-seq technology is expected to play a key role in studying the mechanism of recurrent and relapsed cancers by revealing the primary and secondary tumor clones between treatments. This line of investigation could serve as the foundation of precision medicine. It will reveal useful biomarkers for the diagnosis and prognosis of relapsed patients and provide important clues for the development of therapeutic strategy for the treatment of relapsed cancers.
(7) A technical system for the efficient discovery of pharmacodynamic substances in traditional Chinese medicine
The pharmacodynamic substances of traditional Chinese medicine (TCM), which are extracted and isolated from Chinese medicinal materials, Chinese decoction pieces, Chinese patent medicines, or prescriptions, are chemical substances with specific pharmacological activities for the treatment or prevention of diseases or regulation of the physiological functions of the body. It includes both the original components of traditional Chinese medicine and the newly generated metabolites of traditional Chinese medicine. The determination of effective substances from the complex material system of TCM remains one of the core scientific and technological challenges in TCM modernization. The development of modern analysis and separation technologies has encouraged the gradual employment of new technical means such as chromatography-mass spectrometry analysis, chromatographic separation, NMR structure identification, and multi-omics analysis for the exploration of TCM pharmacodynamic substances. This provides technical support for promoting the study of the material basis of TCM and the discovery of active lead compounds and innovative drugs based on TCM and natural medicine resources. Relevant technologies are also being constantly updated. For example, direct infusion-tandem mass spectrometry (DI-MS/MSALL), which performs analysis at high speed and can simultaneously detect chemical components with different polarities, has replaced liquid chromatography mass spectrometry (LC- MS). A precision single-cell microfluidic technology platform has been developed. With the combination of advanced analytical techniques such as high-content imaging and mass spectrometry, the active components of TCM can be directly screened from complex systems without pre-separation of the components. Cell membrane chromatography in bio-chromatography has become a hot spot in the field of traditional Chinese medicine in recent years. It is used for the separation and identification of medicinal components based on whether the components in the extract have specific affinity with active cells. In vitro cell membrane chromatography can quickly and effectively screen the effective parts and active components of traditional Chinese medicine compounds, thus allowing the high-throughput screening of traditional Chinese medicines.
In recent years, the identification technology of in vivo metabolites of TCM based on “omics” has garnered much attention. The robust applications of transcriptomics, proteomics, and metabolomics can be found in TCM pharmacodynamic research through the comparison of miRNA expression, gene expression, protein expression or metabolites before and after intervention with TCM. It further clarifies the mechanism of action of TCM pharmacodynamic substances and offers the potential to systematically evaluate the efficacy of the active ingredients or components. The rapid development of omics provides a new means for the integration of traditional Chinese medicine with modern technology and systems biology. High-throughput and high-content screening by using innovative platforms substantially increases the efficiency for activity evaluation and the screening rate of active compounds. High-throughput screening enables the large-scale activity screening of components, while high-content screening simultaneously increases the dimensions of the screening. This may be a significant boost for the rapid discovery of pharmacodynamic substances in the complexities of TCM and allows the analysis of gene, protein, and metabolite spectra of biological systems and the large-scale study of the biological functions. Network pharmacology based on computer simulation is widely used in the research of the pharmacodynamics of TCM. By computing a complex biological network of “disease phenotype-gene target-drug ingredients”, the target spectrum of TCM ingredients is elucidated, pharmacologically active ingredients and mechanisms are predicted, and prescription compatibility rules are discovered.
As the human perception of complex diseases gradually expands, the traditional reductionist way of thinking (from molecules to genes then to cells) is slowly being replaced by the application of systems theory in methods such as systems biology and network biology. Multi-modal and cross-scale observation of the effects of TCM on cell phenotype, tissue/ organ structure, and body function will uncover the effects of various constituents/components on multiple subsystems of the body and their synergistic effects, which may open new channels in the exploration of pharmacodynamic substances of TCM. Driven by big data, by using model construction and biological information technology, and considering the multi-component and multi-target characteristics of TCM in treating diseases, an in-depth analysis of the multi- component, multi-target, and multi-disease relationship of TCM can be carried out through AI, and an interaction network of drug component, component target, and disease gene can be constructed. The results will help predict the active ingredients, potential efficacy, clinical indications, and mechanism of action of TCM compounds, which may plot an untraveled course for the R&D of new TCM drugs.
(8) Invasive brain–machine interface technology
Brain–machine interface (BMI) creates a direct information route between the brain and external devices. It is an important tool for the cutting-edge neuroscience research and clinical diagnosis and treatment of major brain diseases. The key challenge of the development of useful BMIs is how to achieve a balance between minimizing brain damage and maximizing brain utilization, which highly depends on the specific application. In comparison with their non-invasive counterparts, invasive BMIs are in close contact with neurons and therefore have intrinsic advantages in key performance, such as the quality of neural signals and the accuracy of neural modulation. However, the brain trauma caused by implantation surgery and the long-term in vivo safety of implanted devices are the current bottlenecks.
BMI is a highly sophisticated system, which involves many key components such as electrodes (more contacts, thinner, and more flexible), chips (lower power consumption), algorithms (more robust), and implantation tools (faster, less brain damage and more automatic). In terms of scientific research, BMIs can promote the sustainable progress of fundamental neuroscience by mapping the brain validity connection network and analyzing new neural circuits through high- throughput neural data recording in multiple brain regions. In the diagnosis and treatment of brain diseases, BMIs can build the connection between human brain and neural prosthesis by extracting and decoding neural activities of the brain spatially and temporally to restore and/or replace the motor function of limbs. It can also simulate and transmit corresponding signals to the target position and provide nerve stimulation for nerve repair. In military applications, by using high-performance BMI technology, the information communication between the brain and the electronic system is optimized in the form of direct information interaction to improve the efficiency of information analysis and execution. It allows human beings to effectively participate in decision-making and keep up with the pace of ever-evolving machines.
At present, more than 200 companies provide BMI-related products and services around the world, mainly in the USA (more than 50% of the global market), the European countries (Germany, UK, and Switzerland), and China. The global BMI market is expected to grow at a compound annual growth rate of 13.8% from 2020 to 2027, and the industry scale will grow to more than 3 billion US dollars by 2027. Clinical validation for major disease treatment will be the key arena for BMI technologies. However, insufficient recording channels, large implantation trauma, and lack of close-loop control (i.e., record and stimulate) are key challenges that need to be solved. High-throughput, minimally invasive implantable closed-loop BMI technology has gained widespread attention and is gradually becoming the key research direction worldwide at present and in the next 5–10 years.
In the field of invasive BMIs, China still lacks original core technology and many key performances of BMI for real- world applications, such as the bandwidth, accuracy, and in vivo stability, and the technology still lags behind that of the USA. In recent years, increasing domestic units have begun to turn to the research of invasive BMI technology. In 2021, the Shanghai Institute of Microsystems and Information Technology, Chinese Academy of Sciences developed the “a minimally invasive implantable high-throughput flexible BMI” technology, which has over 1 000 recording channels and can be implanted in the brain with a surgical opening of less than 1 mm. The technology has been verified in rodent and non-human primate animal models. At present, various key components (e.g., electrodes, chips, wireless communication and powering units) need to be synergistically integrated to achieve a high-performance BMI system with an appropriate implantation strategy. The technology needs to enter the clinical trial stage legally immediately to accelerate the verification of key technologies and the optimization of the overall system.
(9) Stem cell expansion system
Stem cells are a kind of pluripotent cells that can self- renewing and differentiate into various functional cells under specific conditions. They are essential for the study of tissue and embryo development, regenerative medicine, and the development of novel drugs. Stem cell expansion system (SCES) is a sterile culture technology and equipment that is used to increase the number and maintain the characteristics of stem cells after isolation, purification, and identification through morphological and molecular biological approaches. This technology precisely regulates the cell-cell, cell-cytokine and cell-extracellular matrix interactions by bioinspired design the stem cell niche in vivo (including physical, chemical and biological signal parameters). It aims to promote stem cell self-replication on a large scale, reduce cell damage, senescence, and stemness loss, and maintain cell homeostasis and differentiation potential for improving the quantity, function, and safety of stem cells. At the same time, the individualized stem cell expansion process and quality control standards should be established in line with the application demands to improve the stem cell expansion efficiency and reduce the production cost. SCES is rapidly developing towards diversification, standardization, and large-scale use, and it has become one of the main technology platforms for stem-cell-based biomedical basic research and translational medicine. The effective SCES of human induced pluripotent stem cells strongly supports the construction of human organs and intelligent human multi-organ chip systems, showing its promising applications in the areas of human physiological and pathological simulation and new drug development. A variety of SCES of adult stem cells has gradually entered the clinical transformation of stem-cell-based regenerative medicine to overcome the limitations of traditional medical treatment, including hematopoietic stem cells (HSCs) that are used for the treatment of leukemia, mesenchymal stem cells (MSCs) that have been used for immune regulation and tissue repairs of uterus, skin, and cartilage, and human neural stem cells (NSCs) that have been used to repair various nervous system injuries. Considering the wide application prospects of SCES in the field of biomedical basic research and clinical translational, the development of SCES has attached huge investment in China and developed nations such as Europe, the USA, Japan, and South Korea.
With the opening of national policies in recent years, many technology start-up companies have been established at home and abroad. These companies have developed and optimized a series of new reagents and automated equipment that can realize the safe application of stem cells to clinical and drug screening, such as a complete kit of cell culture reagents with safe and identified components, fully enclosed culture equipment that reduces artificial error and contamination risk, and cell-free treatment programs by producing engineered exosomes. These technologies and products have formed a certain scale, and their market will increase with as the stem cell research continues to advance quickly. The key breakthroughs in SCES include the development of large-scale manufacture processes of stem cell expansion, empowerment, separation, and related reagents to perfect a comprehensive and accurate quality control standard system, further innovate the bioactive stem cell carrier materials, and the fully enclosed automatic cell production equipment that monitors cell status in real-time and regulates the cell microenvironment. A new era of visualization, quantification, and controllable biologically active drugs based on stem cells will usher because of the deep integration of SCES with material engineering, imaging technology, and numerical control technology.
(10) Intelligent tumor risk assessment based on multimodal biomedical big data
The cancer-risk intelligent assessment is developed based on massive multi-source (heterogeneous) multimodal biomedical data under macroscopic and microscopic scales by using high-performance computing systems, bioinformatics technology, machine/deep learning methods, and other “IT+BT” technologies. The cancer-risk intelligent assessment system mainly aims to boost the prevention, diagnosis, and treatment of malignant tumors by dynamically providing a full-process, interpretable, high-precision, and interactive risk assessment/decision-making guidance. The intelligent cancer-risk assessment system requires the establishment of scalable, high-quality standardized datasets, intelligent hospital resource planning systems, daily health monitoring/ follow-up systems, cancer imaging/omics/pathological detection methods, trusted biocomputing, and multi-scale software and hardware platforms such as data visualization/ analysis tools (i.e., deconvolution algorithms for tumor cells and their microenvironment), natural language retrieval systems, and professional knowledge base/databases. The construction of new tools for cancer-risk assessment can be accelerated by providing theories, technologies, and platforms to facilitate the collection, storage, sharing, fusion, deep mining, model/marker validation, and clinical applications of cross-scale multimodal biomedical big data. The intelligent cancer-risk assessment system is valuable for the dynamic monitoring, real-time warning, and early screening of various malignant tumors, which is the basis of individualized treatment and early intervention for disease progression. It can provide different disease risk reports for healthy people and patients from real-world clinical practice/ clinical trial. Currently, the commercial products of AI-assisted diagnosis (e.g., medical imaging) and high-throughput multi- omics detection have been widely promoted and applied in China and other countries. These commercial products provide substantial case and data resources for the precision diagnosis and development of novel targeted therapies such as small molecule therapeutics/cellular immunotherapy. With the population aging trend and the increasing incidence of cancers, the demand for intelligent health diagnosis products will continue to increase. The economic benefits are expected to grow from tens of billions to hundreds of billions of dollars in the next few years. Big scientific projects represented by TCGA, the Cancer Cell Line Encyclopedia, the UK Biobank, the Human Proteome Project (HPP), and the International HPP (IHPP) have provided unprecedented opportunities for the early prevention and precise diagnosis and treatment of cancer. In the future, additional advancements will be crucial for the products of intelligent tumor risk assessment, such as the construction of the sharing platform for all cancer/data types, the integrated analysis of large-scale electronic health record systems, and the development of dynamic modeling methods for multimodal data and millions of healthy people/ disease cohorts. In addition, omics-based molecular diagnosis (i.e., single-cell/single-molecule detection), AI-based drug virtual screening technologies, and other cloud computing and information technologies are still iterating. These emerging technologies and the establishment of intelligent cancer-risk assessment systems will substantially promote the understanding of tumor susceptibility mechanisms, the discovery of targeted agents and their sensitive patients, and the design of clinical trials. It will become one of the indispensable aspects for reducing the economic burden of tumor-susceptible populations/patients and improving patients’ long-term survival and quality of life.
《2.2 Interpretations for three key engineering development fronts》
2.2 Interpretations for three key engineering development fronts
2.2.1 Organoid-based drug screening
Organoids are tissue analogs with a certain spatial structure formed by in vitro three-dimensional (3D) culture of human tissue stem cells or patient tumor tissues. These analogs have stable phenotypic and genetic characteristics, thus enabling long-term and stable subculture. In comparison with traditional two-dimensional culture models, organoids effectively simulate the physiological functions of source organs or tissues in structure and function. Moreover, compared with animal models, the construction of organoids is less expensive and simpler in terms of operation, and faster in culture with a higher rate of success, making it suitable for disease pathology research and high-throughput drug screening. These advantages also justify the widespread use of organoids in precision medicine, regenerative medicine, drug screening, and disease modeling. Since the first mouse intestinal organoid is developed in 2009, numerous organoids with some key physiological structures and functions and the corresponding tumor tissue organoids have been cultured and established, including the kidney, liver, lung, intestine, brain, prostate, and pancreas. Guiding clinical medication and precision treatment is an important goal of organoid technology. Organoids have been listed in Science’s top 10 breakthroughs of 2013 and chosen as the Method of the Year 2017 by Nature Methods in 2018. As of September 2020, 63 clinical trials with the use of organoids were filed in the Food and Drug Administration (FDA). Since 2017, 20 clinical studies that involve organoids and cover eight cancer types have been registered and approved by ethics committees in China. Organoids enjoy considerable development potential worldwide.
The following critical issues remain to be addressed for organoid-based drug screening: construction of various organoid and disease models, construction of complex organoid models, such as organoids that recapitulate the tumor microenvironment, development of standardized, inter- batch consistent, and cost-effective organoid culture medium ingredients and basement membrane matrix, standardization and automation of organoid culture and drug screening procedures, development of multi-dimensional analytical methods for organoids, establishment of high-content imaging and analysis techniques for organoids, tracking, supervision, and management specifications of human- derived organoids, and societal acceptance and ethical review system of organoids. The key areas with critical implications for organoid-based drug screening include:
1) Establishment of diversified organoid and disease models. Organoids (including tumor organoids) derived from the heart, brain, lung, liver, intestine, stomach, kidney, breast, ovary, bladder, tongue, pancreas, prostate, thyroid gland, thymus, and retina have been used for drug screening. For example, MCLA-158, a bispecific antibody from Merus, was identified from the screening of patient-derived colorectal cancer organoids and is now under phase 1/2 clinical studies. The latest data show that all seven patients enrolled with head and neck squamous cell carcinoma had tumor reduction. Another typical example is a cystic fibrosis model constructed via single CFTR mutation in an intestinal organoid. Vertex’s blockbuster cystic fibrosis drug, ORKAMBI, was approved in the Netherlands based entirely on the results obtained from this type of organ models. However, less than one-third of all 78 organs in the human body have been constructed as organoids. Non-tumor disease models based on organoids are even more limited. The establishment of diversified organoid and disease models that cover the spectrum of human diseases is the premise of drug screening and development and thus exhibits various possibilities.
2) Construction of vascularized, immune, and systematic organoid models. Various tumor organoids, which are constructed to screen the majority of cytotoxic and targeted drugs, are not applicable to angiogenesis inhibitors and immunotherapy that are now under the spotlight, because they lack tumor microenvironment details, such as blood vessels, immune cells, and stromal cells and thus cannot realize the effects of angiogenesis inhibitors and immunotherapy in full. Tumor organoids with vascular structures have been constructed, and anti-tumor immune cells have been produced by co-culturing immune cells with tumor organoids. “Assembloids” that are composed of multiple organ cell types, which are spatially organized to precisely simulate human tissues or organs, have also been reported in studies over the past few years. Although these models enhanced the complexity of tumor organoid microenvironment, they cannot be compared to the one in vivo, and they are rarely used for drug screening. Systematic organoid models require the connection of multiple types of organoids to mimic the circulation of materials, energy, and body fluids involving their counterparts in vivo, enabling a thorough evaluation of the efficacy and toxicity of drugs. For example, heart, liver, kidney, and intestine organoids are enriched in a chip, in which a culture medium is perfused to simulate the circulatory system. Candidate drugs can then be analyzed for toxicities to several important organs at one time. The construction of vascularized, immune, and systematic organoid models further improves the accuracy of organoid simulation of in vivo situations and is expected to provide more comprehensive pharmacodynamics and toxicity information for drug screening.
3) Optimization of an organoid culture system for drug screening. Drug screening requires large-scale and stable production of organoids, and its major constraints are organoid culture medium and basement membrane matrix with complex ingredients. Organoid culture medium usually contains miscellaneous critical growth factors, such as EGF, FGF2, R-Spondin 1, Noggin, and Wnt3a. These growth factors have been commercialized but are often exorbitant, with varying quality between batches. Moreover, their manufacturers may apply different specifications. The downside partially limits the large-scale application of organoids for drug screening. The basement membrane matrix has even more constraints than the organoid culture medium. The most commonly used basement membrane matrix, Matrigel, provides essential three-dimensional support and some growth factors for organoid growth. However, the product, which is derived from extracts of Engelbreth-Holm- Swarm (EHS) mouse sarcoma tumors, is characterized by complex ingredients, large batch-to-batch differences, and the potential rejection of some human-derived organoids. Some researchers and companies have developed purely chemically synthesized substitutes for the basement membrane matrix and performed several organoid culture studies. However, these novel substitutes have not been fully proven to be functionally equivalent to Matrigel and are not readily accessible. Most growth factors can now be produced in China, but this is not the case for basement membrane matrix, because virtually all GMP-grade products need to be imported. In addition, the development and application of 3D bioprinting technology in organoid design and construction provide possible solutions for the large-scale, standardized, and precise production of complex organoids.
4) Development of multi-dimensional analytical methods for organoids and standardized evaluation of drug screening. Conventional methods include cell imaging, pathological detection, and gene sequencing. However, a series of analytical methods that are further applicable to large- scale drug screening should be developed to produce and evaluate organoids in a multi-dimensional and standardized manner. For example, ordinary microscopes hardly obtains a photograph of organoids in three-dimensional space. Meanwhile, a high-content imaging system provides abundant physiological or pathological phenotype information about organoids, although some commercial high-content imaging systems are expensive and limited by the slow running speed and unstable operation. In addition, researchers usually cannot obtain all the information from high-content images on organoids through only visual observation or simple software analysis. For high-throughput drug screening, a standardized evaluation system needs to be established. By using image recognition and AI, standardized analysis algorithms can be developed to evaluate parameters, such as organoid activity, size, composition, and spatial structure, and drug effects and safety.
Cell and animal models are an important foundation for disease modeling and drug development. A large number of rare diseases for which drug development is not possible due to lack of relevant animal models. Traditional cell models are quite different from actual physiological conditions, while animal models face issues of long duration of modeling, high cost, and difficulty in automatic culture. Therefore, drug R&D is challenged by immense cost, long duration, and high probability of failure. Organoids hold promise for more efficient and precise drug screening and R&D. Since 2015, Pfizer, Johnson & Johnson, Sanofi, AstraZeneca, and other leading global drug companies have increased their presence in organoids through services procurement, collaboration and licensing, and investment. In 2022, the FDA approved the world’s first new drug for a clinical trial (NCT04658472) with preclinical data based on an “organ chip”. The approval represents the FDA’s acceptance of organoid studies and provides a new option for drug studies targeting diseases that lack appropriate animal models. As a late starter in this field, China still manages to catch up with the advanced level by virtue of its prior knowledge of scientific research and policy and regulatory support. From 2009 to 2019, only 8% of the published papers associated with organoids came from Chinese teams. In 2020, this figure rose to 14%, second only to the USA. In January 2021, China’s Ministry of Science and Technology issued the notice on soliciting opinions for the guidelines of application in 2021 for six key specialized projects of the 14th Five-Year Plan’s National Key R&D Program including “mathematics and applied research”. As stated in this Notice, “stem cell-based human major refractory disease models” are one of the first key projects in the 14th Five- Year Plan. In December 2021, the NMPA issued the “Technical Guidelines for Non-Clinical Research and Evaluation of Gene Therapy Products (Interim)”, which first included organoids in the validation guidelines for gene therapy and cell therapy. In July 2022, the first expert consensus on organoids to guide precision oncology was established in China. Considering that the organoid industry enjoys high-speed development and broad prospects, the product transformation and clinical application of organoids need to be accelerated in China.
As of 2021, 198 core patents have been published in the front of “organoid-based drug screening”, and the top three countries with the greatest output of core patents are China, the USA, and South Korea (Table 2.2.1). Up to 30.81% of such patents are filed by Chinese applicants, making China one of the frontrunners in this field (Table 2.2.2). However, collaboration is absent between the top core patent-owning countries. The top core patent-owning institutions include the Yonsei University, Royal Netherlands Academy of Arts and Sciences, Swiss Federal Institute of Technology in Lausanne, and Keio University (Table 2.2.2). Among these institutions, collaboration is only present between Keio University and Osaka University in Japan (Figure 2.2.1).
So far, organoid technology has been widely used in many fields, including disease modeling, drug development and drug screening. Under 3D culture conditions, many kinds of organs such as lung, stomach, intestine, liver, kidney and other organoids have been successfully cultivated. Guiding clinical medication and precision treatment is an important goal of organoid technology. Engineered organoids, a combination of bioengineering techniques is used to standardize and automate the production, control and
《Table 2.2.1》
Table 2.2.1 Countries with the greatest output of core patents on “organoid-based drug screening”
| No. | Country | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations |
| per patent | ||||||
| 1 | China | 57 | 30.81 | 74 | 15.64 | 1.3 |
| 2 | USA | 48 | 25.95 | 158 | 33.4 | 3.29 |
| 3 | South Korea | 32 | 17.3 | 8 | 1.69 | 0.25 |
| 4 | Japan | 15 | 8.11 | 43 | 9.09 | 2.87 |
| 5 | Netherlands | 6 | 3.24 | 77 | 16.28 | 12.83 |
| 6 | Switzerland | 5 | 2.7 | 54 | 11.42 | 10.8 |
| 7 | UK | 5 | 2.7 | 40 | 8.46 | 8 |
| 8 | Australia | 5 | 2.7 | 3 | 0.63 | 0.6 |
| 9 | Germany | 3 | 1.62 | 5 | 1.06 | 1.67 |
| 10 | Sweden | 2 | 1.08 | 3 | 0.63 | 1.5 |
《Table 2.2.2》
Table 2.2.2 Institutions with the greatest output of core patents on “organoid-based drug screening”
| No. | Institution | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations |
| per patent | ||||||
| 1 | Yonsei University | 7 | 3.78 | 2 | 0.42 | 0.29 |
| 2 | Royal Netherlands Academy of Arts and Sciences | 6 | 3.24 | 77 | 16.28 | 12.83 |
| 3 | Swiss Federal Institute of Technology in Lausanne | 5 | 2.7 | 54 | 11.42 | 10.8 |
| 4 | Keio University | 5 | 2.7 | 39 | 8.25 | 7.8 |
| 5 | Harvard University | 3 | 1.62 | 51 | 10.78 | 17 |
| 6 | Osaka University | 3 | 1.62 | 15 | 3.17 | 5 |
| 7 | Cincinnati Children’s Hospital Medical Center | 3 | 1.62 | 12 | 2.54 | 4 |
| 8 | Shanghai Meifeng Biotechnology Co., Ltd. | 3 | 1.62 | 8 | 1.69 | 2.67 |
| 9 | Rutgers University | 3 | 1.62 | 6 | 1.27 | 2 |
| 10 | Beijing Ketu Medical Technology Co., Ltd. | 3 | 1.62 | 4 | 0.85 | 1.33 |
《Figure 2.2.1》
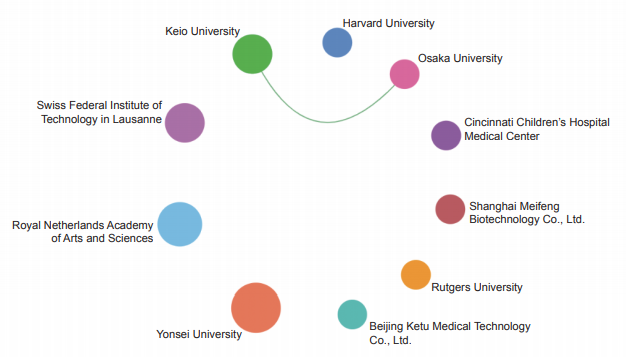
Figure 2.2.1 Collaboration network among institutions in the engineering development front of “organoid-based drug screening”
analysis of human organoid development, homeostasis and disease modeling by mimicking tissue organoids that perform different functions in vivo, thus reproducing the complex and dynamic microenvironment of developing organs. With the establishment of advanced model systems for bioengineering, engineered organoid technologies bring new functionalities into life medicine research and clinical applications, which hold great promise in various research fields, such as in drug research for toxicity detection, pharmacodynamic evaluation, and new drug screening. With the combination of organoids with other sophisticated engineering technologies such as organ chip, micro mobile array, scRNA-seq, CRISPR-Cas9, high-throughput screening, 3D printing and intelligent biomaterials, engineered organoids will become more mature in terms of stability, accuracy, reproducibility and scalability. Therefore, the establishment of diversified organoid and disease models, the construction of vascularized, immunized and systematic organoid models, the optimization of organoid culture system for drug screening and the development of multi-dimensional analytical methods for organoids and standardized evaluation of drug screening will further deepen the application and development of organoid in the field of drug screening (Figure 2.2.2).
《Figure 2.2.2》

Figure 2.2.2 Roadmap of the engineering development front of “organoid-based drug screening”
2.2.2 In vivo gene editing technology
Approximately 70 years since the discovery of the DNA double helix, the techniques for determining, analyzing, and altering genome sequences and gene expression patterns in cells and organisms have been developed. These molecular tools are the foundation of molecular biology, thus improving the biomedical industry by increasing the understanding of the genetics of normal and disease traits. The ability to diagnose genetic diseases has grown rapidly as the cost of genome sequencing has decreased, the comparative analysis of human genome sequences has increased, and the application of high-throughput genomic screening has increased. Preliminary research suggests that genome editing tools can be used to inactivate or correct disease-causing genes in patients, leading to novel, life-saving treatments for people with genetic disorders.
The commonly used tools for gene-editing technology include Zinc Finger Endonuclease (ZFN), Transcription Activator Like Effector Nuclease (TALEN), and CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats-associated) system. CRISPR-Cas9 was first discovered in bacteria and archaea. It is an adaptive immune defense mechanism formed during the long-term evolution of bacteria to fight invading viruses and foreign DNA. Scientists have used the CRISPR-Cas9 gene-editing technology to make specific DNA modifications to target genes and have made significant progress in applications involving gene therapy, such as blood diseases, tumors, and other genetic diseases. By using the Cas9 enzyme to discover, cut, and replace specific sequences of DNA, CRISPR-Cas9 gene editing can make permanent and precise changes to a patient’s chromosomes, repair potential genetic mutations, and cure diseases with genetic origins. Therefore, it is considered as a “game changer” in biology.
Recent advances include the development of robust tools for gene editing in mammalian cells, including programmable nucleases, base editors, and prime editors. These gene- editing agents have been widely applied for the treatment of numerous disorders with a genetic component across various animal models.
At present, product development by using CRISPR gene- editing technology as a gene therapy method covers the fields of blood diseases, solid tumors, rare diseases, and regenerative medicine. Therapeutic gene editing has greatly facilitated the applications of gene-editing therapies into clinical practice.
Jennifer Doudna, who won the Nobel Laureate in Chemistry, pointed out in the 2020 Nature review that “Delivery remains perhaps the biggest bottleneck to somatic-cell genome editing.” According to whether the gene-editing process occurs in vivo or in vitro, CRISPR can be divided into in vitro gene editing and in vivo gene editing. Most current gene- editing clinical trials are limited to in vitro gene editing, and the process is quite complex, including the isolation of cells from patients, in vitro editing, and reinfusion to patients. At present, this kind of research has made important progress in blood diseases and has been applied for the treatment of solid tumors. However, most diseases are not suitable for ex vivo manipulation. In vivo gene editing involves the use of vectors to directly deliver CRISPR to human diseased organs and tissues and directly modify diseased genes in the human body.
The in vivo gene-editing process is simple and oriented to a wide range of indications, and the cost is only about one-tenth of the in vitro gene-editing treatment, providing the greatest hope for the development of many drugs for genetic diseases that patients can afford. However, the key to making therapeutic in vivo gene editing a reality is the safe and effective delivery of gene-editing agents to relevant organs and tissues in the body.
To overcome the off-target risk and immune response risk of in vivo gene editing, the ideal in vivo delivery of gene editing should be transient and cell-targeted. The delivery technologies currently used to achieve in vivo gene editing contain adeno-associated virus (AAV) delivery, lipid nanoparticle (LNP) delivery, and virus-like particle (VLP) delivery. These delivery technologies have been validated in mice, non-human primates (NHPs), and other animals, and are entering the stage of clinical validation.
1) Virus Delivery. Viruses naturally evolved to overcome barriers to in vivo delivery and can natively deliver nucleic acid cargos to many cell types. Considering these favorable characteristics, viruses are promising vehicles for delivering gene-editing agents. Many viral vectors have been developed for in vivo gene therapy applications. Most in vivo gene editing applications use adeno-associated viruses (AAVs), and a few pre-clinical studies have used lentiviruses or adenoviruses. To date, viral vectors offer some of the highest gene-editing efficiencies observed in many organs because of their inherent ability to efficiently transduce different types of cells and deliver their nucleic acid vectors in vivo. However, the progress of AAV-CRISPR therapy also faces a series of obstacles that are prevalent in the development of AAV gene therapy. Future improvements to viral vectors will require careful efforts to overcome the challenges outlined above, including the immunogenicity of the vector, prolonged expression of the gene editing agent, off-target gene editing, potential for genomic integration, manufacturing cost, and dose-limiting toxicity. Vector engineering approaches to improve the potency and specificity could reduce the required dose and the cost of manufacturing of viral delivery platforms. Methods to durably silence cargo expression after on-target editing will also substantially improve the safety profile of viral delivery.
2) Lipid nanoparticle (LNP) Delivery. LNPs have grown increasingly popular as non-viral vehicles for delivering gene- editing agents in vivo. For decades, LNPs have been used to deliver nucleic acid cargos, including siRNAs and therapeutic mRNAs. Their encapsulated payloads are delivered into target cells by first entering cells through endocytosis, and then escaping endosomes by disrupting endosomal membranes after endosome acidification, and subsequently gaining access to the target cell cytosol. Following extensive development and optimization, LNPs have been approved for use in humans by the US FDA, including the intravenous administration to deliver therapeutic siRNAs to hepatocytes, for the treatment of transthyretin amyloidosis (ATTR). However, most nucleic acids delivered by LNP are degraded through the endosomal pathway and still face endosomal escape. The further development of LNPs needs to overcome the challenges of extrahepatic tissue delivery.
3) Virus-like particle (VLP) Delivery. VLPs have emerged as potentially promising vehicles for delivering gene-editing agents. VLPs are non-infectious assemblies of viral proteins that package desired cargo mRNAs, proteins, or RNPs in addition to or instead of viral genetic material. Considering that VLPs are derived from existing viral scaffolds, they exploit natural properties of viruses that enable efficient intracellular delivery, including their ability to encapsulate cargos, escape endosomes, and achieve efficient intracellular delivery. VLPs can be specifically delivered to different types of cells through reprogramming on the particle surface. However, unlike viruses, VLPs transiently deliver gene-editing agents as mRNA or protein instead of as DNA, which substantially reduces the risks of off-target gene editing and viral genome integration. Given that more than 50% of the human population has a pre- existing immune response against Cas9, the characteristics of VLP transient delivery can effectively reduce the risk of gene- edited cells being cleared by the human immune system. Therefore, VLPs are attractive vehicles for delivering gene editing agents, because they can offer key benefits of both viral and non-viral delivery.
In June 2021, the New England Journal of Medicine reported the first clinical research data supporting the safety and efficacy of CRISPR gene editing in vivo. The indication for this study is transthyretin amyloidosis (ATTR), a rare autosomal dominant disorder, which is a life-threatening disease characterized by the progressive accumulation of misfolded transthyretin (TTR) protein in tissues, predominantly the nerves and heart. NTLA-2001 is an in vivo gene-editing therapeutic agent that is designed to deliver liposome- encapsulated CRISPR-Cas9 complexes by intravenous infusion, reducing the concentration of TTR in serum. The research has opened up new avenues for the treatment of human diseases and has been hailed as “opening a new era of medicine”. In addition, Editas Medicine has conducted a clinical study of Leber’s congenital amaurosis type 10 based on AAV delivery of CRISPR (EDIT-101). In China, BDgene Therapeutics Co., Ltd. has carried out clinical research on CRISPR antiviral treatment of viral keratitis based on VLP delivery.
New techniques of in vivo gene editing are also emerging. In June 2022, Precision BioSciences announced that it had reached an exclusive worldwide collaboration and licensing agreement with Novartis for in vivo gene editing R&D. Precision will develop a custom ARCUS nuclease based on a naturally occurring genome-editing enzyme, I-CreI, which has evolved from C. reinhardtii to perform highly specific cleavage in cellular DNA. Precision scientists reengineered I-CreI to form the ARCUS nuclease to edit new DNA sequences for the insertion, removal, or repair of DNA in living cells and organisms. The approach holds promise for the treatment of certain hemoglobin disorders, such as sickle cell disease and beta-thalassemia.
The clinical application of gene editing has a double standard of safety and efficacy. Viral vectors have uncertainties in safety because of the long-term expression of gene editing enzymes, while nanomaterials face efficiency challenges. An ideal gene- editing delivery tool needs to be both transient and efficient to ensure the safety and efficacy of the treatment. Therefore, the CRISPR gene-editing technology needs to be carefully evaluated. Notably, the “God’s knife” of CRISPR gene editing must also resolve ethical and moral conflicts.
The era of therapeutic gene editing in humans has arrived. Extensive development and optimization of CRISPR-Cas technology has yielded powerful gene-editing tools, including nuclease, base, and guide editing. The combination of these gene editing tools with effective in vivo delivery methods (viral vectors, LNPs, and VLPs) for the treatment of disease has been applied from proof-of-concept in animal models to clinical treatment in humans. With the development of in vivo gene editing therapies and future advances in gene delivery technology, it will provide a reliable approach and a solid foundation for the clinical treatment of genetic diseases in different organs.
The countries with the largest number of core patents are the USA, China, and Switzerland (Table 2.2.3). As shown in the cooperation network among main countries, the USA, Switzerland, China, the UK, Germany, Netherlands, and Japan have cooperation (Figure 2.2.3). The top institutions in terms of core patents output are Harvard University, The Broad Institute Incorporation, and University of California (Table 2.2.4). A cooperative relationship was observed between The Broad Institute Incorporation, Massachusetts Institute of Technology (MIT), Harvard University, and Brigham and Women’s Hospital. Partnership was also present between CRISPR Therapeutics and Vertex Pharmaceuticals Incorporated (Figure 2.2.4).
According to statistical analysis results, China is currently in a situation of running parallel with similar foreign countries for the development of “in vivo gene editing technology”.
《Table 2.2.3》
Table 2.2.3 Countries with the greatest output of core patents on “in vivo gene editing technology”
| No. | Country | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations |
| per patent | ||||||
| 1 | USA | 493 | 67.91 | 4 594 | 81.05 | 9.32 |
| 2 | China | 103 | 14.19 | 209 | 3.69 | 2.03 |
| 3 | Switzerland | 51 | 7.02 | 538 | 9.49 | 10.55 |
| 4 | South Korea | 16 | 2.2 | 32 | 0.56 | 2 |
| 5 | UK | 15 | 2.07 | 55 | 0.97 | 3.67 |
| 6 | France | 14 | 1.93 | 106 | 1.87 | 7.57 |
| 7 | Germany | 12 | 1.65 | 28 | 0.49 | 2.33 |
| 8 | Netherlands | 7 | 0.96 | 118 | 2.08 | 16.86 |
| 9 | Canada | 7 | 0.96 | 22 | 0.39 | 3.14 |
| 10 | Japan | 5 | 0.69 | 88 | 1.55 | 17.6 |
《Figure 2.2.3》
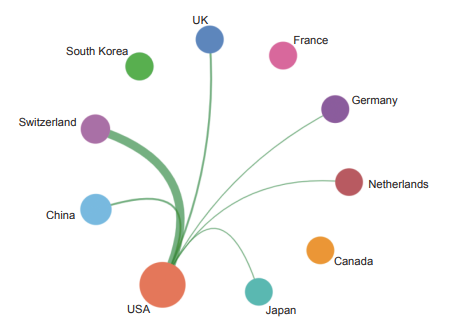
Figure 2.2.3 Collaboration network among major countries in the engineering development front of “in vivo gene editing technology”
《Table 2.2.4》
Table 2.2.4 Institutions with the greatest output of core patents on “in vivo gene editing technology”
| No. | Institution | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations |
| per patent | ||||||
| 1 | Harvard University | 61 | 8.4 | 1 229 | 21.68 | 20.15 |
| 2 | The Broad Institute Incorporation | 54 | 7.44 | 644 | 11.36 | 11.93 |
| 3 | University of California | 48 | 6.61 | 469 | 8.27 | 9.77 |
| 4 | CRISPR Therapeutics | 37 | 5.1 | 301 | 5.31 | 8.14 |
| 5 | Massachusetts Institute of Technology | 31 | 4.27 | 614 | 10.83 | 19.81 |
| 6 | Dana-Farber Cancer Institute | 19 | 2.62 | 85 | 1.5 | 4.47 |
| 7 | Brigham and Women’s Hospital | 18 | 2.48 | 98 | 1.73 | 5.44 |
| 8 | Beam Therapeutics | 17 | 2.34 | 70 | 1.24 | 4.12 |
| 9 | Stanford University | 14 | 1.93 | 77 | 1.36 | 5.5 |
| 10 | Vertex Pharmaceuticals Incorporated | 11 | 1.52 | 112 | 1.98 | 10.18 |
《Figure 2.2.4》
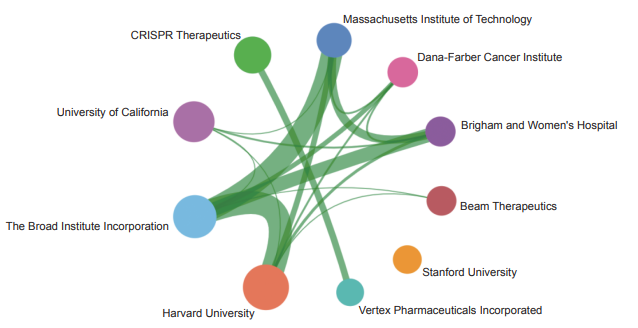
Figure 2.2.4 Collaboration network among institutions in the engineering development front of “in vivo gene editing technology”
Considering that the CRISPR gene-editing technology has great advantages and prospects in the fields of disease treatment, disease detection, and genetic breeding, the commercial application of CRISPR gene-editing technology has broad prospects. The original gene therapy vector independently developed by Chinese scholars reflects China’s scientific and technological progress in the field of gene therapy.
In vivo gene editing has more diversity and flexibility in the selection of adaptation disease, which can cover almost all diseases. In vivo gene editing has higher requirements on vector technology. In particular, vector technology needs to be safety and effective. In order to improve the safety of gene editing therapy in vivo, one is to develop more precise gene-editing enzymes, the second is to realize efficient gene editing enzyme delivery system. At present, VLP and LNP can achieve mRNA delivery in vivo, which has been clinically verified and is an ideal delivery vector for gene editing therapy in vivo. In the future, the tissue targeting of vectors needs to be further solved. Future directions will focus on the optimization of nuclease-mediated gene editing technology, the development of novel base editing therapies, and the realization of tissue-specific gene editing enzyme and instantaneous delivery technology. At present, the R&D of in vivo gene editing technology mainly focus on rare diseases, ocular diseases, and the development of neurological diseases is still in a very early stage. In the long run, gene editing in vivo has made it possible to cure HBV, HPV, HIV, HSV, and other viral infections. In addition, the possibility of clinical breakthroughs in the treatment of metabolic diseases and neurological diseases by gene editing in vivo is promising. Huntington’s disease that have no substantial breakthroughs for a long time may be cured by gene editing in vivo (Figure 2.2.5).
2.2.3 Development and application of single base editors in biomedicine
Base editor (BE) is a new genome-editing tool developed by combining the CRISPR-Cas system with different deaminases. With the guidance of CRISPR-Cas system, the fused deaminase is directed to bind the target locus and catalyze the site- specific deamination within editing window. It stimulates cellular base excision repair (BER) and DNA replication of that strand to achieve precise base substitution. In 2016, the first base editor (BE), namely, the cytosine base editor (CBE), which is a derivative of the CRISPR-Cas system, efficiently and precisely converted the C–G base pair to a T–A base pair without inducing DNA double-stranded breaks (DSBs) and without participate of DNA templates. CBE has become a milestone as it rapidly brought genome-editing technology into the era of single-base resolution. The following year, another BE, namely, adenine base editors (ABEs), induced a A–T base pair to a G–C base pair substitution efficiently, further booming development of base editor tools. In recent years, researchers have worked on the development and optimization of BEs and have generated many new BEs with richer variants, improved functions, and expanded application scenarios. In term of functions, in addition to CBEs and ABEs, researchers have developed glycosidase base editors (CGBEs) that enable substitution from C–G base pair to G–C base pair
《Figure 2.2.5》
Figure 2.2.5 Roadmap of the engineering development front of “in vivo gene editing technology”
and from C–G base pair to A–T base pair substitution and dual- base editors that enable concurrent adenine and cytosine editing, such as STEME, ACBE, and AGBE. According to editing substrates, the aforementioned BEs are nuclear DNA base editors, and RNA base editors (e.g., REPAIR and RESCUE) and mitochondrial DNA base editors (e.g., DdCBE and TALED) have been developed. Theoretically, the BEs reported currently can correct approximately 2/3 of pathogenic single nucleotide variants, but they cannot introduce all six possible base pair conversions. To break through this limitation, David R. Liu and colleagues have developed the prime editors (PEs) that can mediate all 12 possible base-to-base conversion without requiring DSBs, which substantially expands the scope of genome editing at single-base resolution. Based on the existing data, PE applications are limited by low editing efficiency, keeping it from being a core tool in the field of base editing.
DNA base editors enable the permanent and irreversible conversion of one target DNA base into another in genome, while RNA base editors merely modify RNA sequences and do not change the genome sequences, thus requiring continuous administration. Both tools have their advantages and disadvantages, and the optimal choice of BEs depends on the intended application.
As a new genome-editing tool, BEs have the advantages of high efficiency, precision, safety, and non-cell cycle dependence over conventional artificial nuclease system. However, some key technical hurdles were found in the practical applications of BEs, mainly including the low product purity caused by random base repair, variable editing efficiency affected by sequence context, genome targeting scope limited by PAM compatibilities, bystander editing with enlarged editing windows, Cas-dependent predictable off-target editing caused by non-specific binding of guide RNA (gRNA), random genome- and transcriptome-wide off-target editing induced by deaminases, in vivo delivery difficulty caused by large size of base editing components, and inability to control tissue- or organ-specific targeting. Many researchers have made remarkable progress in the optimization of existing base editing tools or development of new ones with enhanced editing performance by improving editing window, editing efficiency, precision, DNA specificity, PAM compatibility, and delivery vehicle. These advances are crucial for applications with more stringent requirements for the safety and efficacy, such as gene therapy, in which technical barriers have been cleared up, thus promoting the translation of BEs from laboratory research to clinical practice.
The emergence of BE has met the demands for precise gene editing in the field of life science and brings gene function study to the single-base resolution. Since BEs are developed, they have been applied in animals, plants, and microorganisms successfully. BEs have also exhibited broad application prospects in biomedicine field.
To date, the hotspots of BE applications in the field of biomedicine include the following:
1) Generation of disease animal models. Among known human pathogenic mutation, single nucleotide variants account for approximately 58%, and more than 60% of them are C–G to T–A or A–T to G–C mutations. Therefore, BEs could act in generating animal models for human genetic diseases, which mimic the clinical features of patients in both genotype and phenotype. Based on Bes, many genetic disease animal models such as zebrafish, mice, rabbits, dogs, pigs, monkeys, and human cell models have been established via embryo microinjection or somatic cell nuclear transfer approaches, contributing to the investigation of mechanisms of diseases and development of novel therapeutics.
2) Gene therapy. The vast majority of human somatic cells are non-dividing and cannot be efficiently edited through conventional homology-directed repair. Given that BEs perform base conversions through cellular mismatch repair machinery, a process that does not depend on the cell cycle, they can create efficient nucleotide mutation in non-dividing cells. Accordingly, Bes is considered as an important technical tool for precision genome editing. By delivering base editing components to cells ex vivo or directly to the patient in vivo, the disease-causing mutations can be directly corrected, restoring normal function for therapeutic purposes.
3) Directed protein evolution and genomic diversification. BEs can be used to induce the mutagenesis of coding sequence of specific functional domain of protein. By using a specific screening strategy, the key amino acid sites associated with protein function can be identified, especially for those caused by point mutations related to drug resistance. A similar approach can be used to generate mutant libraries of human disease-related genes to discover key loci that determine the function of the target genes.
4) Genetic lineage tracing. Lineage tracing by using cellular marker technologies is an important tool for tracking cellular origins and fate transitions during development, disease, and regeneration. By combining the DNA barcode with single- cell sequencing technology, the information of single-cell transcriptome and associated cell lineage can be captured simultaneously, thus enabling single-cell resolution lineage tracing. Substitution mutations created by BEs can record abundant mutations per barcode. In comparison with the common CRISPR barcode strategy, BEs can accumulate more mutation information, because they do not generate indels, which is useful to reduce the consumption of barcodes. Thus, lineage tracing with BEs is effective for the reconstruction of the developmental process of tissues and organs in complex multicellular organisms.
Considering the applications mentioned above, as a new gene-editing tool, BEs have realized the treatment of many diseases, such as genetic mutation, cancer, and viral infections. In 2018, the National Institutes of Health launched the Somatic Cell Genome Editing program to accelerate the development of safer and more effective genome-editing therapeutics by editing the genomes of disease-relevant somatic cells in patients, which will enormously promote the clinical translation of genome- editing tools, including BEs.
Before formal clinical trials, BEs have been used in gene therapy research in animal models with two strategies, namely, ex vivo cell base editing followed by transplantation and direct in vivo delivery of base editing elements for disease treatment. ABEs have been used for the treatment of type I hereditary tyrosinemia by ex vivo editing of mouse liver precursor cells followed by transplantation for the treatment of sickle cell disease caused by the ex vivo editing of mouse hematopoietic stem cells followed by transfusion. In terms of in vivo delivery of vectors, ABEs have been used for the treatment of Hutchinson-Gilford progeria syndrome or hereditary retinal disease and Duchenne muscular dystrophy mice, to reduce cholesterol levels by editing monkey PCSK9 gene. CBEs has also been used for the treatment of amyotrophic lateral sclerosis mice. RNA base editor has been used for the treatment of genetically deaf mice.
These experimental advances in animals have laid a solid preliminary foundation for BEs to enter clinical trials. In July 2022, Verve Therapeutics in New Zealand announced that the first patient has been administrated with VERVE-101, a first- in-class base-editing medicine designed for the treatment of heterozygous familial hypercholesterolemia. This study involves the first in vivo base-editing clinical trial in the world and is considered as a new milestone and a crucial step in the translation of base-editing therapies from bench to beside. Subsequent clinical trials by using BEs for sickle cell disease, β-thalassemia, and CAR-T cell editing, are also forthcoming. Notably, BEs hold an extensive application prospect in biomedicine. The use of BEs to conduct precise and efficient editing at the DNA or RNA level will be the future trend of precise gene therapy.
The market potential for gene therapy is huge, making it a popular track for capital investment. The global gene therapy market size is expected to reach 30.54 billion dollars by 2025, and China market alone will be 2.59 billion dollars. At present, the frontier of “development and application of single base editors in biomedicine” has more than 240 patents. The USA, China, and South Korea have a high number of patents, among which 36.18% of the patents are filed by Chinese authors, which shows that China has made remarkable achievements in this frontier field (Table 2.2.5). However, in terms of the proportion of citation frequency and average citation frequency of patents, that of China is significantly lower than that of USA. From the perspective of the cooperation network among the countries with core patents, the USA has cooperation with Switzerland, China, and the UK (Figure 2.2.6). The TOP institutions in terms of core patent outputs are Harvard University and Harvard- MIT Broad Institute and Beam Therapeutics (Table 2.2.6). Collaborations are present between Harvard-MIT Broad Institute and Harvard University, MIT, Brigham and Women’s Hospital, Massachusetts General Hospital, and Beam Therapeutics (Figure 2.2.7). China is closely following the world frontier in the development and application of BEs with fruitful achievements, but lack of original innovation of the root technology. Thus, China still faces challenging to step up from “following player” and “paralleling player” to “leading player”
The rapid advancement of genome editing agents over the past decade has yielded many new therapeutic possibilities to treat genetic diseases that were once considered incurable. Base editing is one of the most recent advances in the field of genome editing, which has attracted much attention since
《Table 2.2.5》
Table 2.2.5 Countries with the greatest output of core patents on “development and application of single base editors in biomedicine”
| No. | country | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations |
| per patent | ||||||
| 1 | USA | 138 | 56.1 | 650 | 76.47 | 4.71 |
| 2 | China | 89 | 36.18 | 142 | 16.71 | 1.6 |
| 3 | South Korea | 5 | 2.03 | 21 | 2.47 | 4.2 |
| 4 | Switzerland | 4 | 1.63 | 21 | 2.47 | 5.25 |
| 5 | UK | 3 | 1.22 | 15 | 1.76 | 5 |
| 6 | France | 3 | 1.22 | 3 | 0.35 | 1 |
| 7 | Germany | 2 | 0.81 | 0 | 0 | 0 |
| 8 | Italy | 1 | 0.41 | 4 | 0.47 | 4 |
| 9 | Lithuania | 1 | 0.41 | 4 | 0.47 | 4 |
| 10 | Canada | 1 | 0.41 | 1 | 0.12 | 1 |
《Figure 2.2.6》
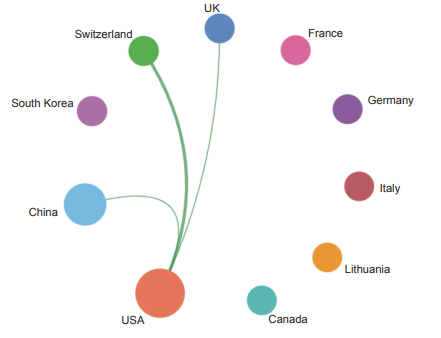
Figure 2.2.6 Collaboration network among major countries in the engineering development front of “development and application of single base editors in biomedicine”
《Table 2.2.6》
Table 2.2.6 Institutions with the greatest output of core patents on “development and application of single base editors in biomedicine”
| No. | Institution | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations |
| per patent | ||||||
| 1 | Harvard University | 26 | 10.57 | 373 | 43.88 | 14.35 |
| 2 | Harvard-MIT Broad Institute | 26 | 10.57 | 122 | 14.35 | 4.69 |
| 3 | Beam Therapeutics | 24 | 9.76 | 77 | 9.06 | 3.21 |
| 4 | Shanghai university of science & technology | 20 | 8.13 | 91 | 10.71 | 4.55 |
| 5 | Massachusetts General Hospital | 18 | 7.32 | 70 | 8.24 | 3.89 |
| 6 | MIT | 14 | 5.69 | 60 | 7.06 | 4.29 |
| 7 | Arbor Biotechnology Inc | 8 | 3.25 | 36 | 4.24 | 4.5 |
| 8 | Sun-Yat-Sen University | 8 | 3.25 | 24 | 2.82 | 3 |
| 9 | Brigham and Women’s Hospital | 7 | 2.85 | 8 | 0.94 | 1.14 |
| 10 | University of California | 5 | 2.03 | 25 | 2.94 | 5 |
its publication in 2016. Up to now, scientists have developed more than 100 optimized base editors with improved editing efficiency, precision, specificity, targeting scope, and capacity to be delivered in vivo to data. Recent advances in the DNA and RNA base editors are revealing exciting therapeutic opportunities for these technologies. Together, improving and expanding base editor technologies as well as establishing various systems suitable for human in vivo delivery of base editors will collectively represent the next step forward in the field of precision medicine. The roadmap of the engineering development front of “development and application of base editors in biomedicine” is shown in Figure 2.2.8.
《Figure 2.2.7》
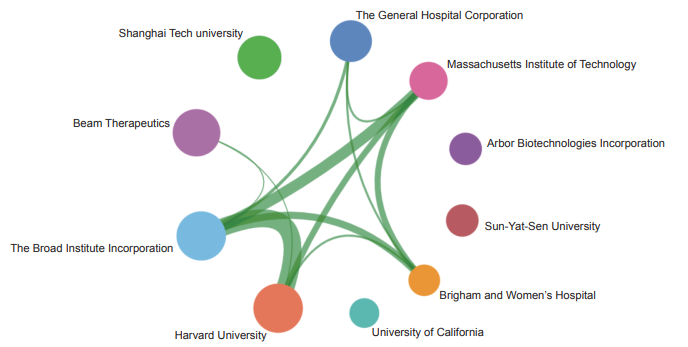
Figure 2.2.7 Collaboration network among major institutions in the engineering development front of “development and application of single base editors in biomedicine”
《Figure 2.2.8》
Figure 2.2.8 Roadmap of the engineering research front of “development and application of single base editors in biomedicine”
Participants of the Field Group
Leaders
CHEN Saijuan
Academicians and Experts
ZHANG Boli, WANG Chen, CHEN Xiangmei, ZHANG Zhiyuan, WANG Qi, NING Guang, GAO Tianming,
XU Binghe, ZHAO Yiming, JIANG Jiandong, WANG Rui, JIANG Baoguo, TIAN Jingzhou, CHEN Keji,
CHENG Tao, YING Zhinan, ZHENG Liming, PAN Xinhua, LI Chengyao, CHEN Xiaoguang, XU Xiangming,
HUANG Xiaojun, YU Jun, MA Changsheng, WANG Songling, SHI Songtao, LIN Ye, YAN Fuhua, YU Qing,
ZHAO Xinyi, ZHANG Qiang, LI Qihan, HAO Haiping, LIU Changsheng, NIE Guangjun, XI Jianzhong, ZHENG Yufeng,
CHEN Liangyi, LIU Bin, KOU Yuhui, GUO Dean, LI Qiao, NI Jian, SONG Xiaoting, SUN Xiaobo, ZHANG Tong,
QIAO Yanjiang , TU Pengfei, LIU Jianping, LIU Baoyan, TONG Peijian
Secretary Group
ZHANG Wentao, ZHAO Xilu, XI Xiaodong, YAN Xiaoyu, CHEN Yinyin, YIN Wei, ZHANG Yuliang, LU Wenqing
Data Support Group
QIU Xiaochun, DENG Peiwen, WU Hui, FAN Rong, KOU Jiande, LIU Jie, TAO Lei, JIANG Hongbo, CHEN Daming,
LU Jiao, MAO Kaiyun, FAN Yuelei, YUAN Yinchi, ZHANG Yang, WANG Chaohui, CHU Jingshen
Report Writers
ZHU Bo, ZHUANG Guanglei, LIU Guanghui, SONG Moshi, LI Jingyi, JIANG Jiandong, LI Peilong, CHEN Shuqing,
XI Jianzhong, TIAN Jie, XIE Qi, CAI Jiayu, LAI Liangxue, LI Hongbing, RAO Ju, LIU Feng, TAO Hu, LV Baoliang,
YANG Jun, LI Jianfeng














 京公网安备 11010502051620号
京公网安备 11010502051620号




