《1 Engineering research fronts》
1 Engineering research fronts
《1.1 Trends in Top 10 engineering research fronts》
1.1 Trends in Top 10 engineering research fronts
The Top 10 engineering research fronts in the field of medicine and health include basic medicine, clinical medicine, biomedical engineering, bioinformatics, immunology, and developmental biology (Table 1.1.1). These 10 fronts involve “multi-omics traits of complex diseases”, “mechanism of persistent virus infection and reactivation and analysis of intervention targets”, “the core human microbiome and host−microbiome interaction”, “reprogramming of aging”, “regulation and remodeling of immune homeostasis in organ transplantation”, “monoclonal antibody therapy for Alzheimer’s disease”, “biomacromolecular phase separation and membraneless organelles”, “research on the mechanism of organoid construction and development in primates”, “the human pangenome and disease pangenome”, and “mechanisms of chromatin dynamic modification on tissue and organ development”. All core papers on these fronts published between 2017 and 2022 are listed in Table 1.1.2.
(1) Multi-omics traits of complex diseases
Complex diseases, characterized by the interplay of genetic and environmental factors, pose significant challenges to healthcare due to the absence of precise treatment modalities. These conditions adversely impact patients’ quality of life and exert substantial social and economic burdens. The advent of high-throughput sequencing technologies has elevated multi-omics to a pivotal role in the study of complex diseases, enabling nuanced trait dissection and facilitating clinical applications. Specifically, multi-omics approaches contribute to the identification of predictive biomarkers and the screening of therapeutic targets, thereby becoming a focal point in contemporary medical research that has garnered significant investment and international collaboration, including active participation from China.
《Table 1.1.1》
Table 1.1.1 Top 10 engineering research fronts in medicine and health analysis of intervention targets
| No. | Engineering research front | Core papers | Citations | Citations per paper | Mean year |
| 1 | Multi-omics traits of complex diseases | 9 428 | 848 396 | 89.99 | 2018.5 |
| 2 | Mechanism of persistent virus infection and reactivation and analysis of intervention targets | 504 | 40 148 | 79.66 | 2018.2 |
| 3 | The core human microbiome and host−microbiome interaction | 82 | 6 879 | 83.89 | 2018.6 |
| 4 | Reprogramming of aging | 106 | 7 255 | 68.44 | 2019.1 |
| 5 | Regulation and remodeling of immune homeostasis in organ transplantation | 174 | 13 417 | 77.11 | 2018.8 |
| 6 | Monoclonal antibody therapy for Alzheimer’s disease | 170 | 9 066 | 53.33 | 2019 |
| 7 | Biomacromolecular phase separation and membraneless organelles | 614 | 74 515 | 121.36 | 2018.7 |
| 8 | Research on the mechanism of organoid construction and development in primates | 40 | 1 784 | 44.6 | 2020.3 |
| 9 | The human pangenome and disease pangenome | 165 | 17 103 | 103.65 | 2018.6 |
| 10 | Mechanisms of chromatin dynamic modification on tissue and organ development | 290 | 32 576 | 112.33 | 2018.2 |
《Table 1.1.2》
Table 1.1.2 Annual number of core papers published for the Top 10 engineering research fronts in medicine and health
| No. | Engineering research front | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
| 1 | Multi-omics traits of complex diseases | 2626 | 2421 | 2178 | 1439 | 631 | 133 |
| 2 | Mechanism of persistent virus infection and reactivation and analysis of intervention targets | 193 | 138 | 96 | 49 | 24 | 4 |
| 3 | The core human microbiome and host−microbiome interaction | 20 | 20 | 21 | 15 | 6 | 0 |
| 4 | Reprogramming of aging | 22 | 24 | 19 | 16 | 17 | 8 |
| 5 | Regulation and remodeling of immune homeostasis in organ transplantation | 37 | 45 | 37 | 25 | 26 | 4 |
| 6 | Monoclonal antibody therapy for Alzheimer’s disease | 35 | 40 | 29 | 29 | 23 | 14 |
| 7 | Biomacromolecular phase separation and membraneless organelles | 118 | 174 | 162 | 115 | 40 | 5 |
| 8 | Research on the mechanism of organoid construction and development in primates | 2 | 3 | 6 | 5 | 17 | 7 |
| 9 | The human pangenome and disease pangenome | 43 | 43 | 37 | 29 | 11 | 2 |
| 10 | Mechanisms of chromatin dynamic modification on tissue and organ development | 99 | 82 | 69 | 31 | 9 | 0 |
Looking to the future, it is imperative for research institutions to establish standardized, large-sample, multi-center cohorts and biobanks that are spatiotemporally paired. Comprehensive assessments should be conducted using omics technologies and phenotypic data to elucidate the traits of complex diseases. Advanced computational methods, such as network analysis and machine learning, should be employed to delineate intricate disease networks and identify potential pathways for intervention. Furthermore, the development of both in vitro and in vivo experimental models is crucial for exploring innovative strategies for precision diagnosis and treatment.
(2) Mechanism of persistent virus infection and reactivation and analysis of intervention targets
Persistent viral infection occur repeatedly and are difficult to cure, and can even trigger tumors, posing a serious threat to human health. The mechanisms that lead to persistent viral infection and reactivation are extremely complicated, involving factors such as viral gene replication regulation and its interaction with the host immune system. By elucidating the relevant mechanisms, new targets and strategies can be created for the prevention and control of related infectious diseases, not only provide more effective treatments for known viral diseases, but also provide strategic reserves for dealing with future emerging viruses.
The mechanism of persistent viral infection involves the long-term persistence of the viral genome, the evasion of cellular and host defense mechanisms, and the inhibition of the virus’s cytopathic effect. Persistent viral infection can result in the virus being reactivated, which can be lead to pathological effects and complicate the disease process.
The main interventions for latent infection currently include targeting viral replication and targeting the virus-specific immune response. The analysis of intervention targets and the mechanism of persistent virus infection and reactivation are crucial frontiers in virology research, and countries with advanced science and technology have made remarkable achievements. China has the ability to catch up with similar international research in this field. It is necessary to develop new technologies, such as gene editing, multi-omics, organoid culture, and novel humanized animal models, from a systems biology perspective to reveal the mechanisms of viral latency and persistent infection, viral reactivation, inflammation-cancer transformation, virus-host interactions, and pathogenesis at the molecular, cellular, organ, animal, and human levels. Discovering effective intervention targets and new diagnostic and therapeutic markers, as well as developing novel antiviral strategies, is crucial simultaneously.
(3) The core human microbiome and host−microbiome interaction
The human microbiome serves as a cornerstone in the maintenance of host health, and advances in high-throughput omics sequencing have catalyzed a plethora of studies aimed at elucidating the composition and functions of human gut microbiota. These studies also seek to decode host-microbiota interactions and formulate microbiome-centric intervention strategies. However, the concept of a core microbiome, comprising microbiota shared among diverse individuals and exerting broad health impacts, remains inadequately defined. Consequently, the identification of this core microbiota and the development of microbiome-targeted therapies have emerged as critical frontiers in medical research, attracting significant international funding.
China, characterized by its large population and diverse dietary, cultural, and lifestyle factors, necessitates the establishment of a high-caliber human microbiome research platform tailored to its demographic. Employing cutting-edge technologies such as whole-genome sequencing, high-throughput culturomics, and artificial intelligence algorithms is essential for advancing our comprehension of microbiota-host interactions. Additionally, the promotion of engineered live biotherapeutic products and the incorporation of precision medicine strategies for disease prevention through microbiome modulation are imperative. Collectively, these endeavors are pivotal for broadening the clinical applicability of microbiota-based interventions and propelling the advancement of microbiome-centered medicine.
(4) Reprogramming of aging
The global rise in aging populations and the concomitant prevalence of age-related diseases present formidable challenges to healthcare systems worldwide. Aging is a multifaceted process characterized by programmatic functional decline, heterogeneity, complexity, and susceptibility to disease. Recent advancements in aging reprogramming technologies have shown promise in decelerating or even reversing age-associated declines at cellular, tissue, and organismal levels, thereby laying the groundwork for targeted interventions in aging and age-related pathologies.
Current research in aging reprogramming encompasses several key domains: ① integration of interdisciplinary technologies spanning biology, medicine, and computer informatics to elucidate aging regulatory mechanisms and identify biomarkers at multiple biological scales; ② application of gene-editing technologies and targeted delivery methods to modulate the expression of aging-related and rejuvenation-associated genes; ③ introduction of reprogramming factors to alter chromatin states and epigenetic markers linked to cellular senescence; ④ utilization of senolytic drugs, inhibitors of the senescence-associated secretory phenotype (SASP), and senolytic vaccines to eliminate senescent cells and facilitate tissue regeneration; and ⑤ enhancement of gut microbiota homeostasis and the modulation of nutrition-sensing pathways to reshape the tissue microenvironment.
Chinese researchers have made significant strides in understanding the mechanisms of aging and developing intervention strategies, notably in the identification of aging biomarkers, the regulation of aging-associated genes, the screening of geroprotective drugs, and the advancement of epigenetic reprogramming techniques. However, the majority of these studies have been conducted in animal models, with a paucity of translational research and clinical validation. As such, rigorously designed clinical trials are imperative to assess the efficacy and safety of aging reprogramming strategies, thereby accelerating both basic and translational research in human aging interventions and addressing the challenges posed by demographic aging.
(5) Regulation and remodeling of immune homeostasis in organ transplantation
Organ transplantation represents a pivotal therapeutic approach for individuals facing end-stage organ failure. Nevertheless, the persistent challenges of organ rejection and the adverse effects stemming from prolonged immunosuppressive drug usage loom large over clinical transplantation. The maintenance of immune equilibrium and its post-transplantation reconfiguration stand as pivotal determinants of transplant outcomes and patient prognoses. Notably, the pursuit of immune tolerance induction presents discernible therapeutic advantages, encompassing a diminishment of the toxic ramifications linked to immunosuppressive agents and an enhancement of overall quality of life.
The targeted instigation of immune tolerance stands at the forefront of biomedical and scientific inquiry, closely aligned with significant clinical demand. The immune response provoked by organ transplantation exhibits multifaceted and variegated dimensions. Within distinct immune responses, whether of the acute or chronic variety, or mediated by cells or antibodies, immune cells within transplanted organ grafts, peripheral lymphoid organs, and peripheral blood manifest intricate and heterogeneous functionalities. An array of immune cell types and their subpopulations delineate disparate phenotypic traits and execute both immune-boosting and immune-suppressing roles, culminating in an intricate and delicately regulated immune milieu.
Delving into the vanguard issues associated with the orchestration of immune homeostasis and its remodeling in the context of organ transplantation necessitates, but is not confined to, a comprehensive delineation of the temporal and spatial features characterizing the dynamic alterations in systemic and localized immune responses before and after transplantation. Additionally, an in-depth exploration of the attributes exhibited by immune cells, molecular kinetics, and the principal regulatory networks underlying distinct forms of transplant rejection is indispensable.
By dissecting the temporal and spatial progression of dynamic changes and the remodeling of immune cells originating from both donors and recipients, alongside their correlation with prognostic indicators, the establishment of a diagnostic and treatment framework for immune tolerance induction and immune homeostasis remodeling becomes a paramount objective. Further, comprehending the chronological interplay between immune homeostasis regulation, remodeling, and the organism’s capacity for anti-tumor and anti-infection immunity opens avenues for the development of pertinent diagnostic and therapeutic strategies.
A more nuanced comprehension of the distinctive attributes and clinical import associated with diverse immunosuppressive agents in the context of immune homeostasis and remodeling serves to guide judicious clinical regulation. The efficient and judicious reconstruction and upkeep of immune homeostasis post-organ transplantation carry the potential to markedly enhance the practical application of this therapeutic modality within clinical transplantation.
(6) Monoclonal antibody therapy for Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the insidious erosion of cognitive function and behavioral faculties. In the backdrop of a globally aging populace, it has burgeoned into one of the foremost afflictions exerting a substantial toll on both social and economic health across the world. The pathogenesis of Alzheimer’s remains an intricate conundrum, its precise mechanistic underpinnings still eluding comprehensive elucidation. Currently, the amyloid cascade hypothesis, prominently featuring amyloid β-protein (Aβ), occupies a preeminent status in the pantheon of theories explicating the pathogenesis of AD. In accordance with this paradigm, three principal domains of pharmacological inquiry have emerged, revolving around the modulation of Aβ: the abatement of Aβ production, mitigation of Aβ aggregation, and fortification of Aβ clearance. Amongst these pursuits, passive immunotherapy aimed at augmenting Aβ clearance, specifically through the conduit of anti-Aβ humanized monoclonal antibody therapy, and has ascended to the vanguard.
Over the years, global pharmaceutical conglomerates have infused colossal financial investments into the realm of Alzheimer’s disease therapeutics. Regrettably, the developmental trajectory has proven to be a vexing odyssey, fraught with perils, with the attrition rate surpassing that observed in other therapeutic domains. The overwhelming majority of these endeavors have culminated in disappointment, primarily stemming from safety concerns or an absence of cogent clinical efficacy substantiation. Notably, in the annals of 2021, the US Food and Drug Administration (FDA) granted accelerated approval to the human IgG1 monoclonal antibody Aducanumab, a move that transpired amidst contentious discourse.
In the subsequent year of 2022, the outcomes of Phase Ⅲ clinical trials assessing the efficacy of lecanemab, an antibody targeting Aβ oligomers, were unveiled. The year 2023 witnessed the FDA’s imprimatur for lecanemab’s market deployment. Notably, the results unveiled a 27% retardation in the advancement of early-stage disease, achieving the primary clinical endpoint alongside the full constellation of pivotal secondary endpoints. Furthermore, the incidence of treatment-associated adverse effects registered a notable decline. It is worth accentuating that lecanemab not only decelerates the inexorable progression of the malady but also engenders an amelioration in clinical symptomatology. While additional scrutiny concerning lecanemab’s efficacy and safety remains incumbent, it undeniably furnishes a compelling exemplar fortifying the edifice of the Aβ hypothesis. It signifies one of the most momentous strides in the sphere of Alzheimer’s disease therapeutics in recent memory.
Historically, therapeutic interventions for Alzheimer’s disease predominantly fixated upon palliative measures bereft of the capacity to impede disease advancement. The affirmative findings stemming from the Phase Ⅲ clinical investigations of Lecanemab undeniably kindle newfound optimism among the global cohort of researchers, clinicians, and patients embroiled in Alzheimer’s disease research. This harbors the potential to catalyze augmented investments within the domain of monoclonal antibody drugs for Alzheimer’s disease, thereby affording succor to an expansive patient cohort and fostering the maturation of clinical diagnostic and therapeutic paradigms.
(7) Biomacromolecular phase separation and membraneless organelles
Cells harbor exceedingly intricate structural arrangements that orchestrate the meticulous and orchestrated execution of intricate biochemical processes. As such, a profound comprehension of the nuanced internal architectures of cells assumes paramount importance in unraveling the intricacies of cellular functions and regulatory pathways. Within eukaryotic cells, alongside membrane-bound organelles ensconced by phospholipid bilayers, a diverse array of membraneless organelles emerges through the spontaneous aggregation of biomacromolecules. These membraneless entities, characterized by their dynamic adaptability, exhibit alacrity in mounting responses and orchestrating a gamut of pivotal physiological functions, encompassing the regulation of transcription, translation, and signal transduction. Consequently, the exploration of the mechanisms underpinning membraneless organelles and their synergy with membrane-bound organelles to facilitate cellular compartmentalization stands as an emergent frontier and challenge within the life sciences.
Recent breakthroughs have laid bare the crux of the mechanism governing the genesis of membraneless organelles, which revolves around the phenomenon of phase separation propelled by polyvalent interactions among biomacromolecules. Interdisciplinary teams of scientists across the globe are actively embarked upon the exploration of novel membraneless organelles and their attendant physiological functions. This endeavor is underscored by an ardent quest to unravel the physicochemical attributes characterizing membraneless organelles and the intricacies of the dynamic processes governing their assembly, regulation, and eventual dissolution. It is noteworthy that aberrant phase separation precipitates a direct etiology in several formidable maladies, including but not confined to cancer and neurodegenerative disorders. In this context, scientists are vigorously probing strategies to intervene in anomalous phase separation, harboring the potential to furnish novel therapeutic modalities for diseases that currently elude effective treatment. Notably, Chinese scientists occupy a preeminent position on the global stage in the realms of phase separation and membraneless organelles.
Research endeavors dedicated to the exploration of biomolecular phase separation and the intricacies of membraneless organelles are poised to perpetuate their preeminence within the sphere of life sciences. The trajectory of these investigations will encompass a deeper penetration into the universal regulatory mechanisms and foundational tenets underpinning phase separation. This shall be paralleled by a profound delving into the labyrinthine intricacies underpinning cellular structure and function. Such efforts are anticipated to catalyze revolutionary waves within multifarious domains, spanning medicine, biotechnology, and the realm of pharmaceutical drug development.
(8) Research on the mechanism of organoid construction and development in primates
Organoids, self-organized three-dimensional tissue cultures derived from various stem cell types, have garnered increasing attention for their capacity to closely mimic in vivo tissue structures and functions. These miniature organ-like structures can be generated from a range of stem cell sources, including pluripotent stem cells (PSCs), induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), adult stem cells (ASCs), and tumor cells. With established models for organs such as the brain, retina, lung, and liver, among others, organoids serve as invaluable tools for in vitro modeling of tissue morphogenesis, organogenesis, regenerative medicine, drug testing, toxicology screening, and disease modeling. Their unique attributes include high physiological fidelity, genetic stability, rapid growth, and a high culture success rate, offering advantages over traditional patient-derived tumor xenograft models. Moreover, their histological and gene expression profiles closely mirror those of native tissues, enhancing their clinical and scientific utility. Despite these advancements, challenges persist, including the lack of standardized culture conditions and the limited availability of samples. Future research should focus on integrating organoids with other advanced technologies to enhance research accuracy. In China, organoid research has been recognized as a pivotal area of innovation, featuring prominently in the 14th Five-Year Plan’s national key research and development programs, where they are employed for disease modeling, target identification for diagnosis and treatment, and the exploration of new therapeutic strategies.
(9) The human pangenome and disease pangenome
Approximately two decades ago, the Human Genome Project embarked on a seminal endeavor, proposing a reference genome for the human species. Over time, as the corpus of whole-genome sequencing data expanded, a noteworthy observation surfaced: the existence of genome sequencing disparities among individuals, notably, the genomic diversity inherent to the human population. Evidently, the reference genome, painstakingly derived from a limited cohort of individuals, has struggled to satiate the burgeoning demands of genomic inquiries into various diseases. In response, the concept of the human pangenome has emerged. The pangenome, in essence, constitutes a compendium of DNA sequences that encapsulate genetic variants sourced from diverse individuals within a species or a gene pool that encompasses the entirety of said species.
Pangenomic investigations are underpinned by three fundamental components: core genes, distributed genes, and population- specific genes. Core genes are ubiquitous, shared across all individuals within a species, exemplified in the context of humans by genes universally present in every individual. These core genes exert a commanding influence over the foundational biological processes and phenotypic attributes characteristic of the species. In contradistinction, distributed genes, often termed non- essential genes, exhibit a presence in some individuals while remaining conspicuously absent in others. Concurrently, population- specific genes exclusively manifest within individuals belonging to distinct ethnic groups. While these distributed genes and population-specific genes may not be indispensable for the fundamental biological requisites of the species, they may prove to be integral in response to environmental pressures, thereby delineating or augmenting the species’ capacity to secure a survival advantage.
Distinct from traditional genomic analysis, which primarily serves as a conduit for the identification of genetic mutations, pan- genome research endeavors to unearth hitherto undiscovered genetic constituents, encompassing expansive structural variants (SVs) and even novel presence-absence variations (PAVs) within the genetic repertoire. These variations have the potential to confer heightened susceptibility to specific diseases. On a global scale, this scientific pursuit has yielded remarkable outcomes, encompassing the delineation of a preliminary human pangenome reference and the assembly of a pangenome reference spanning 36 distinct Chinese ethnic minorities. Furthermore, Chinese researchers have pioneered the development of an automated pangenomic analysis pipeline (known as HUPAN) and culminated in the inaugural pangenomic analysis of gastric tumors specific to the Chinese population. Nevertheless, the field of pan-genomic research continues to grapple with certain technical bottlenecks. A nuanced exploration of the intricate relationship between novel genetic variants and their implications in human diseases mandates in-depth scrutiny and exploration.
(10) Mechanisms of chromatin dynamic modification on tissue and organ development
Dynamic chromatin modifications refer to chemical modifications that occur at the chromatin level, which can affect the regulation of gene expression. Common chromatin dynamic modifications include DNA methylation, histone modification, and non-coding RNA. Dynamic chromatin modifications play a crucial role in cell development, physiological adaptation, and disease occurrence and progression. They can regulate the expression patterns of genes, enabling cells to adapt to various environmental and physiological demands. Additionally, abnormal modifications of chromatin dynamics are closely related to the occurrence and development of various diseases, including cancer, neurological diseases, and cardiovascular diseases. At present, the impact of chromatin dynamic modifications on tissue and organ development can be categorized into four aspects. First, gene expression regulation, where chromatin dynamic modifications can alter chromatin structure, thereby affecting gene accessibility and transcriptional activity. Second, tissue specificity, where different cells and tissues exhibit differences in gene expression patterns, is partly due to chromatin dynamic modifications. Third, genome stability, as chromatin dynamic modifications, can protect the genome from damage caused by external environmental and internal factors. Finally, transcriptional regulation, such as dynamic chromatin modifications, can influence the binding specificity of transcription factors and other regulatory factors to regulate the transcriptional activity of genes. In recent years, with the use of emerging technologies and methods such as high-throughput sequencing, genome editing, and multi-omics technology, both domestic and international research on the mechanisms underlying the impact of chromatin dynamic modifications on tissue and organ development has achieved a deeper and more comprehensive understanding. These findings provide an important reference for investigating the mechanisms and functions of dynamic chromatin modifications, building systematic associations, and decoding epigenetic “association information”.
《1.2 Interpretations for three key engineering research fronts》
1.2 Interpretations for three key engineering research fronts
1.2.1 Multi-omics traits of complex diseases
Complex diseases such as endocrine diseases, neurodegenerative diseases, and cancer are driven by a combination of multiple environmental and genetic factors. However, despite extensive research, the etiopathogenesis of these diseases remains largely unknown, leading to a lack of precise treatment. In the context of an aging population, the increasing prevalence of complex diseases is not only a personal health issue, but also a substantial burden on public health. Therefore, there is an urgent need to develop effective and personalized treatments based on novel drug targets and clinically relevant biomarkers. However, traditional analytical methods, such as epidemiological research and clinicopathological analysis, cannot easily differentiate the complex relationship between causes and effects, and suffer from selection bias. High-throughput sequencing technologies have emerged over the past decades. The research outcomes achieved using multi-omics technologies make them a promising approach for investigating complex diseases.
Multi-omics technology refers to a collection of high-throughput methods for assessing a large number of genomic, transcriptomic, proteomic, metabolomic, and microbiological traits from biological specimens. Recently, new omics dimensions have been investigated. A more comprehensive molecular atlas of diseases reveals insights beyond previous traditional genome-wide association studies (GWAS), especially variants in non-coding sequences and essential metabolic molecules in tumor progression. In addition, researchers are working on methods for integrating multi-omics data. A well-established example is the integration of genomic and expression data to identify genetic variants that influence expression levels, termed as expression quantitative trait loci (eQTL). In summary, taking full advantage of these multi-omics data and analytical techniques is essential to unravel the traits of complex diseases and to realize their clinical application.
Based on a deeper understanding of complex diseases, the application of multi-omics approaches in complex disease research is primarily reflected in two aspects: identification of predictive biomarkers and screening for therapeutic targets. On the one hand, multi-omics data strengthens the link between molecular data and specific diseases through differential analysis, which encourages the exploration of pathogenesis and regulatory factors in complex diseases. On the other hand, the integration of multi-omics data with clinical and epidemiological data can provide stronger evidence for specific molecular mechanisms and causal relationships between external factors and diseases. Currently, researchers are actively overcoming quantitative restrictions in multi-omics cohorts and addressing deficiencies in data standardization for multi-platform datasets. These efforts are aimed at early diagnosis and precise treatment.
In the engineering research front of “multi-omics traits of complex diseases”, the top three countries with core papers published are the USA, China, and the UK. Among them, China accounts for 26.39% of the published papers and is one of the major countries in research on this topic (Table 1.2.1). In terms of collaboration networks among the main countries (Figure 1.2.1), there is strong collaboration among the top ten countries.
The top ten institutions with core papers published in “multi-omics traits of complex diseases” were from the USA, China, the UK, and Denmark. The top three institutions, Harvard University, Chinese Academy of Sciences, and University of California, San Diego (Table 1.2.2) were from the USA and China. The collaboration network among major institutions demonstrates the close collaboration among national research institutions (Figure 1.2.2).
According to the results of the above statistical analysis, China is now in a parallel trend with similar foreign research in the frontier of “multi-omics characteristics of complex diseases”.
Exploring the multi-omics traits of complex diseases in depth is of great significance for precision treatment. The construction of high-quality cohorts serves as the foundation for investigating multi-omics traits. Standardized, large-sample, multicenter, spatiotemporally paired cohorts will provide extensive dimensions and a wide analytical space for subsequent research. At present, some institutions have established tissue-specific multi-omics databases through close international cooperation, further driving the progress of integrative multi-omics. Additionally, leveraging phenotypic data (e.g., imaging information) and developing experimental models in vitro and in vivo are both feasible approaches to overcome sample acquisition challenges for neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease. Furthermore, advanced genomics technologies, such as single-cell transcriptomics and spatial transcriptomics, have led to a deeper understanding of cellular subpopulations related to diseases. For multi-institutional cohorts and multidimensional omics data, employing network analysis and machine learning methods is a dependable approach. It further delineates complex disease networks and explores potential pathways, thus providing valuable insights. These new technologies and abundant multi-omics data will definitely help to improve the prevention, early detection, and treatment of complex diseases in the future. For details, see the development roadmap (Figure 1.2.3).
《Table 1.2.1》
Table 1.2.1 Countries with the greatest output of core papers on “multi-omics traits of complex diseases”
| No. | Country | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | USA | 4174 | 44.27 | 421943 | 101.09 | 2018.5 |
| 2 | China | 2488 | 26.39 | 196360 | 78.92 | 2018.8 |
| 3 | UK | 1082 | 11.48 | 108847 | 100.6 | 2018.6 |
| 4 | Germany | 1002 | 10.63 | 99737 | 99.54 | 2018.7 |
| 5 | Canada | 616 | 6.53 | 65952 | 107.06 | 2018.6 |
| 6 | France | 610 | 6.47 | 62675 | 102.75 | 2018.5 |
| 7 | Italy | 554 | 5.88 | 49721 | 89.75 | 2018.6 |
| 8 | Netherlands | 535 | 5.67 | 58463 | 109.28 | 2018.6 |
| 9 | Australia | 517 | 5.48 | 46441 | 89.83 | 2018.6 |
| 10 | Spain | 456 | 4.84 | 40483 | 88.78 | 2018.6 |
《Figure 1.2.1》
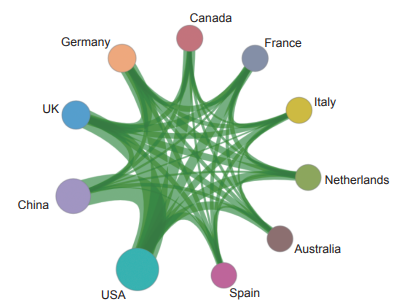
Figure 1.2.1 Cooperation network among major countries in the engineering research front of “multi-omics traits of complex diseases”
《Table 1.2.2》
Table 1.2.2 Institutions with the greatest output of core papers on “multi-omics traits of complex diseases”
| No. | Institution | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | Harvard University | 495 | 5.25 | 65 133 | 131.58 | 2018.8 |
| 2 | Chinese Academy of Sciences | 438 | 4.65 | 40 153 | 91.67 | 2018.9 |
| 3 | University of California, San Diego | 248 | 2.63 | 32 491 | 131.01 | 2018.7 |
| 4 | University of Copenhagen | 226 | 2.4 | 25 923 | 114.7 | 2018.7 |
| 5 | Stanford University | 207 | 2.2 | 26 167 | 126.41 | 2018.9 |
| 6 | University of Michigan | 169 | 1.79 | 16 829 | 99.58 | 2018.8 |
| 7 | Zhejiang University | 166 | 1.76 | 13 933 | 83.93 | 2019.1 |
| 8 | Baylor College of Medicine | 165 | 1.75 | 23 176 | 140.46 | 2018.8 |
| 9 | University of Pennsylvania | 165 | 1.75 | 17 229 | 104.42 | 2018.7 |
| 10 | Imperial College London | 161 | 1.71 | 14 924 | 92.7 | 2018.6 |
《Figure 1.2.2》
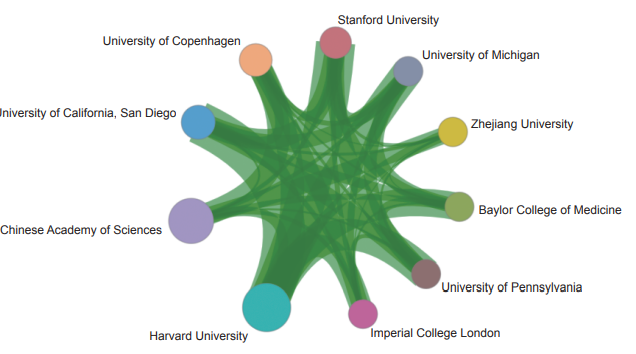
Figure 1.2.2 Collaboration network among major institutions in the engineering research front of “multi-omics traits of complex diseases”
《Figure 1.2.3》
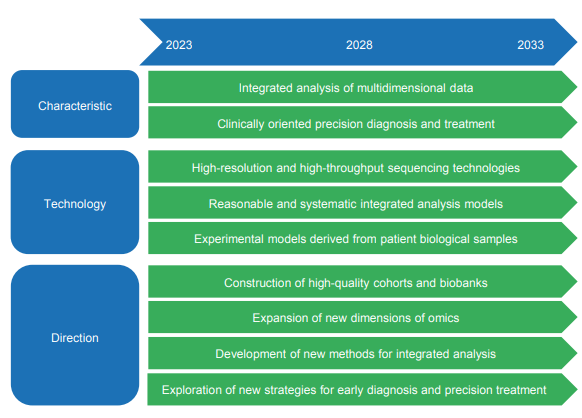
Figure 1.2.3 Roadmap of the engineering research front of “multi-omics traits of complex diseases”
1.2.2 Mechanism of persistent virus infection and reactivation and analysis of intervention targets
The outcome of viral infection is determined by the interaction between viral replication and the host defense system, which results in two types of clinical manifestations: acute infection and persistent infection. Multifarious viruses, such as human herpesviruses (e.g., herpes simplex virus, cytomegalovirus, Epstein-Barr virus), hepatitis B virus, human papillomavirus, human immunodeficiency virus, and hepatitis C virus, can establish persistent infections, making it a crucial area of focus in medical virology research. Viruses that are persistently infected can replicate continuously and produce progeny viruses, such as hepatitis C virus. Others have latent infections that can be reactivated under specific circumstances to produce progeny viruses, such as the herpes virus.
Two factors are required for the development of persistent viral infection: first, the virus suppresses its cytotoxic effects to prevent the death of infected host cells; second, the virus effectively evades host immune clearance, including innate and adaptive immunity, to continue replicating within host cells or remain latent within infected cells. When latent viruses in infected host cells enter the lytic phase, where they reproduce and disseminate once more, this is referred to as viral reactivation. The course of disease caused by persistent viral infection is mostly chronic or repeated, difficult to cure, and even can lead to the development of tumors or autoimmune diseases, seriously endangering human health and causing substantial economic burdens to society. The mechanism of persistent viral infection and reactivation is extraordinarily intricate, and an accurate understanding of the relevant mechanism can provide new targets and strategies for the prevention and treatment of related infectious diseases, not only improving the effectiveness of existing viral diseases treatment, but also providing strategic reserves to deal with future emerging viruses.
Research on the mechanisms of persistent viral infection is primarily concerned with the regulation of viral replication and immune modulation. Studies of viral replication regulation and reactivation mainly include the virus genome encoding product and its structure, function and regulatory mechanism, the maintenance mechanism of viral genome replication in host cells, the regulation mechanism of viral genome transcription and replication, the integration mechanism of viral genome, the transformation mechanism of virus-lytic infection, the mode of virus-induced host cell death and its regulatory mechanism, the carcinogenic mechanism of virus infection, host limiting factors and their mechanisms of action.
The study of viral replication regulation and reactivation mainly include the mechanism of restriction of viral antigen expression, the mechanism of viral antigen mutation, the mechanism of immune pardoning site infection, the mechanism of virus-induced immunosuppression, and the mechanism of acquired immune depletion such as T cells exhaustion. Intervention strategies for persistent viral infection can be divided into two broad categories: targeting viral replication and targeting virus-specific immune responses. Targeted virus replication strategies are mainly aimed at blocking specific stages of the virus life cycle, including targeting the virus itself or host factors required for virus replication. Targeting virus-specific immune responses is mainly about restoring the host’s effective immune response to eliminate viral infection.
Due to the continuous development of virology, immunology, molecular biology, cell biology and other disciplines, prominent progress has been made in resolving the mechanism of persistent viral infection and reactivation. For example, human immunodeficiency virus (HIV) can evade immunological surveillance through genome integration and high variability, leading to persistent infection of lymphoid system cells. Varicella-zoster virus (VZV) can establish a state of immune evasion in various specialized cells and the central nervous system, resulting in persistent infection of the nervous system. Hepatitis B virus (HBV) can trigger T cell exhaustion, leading to persistent infection. Targeted viral replication is the most commonly used intervention strategy for viral infection, which mainly includes antiviral compounds, interferon and therapeutic target cell modification. Intervention strategies targeting virus-specific immune responses have not yet been widely used in clinical practice, but broad-spectrum neutralizing antibodies, toll-like receptor agonists, and therapeutic vaccines have manifested encouraging preliminary results in animal models and clinical trials.
Currently, in the engineering research frontier of “mechanism of persistent virus infection and reactivation and analysis of intervention targets”, the top three countries with the highest number of core publications are the USA, China, and Germany (Table 1.2.3). Among them, China accounts for 34.33% of the core publications, making it one of the major countries in research of this front. From the perspective of collaboration networks among main countries (Figure 1.2.4), there is close cooperation among the top ten countries.
《Table 1.2.3》
Table 1.2.3 Countries with the greatest output of core papers on “mechanism of persistent virus infection and reactivation and analysis of intervention targets”
| No. | Country | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | USA | 285 | 56.55 | 24544 | 86.12 | 2018.2 |
| 2 | China | 173 | 34.33 | 12280 | 70.98 | 2018.3 |
| 3 | Germany | 58 | 11.51 | 4749 | 81.88 | 2018.6 |
| 4 | UK | 56 | 11.11 | 5076 | 90.64 | 2018.7 |
| 5 | France | 51 | 10.12 | 4369 | 85.67 | 2018.4 |
| 6 | Canada | 31 | 6.15 | 3177 | 102.48 | 2018 |
| 7 | Japan | 26 | 5.16 | 1515 | 58.27 | 2018.5 |
| 8 | Australia | 21 | 4.17 | 1764 | 84 | 2018.6 |
| 9 | Netherlands | 18 | 3.57 | 1397 | 77.61 | 2018 |
| 10 | Belgium | 16 | 3.17 | 1087 | 67.94 | 2018.6 |
《Figure 1.2.4》
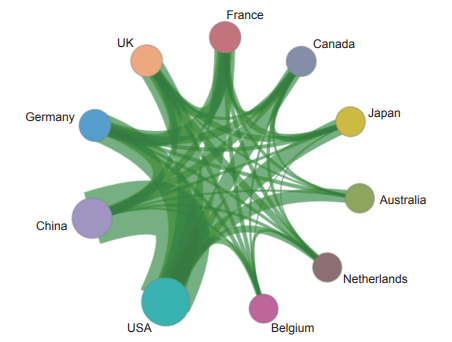
Figure 1.2.4 Cooperation network among major countries in the engineering research front of “mechanism of persistent virus infection and reactivation and analysis of intervention targets”
The top ten institutions with core papers published in the engineering research frontier of “mechanism of persistent virus infection and reactivation and analysis of intervention targets” were from the USA, China, and the UK. The top three institutions, including Harvard University, the US National Institute of Allergy and Infectious Diseases, and Chinese Academy of Sciences (Table 1.2.4) were from the USA and China. The collaboration network among major institutions (Figure 1.2.5) enunciates that there is strong cooperation among American scientific research institutions, and some cooperation among other institutions.
Based on the above statistical analysis results, China is currently in a parallel trend with similar foreign research in the frontier of “mechanism of persistent virus infection and reactivation and analysis of intervention targets”.
In-depth understanding of the mechanisms of persistent viral infection and reactivation urgently requires the strengthening of basic disciplines such as virology, immunology, molecular biology, and cell biology, as well as the development of cutting-edge biotechnologies and their deep integration with materials science and engineering technologies. This will greatly enhance our ability to decipher the mechanisms of persistent viral infection and reactivation, and enable the development of effective antiviral drugs, immunotherapies, cell therapies, and new-generation vaccines, as well as effectively support the prevention, control, diagnosis, and treatment of chronic or major infectious diseases.
《Table 1.2.4》
Table 1.2.4 Institutions with the greatest output of core papers on “mechanism of persistent virus infection and reactivation and analysis of intervention targets”
| No. | Institution | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | Harvard University | 50 | 9.92 | 5 480 | 109.6 | 2018.3 |
| 2 | National Institute of Allergy and Infectious Diseases | 31 | 6.15 | 2 259 | 72.87 | 2018.5 |
| 3 | Chinese Academy of Sciences | 21 | 4.17 | 1 771 | 84.33 | 2018.4 |
| 4 | Sun Yat-sen University | 19 | 3.77 | 1 545 | 81.32 | 2018.2 |
| 5 | University of Washington | 18 | 3.57 | 2 472 | 137.33 | 2018.3 |
| 6 | University of Pennsylvania | 17 | 3.37 | 1 836 | 108 | 2018.4 |
| 7 | University of California, San Francisco | 16 | 3.17 | 1 646 | 102.88 | 2018.4 |
| 8 | Chinese Academy of Agricultural Sciences | 15 | 2.98 | 838 | 55.87 | 2018.9 |
| 9 | Duke University | 14 | 2.78 | 1 079 | 77.07 | 2018.4 |
| 10 | University of Oxford | 13 | 2.58 | 1 303 | 100.23 | 2018.9 |
《Figure 1.2.5》

Figure 1.2.5 Cooperation network among major institutions in the engineering research front of “mechanism of persistent virus infection and reactivation and analysis of intervention targets”
For a long time, the research on the mechanism of persistent viral infection and reactivation has predominantly been based on the study of individual viruses or host genes, single signaling pathways, and single intervention targets. This limited approach has led to an incomplete and shallow understanding of the mechanisms involved, and the translation of basic research findings into clinical practice has been challenging. To address this, a systems biology approach is needed, utilizing new technologies such as multi-omics, artificial intelligence, and high-throughput screening, to provide a panoramic, dynamic, multi-scale, and multidimensional elucidation of the relevant mechanisms. The lack of suitable cell and animal models has also hindered the exploration of these mechanisms. The emergence of technologies such as organoids and humanized animal models can provide new research platforms to overcome these limitations.
In terms of intervention strategies, gene editing technology has been successfully applied to target a variety of viral genomes.
However, challenges still exist in terms of targeting efficiency, off-target effects, delivery platforms, and viral escape mutations. The research and development of therapeutic vaccines should be closely combined with material science and engineering technology to achieve breakthroughs in antigen design, delivery system, adjuvant development and other key aspects. Targeted blocking of immune checkpoints has shown some potential in combating HIV infection, but the selection of biomarkers, the development of drug resistance, and potential adverse reactions are pressing issues that need to be addressed. Multiple studies have shown that adoptive T-cell therapy is effective in clearing viral infections and enhancing antiviral immunity after stem cell transplantation. It has demonstrated promising prospects in preclinical studies for treating respiratory viruses or immunodeficiency viruses, and the indications for such therapies are expected to expand gradually. However, the safety and efficacy of these therapies still need further validation (Figure 1.2.6).
《Figure 1.2.6》
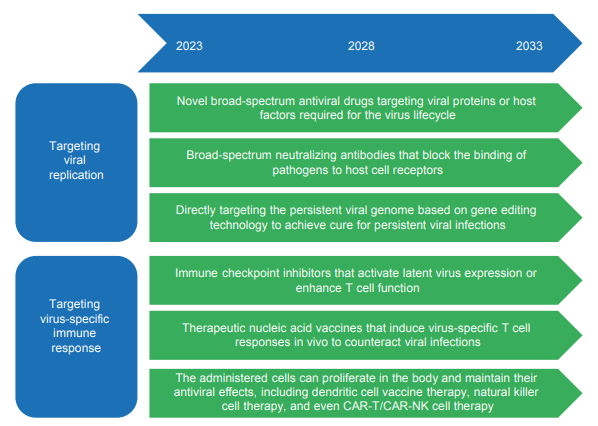
Figure 1.2.6 Roadmap of the engineering research front of “mechanism of persistent virus infection and reactivation and analysis of intervention targets”
1.2.3 The core human microbiome and host−microbiome interaction
The microbiome plays a crucial role in maintaining human health by participating in various physiological processes such as nutrient metabolism, immune regulation, inflammatory responses, and endocrine regulation. The identification of a universal signature of a healthy or unhealthy human microbiome is essential for maintaining a healthy status and preventing chronic diseases. However, due to the large inter-individual variation in human microbiota and the complex interplay between the microbiota and their host, the core human microbiome is yet to be defined.
The core human microbiome refers to the microbiota that is commonly shared among individuals and has significant impacts on human health. Despite the popularity of this term and its growing use, there is a lack of consensus on how a core microbiome should be quantified in practice. Therefore, identification of the core human microbiota and microbiome-targeting therapy represents a crucial frontier in medicine and healthcare enterprises.
To address these issues, researchers have initiated several large-scale, well-phenotyped cohorts to characterize the composition and function of the human microbiota in recent years. There are several landmark human microbiome projects, such as the US National Institutes of Health-funded Human Microbiome Project (HMP) and Dutch Microbiome Project (DMP). These projects mapped the human microbiome in their populations and revealed comprehensive profiles of microbiome compositions and functions using multi-omics data, such as metagenomics, metatranscriptomics, and metabolomics. They also explored the associations of microbial features with the host exposome and diverse diseases to clarify the contributions of host intrinsic factors, modifiable environmental factors, and health-related factors in shaping the human microbiome. To further disentangle meaningful host-microbiota interactions, they integrated human genetics data, leveraged longitudinal data, and utilized in silico mediation analyses to reveal putative causal relationships between the microbiome and their host phenome. To identify potential therapeutic targets for microbiota-directed intervention, experiments using gnotobiotic mice or piglets have been conducted to validate computationally identified targets and further confirm the mechanism underlying microbiota-host interactions. Together, these efforts provide a rich resource for dissecting the link between the human microbiome and health, thereby pinpointing future directions for microbiome- directed interventions.
However, the majority of clinical trials on microbiota-targeted interventions have reported a lack of efficacy. There are several reasons for this finding. First, the quantification of the core human microbiome in prior research primarily relies on abundance-based indicators, which may potentially overlook microorganisms that are ecologically and functionally significant. Second, existing studies investigating the microbiota-host relationship extensively rely on small-scale population studies or animal models. Although these small studies provide valuable insights, they may not fully capture the complexities and variations in larger human populations. Meanwhile, the biological differences between animals and humans can also limit the translation of findings from animal models towards humans; Thirdly, it is important to note that current sequencing technology and analysis methods have limitations in inferring causations from microbiome data; Lastly, due to substantial differences in diet, lifestyle and ethnicity, conclusions drawn from certain population may not be generalizable to other populations.
In the engineering research front of “the core human microbiome and host−microbiome interaction”, the top three countries with core papers published are the USA, China, and France (Table 1.2.5). Among them, China’s core papers account for 28.05%, making it one of the main research countries in this front. According to the cooperation network among major countries (Figure 1.2.7), the top ten countries in the number of core papers have close cooperative relationships.
In the engineering research frontier of “the core human microbiome and host−microbiome interaction”, the top ten institutions with the highest number of core papers were principally from the USA and European countries. The top three institutions, Harvard University, University of California, San Diego, and Wageningen University & Research, were from the USA and the Netherlands (Table 1.2.6). The collaboration network among the major institutions demonstrates that some institutions have cooperative relationships (Figure 1.2.8).
Therefore, there are several major challenges in human microbiome research, including identifying the core human microbiome, deciphering its role in regulating host physiology and pathology, and identifying microbiome-targeting therapies. To address these challenges, the development of a new framework and the incorporation of new technologies are crucial. We espouse the adoption of a multifaceted strategy, integrating diverse layers of omics data, which encompass comprehensive genome sequencing, single- cell sequencing, high-throughput culturomics, and longitudinal data analysis. This integrative approach is pivotal in fostering a deeper comprehension of causative inferences and mechanistic insights within the domain. Furthermore, the refinement of computational methodologies is indispensable, as it will facilitate the harmonious amalgamation of multi-omics data, thereby elucidating plausible mechanisms and pinpointing molecular targets for therapeutic intervention. Furthermore, we underscore the critical need to optimize the design of research studies, effectively expand microbiome inquiries within epidemiological populations, and ensure the reproducibility of translational outcomes. Lastly, we accentuate the urgency of incorporating innovative organ-on-chip technologies, as they hold the potential to enable comprehensive investigations into the interactions between the microbiome and the human organism. The synergistic integration of these approaches shall expedite the discovery of microbiota-related biomarkers and transition microbiome research from the realm of association to causality. Ultimately, this endeavor shall facilitate the design of robust therapeutic interventions aimed at modulating the composition of the gut microbiome.
In light of the aforementioned statistical analyses, it becomes evident that China is aligned with international trends in the research frontier concerning “the core human microbiome and host−microbiome interaction”. To harness the distinct advantages
《Table 1.2.5》
Table 1.2.5 Countries with the greatest output of core papers on “the core human microbiome and host−microbiome interaction”
| No. | Country | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | USA | 33 | 40.24 | 2 980 | 90.3 | 2018.7 |
| 2 | China | 23 | 28.05 | 2 065 | 89.78 | 2018.8 |
| 3 | France | 15 | 18.29 | 1 925 | 128.33 | 2018.7 |
| 4 | UK | 15 | 18.29 | 1 730 | 115.33 | 2018.8 |
| 5 | Australia | 13 | 15.85 | 1 605 | 123.46 | 2018.6 |
| 6 | Germany | 10 | 12.2 | 1 511 | 151.1 | 2018.8 |
| 7 | Canada | 10 | 12.2 | 1 370 | 137 | 2019 |
| 8 | Netherlands | 9 | 10.98 | 1 343 | 149.22 | 2019.4 |
| 9 | Italy | 8 | 9.76 | 702 | 87.75 | 2018.6 |
| 10 | India | 5 | 6.1 | 895 | 179 | 2019.2 |
《Figure 1.2.7》

Figure 1.2.7 Cooperation network among major countries in the engineering research front of “the core human microbiome and host−microbiome interaction”
《Table 1.2.6》
Table 1.2.6 Institutions with the greatest output of core papers on “the core human microbiome and host−microbiome interaction”
| No. | Institution | Core papers | Percentage of core papers/% | Citations | Citations per paper | Mean year |
| 1 | Harvard university | 5 | 6.1 | 279 | 55.8 | 2019.6 |
| 2 | University of California, San Diego | 4 | 4.88 | 359 | 89.75 | 2019.2 |
| 3 | Wageningen University & Research | 3 | 3.66 | 996 | 332 | 2019.3 |
| 4 | University of Minnesota | 3 | 3.66 | 759 | 253 | 2019 |
| 5 | University College Cork | 3 | 3.66 | 534 | 178 | 2019.7 |
| 6 | University of Kiel | 3 | 3.66 | 400 | 133.33 | 2017 |
| 7 | University of Helsinki | 3 | 3.66 | 397 | 132.33 | 2019 |
| 8 | King’s College London | 3 | 3.66 | 227 | 75.67 | 2019.3 |
| 9 | University of Munich | 3 | 3.66 | 222 | 74 | 2019.7 |
| 10 | University of New South Wales | 3 | 3.66 | 217 | 72.33 | 2018.7 |
of the Chinese populace, it is important to further exploit these resources. A high-caliber resource platform, centered on the human microbiome, should be established, concurrently with the meticulous delineation of microbiome maps specific to the Chinese population. This concerted effort is poised to comprehensively characterize the composition and functional attributes of the core microbiome within the Chinese demographic landscape.
These innovative strategies related to human microbiome research include but are not limited to fecal microbiota transplantation, engineered living biotherapeutics based on probiotics, prebiotics, and targeted nutritional interventions. Additionally, integrating host genetic information may also aid in the identification of key genetic loci that interact with the core human microbiome, facilitating the discovery of the core microbiota underlying disease pathogenesis. Consequently, this may propel the advancement of microbiome-based precision medicine, including the development of personalized probiotics, prebiotics, and postbiotics. These future directions are highlighted in the development roadmap of the project “core human microbiome and host−microbiome interaction” (Figure 1.2.9). In summary, it is necessary to continuously propel the development of technology, optimize intervention strategies targeting core microbial communities, and strengthen international cooperation and communication to identify the core microbiome that is responsible for maintaining host homeostasis.
《Figure 1.2.8》
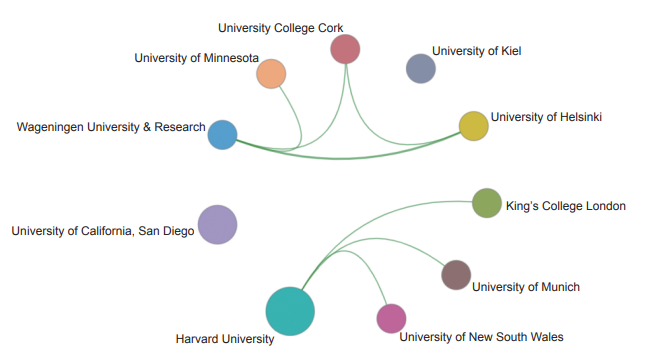
Figure 1.2.8 Collaboration network among major institutions in the engineering research front of “the core human microbiome and host−microbiome interaction”
《Figure 1.2.9》
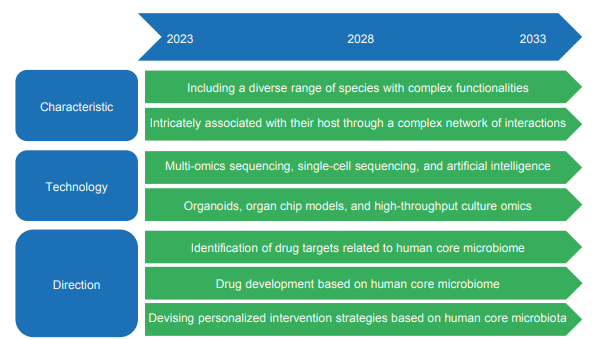
Figure 1.2.9 Roadmap of the engineering research front of “the core human microbiome and host−microbiome interaction”
《2 Engineering development fronts》
2 Engineering development fronts
《2.1 Trends in Top 10 engineering development fronts》
2.1 Trends in Top 10 engineering development fronts
This section of the review describes the Top 10 engineering development fronts in the field of medicine and health, including basic medicine, clinical medicine, pharmacy, traditional Chinese medicine, medical informatics, and biomedical engineering (Table 2.1.1). The emerging fronts are “T-cell receptor-engineered T-cell therapy”, “single-cell spatial transcriptomics technology”, “chimeric antigen receptor natural killer cell therapy”, “single-molecule protein sequencing”, “the application of a large language model in digital healthcare”, and “epigenetic editing technology”. Traditional research has focused on “combining antibody-drug conjugates with immunotherapy for malignancies”, “application of medical nanorobots in cancer treatment”, “technologies for synthetic immunology”, and “small nucleic acid drugs”. All patents related to these 10 fronts, published between 2017 and 2022, are listed in Table 2.1.2.
《Table 2.1.1》
Table 2.1.1 Top 10 engineering development fronts in medicine and health immunotherapy for malignancies
| No. | Engineering development fronts | Published patents | Citations | Citations per patent | Mean year |
| 1 | T cell receptor engineered T cell therapy | 429 | 1 447 | 3.37 | 2020.2 |
| 2 | Combining antibody-drug conjugates with immunotherapy for malignancies | 334 | 1 815 | 5.43 | 2019.9 |
| 3 | Single-cell spatial transcriptomics technology | 162 | 942 | 5.81 | 2020.4 |
| 4 | Chimeric antigen receptor natural killer cell therapy | 332 | 829 | 2.5 | 2020.5 |
| 5 | Application of medical nanorobots in cancer treatment | 2 505 | 5 842 | 2.33 | 2020.3 |
| 6 | Technologies for synthetic immunology | 431 | 726 | 1.68 | 2019.6 |
| 7 | Small nucleic acid drugs | 1 723 | 3 078 | 1.79 | 2019.8 |
| 8 | The application of large language model in digital healthcare | 398 | 2 302 | 5.78 | 2019.7 |
| 9 | The application of large language model in digital healthcare | 2 042 | 7 062 | 3.46 | 2020.7 |
| 10 | Epigenetic editing technology | 97 | 552 | 5.69 | 2020.2 |
《Table 2.1.2》
Table 2.1.2 Annual number of core patents published for the Top 10 engineering development fronts in medicine and health immunotherapy for malignancies
| No. | Engineering development fronts | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
| 1 | T cell receptor engineered T cell therapy | 23 | 31 | 83 | 86 | 101 | 105 |
| 2 | Combining antibody-drug conjugates with immunotherapy for malignancies | 44 | 44 | 35 | 59 | 74 | 78 |
| 3 | Single-cell spatial transcriptomics technology | 13 | 8 | 21 | 27 | 38 | 55 |
| 4 | Chimeric antigen receptor natural killer cell therapy | 14 | 25 | 35 | 62 | 87 | 109 |
| 5 | Application of medical nanorobots in cancer treatment | 227 | 221 | 276 | 426 | 679 | 676 |
| 6 | Technologies for synthetic immunology | 63 | 73 | 72 | 57 | 86 | 80 |
| 7 | Small nucleic acid drugs | 239 | 241 | 271 | 301 | 299 | 372 |
| 8 | The application of large language model in digital healthcare | 54 | 56 | 56 | 76 | 89 | 67 |
| 9 | The application of large language model in digital healthcare | 62 | 99 | 203 | 355 | 630 | 693 |
| 10 | Epigenetic editing technology | 9 | 6 | 22 | 7 | 28 | 25 |
(1) T cell receptor engineered T cell therapy
T-cell receptor-engineered T-cell therapy (TCR-T) represents a novel cellular immunotherapy modality that entails the modification of T lymphocytes through the introduction of exogenous TCRs with the specific capacity to recognize the antigenic peptide- major histocompatibility complex (pMHC) displayed on tumor cells. This strategic alteration redirects T cells to mount targeted immune responses against malignancies. In 2002, the pioneering work of Rosenberg’s group unveiled a seminal discovery: tumor- infiltrating lymphocytes (TILs) could selectively eliminate tumor cells following in vitro expansion and infusion. However, TILs pose accessibility limitations for various tumor types and necessitate prolonged cultivation to obtain clinically relevant quantities. Consequently, researchers embarked on the exploration of whether ordinary peripheral blood lymphocytes (PBLs) could be genetically engineered with TCR genes to combat tumors. This quest culminated in 2006 when Rosenberg’s group demonstrated the clinical efficacy of genetically modified TCR-T cells in the context of melanoma, marking the inaugural proof of concept for TCR-T immunotherapy. Over more than two decades of rapid evolution, TCR-T immunotherapy has garnered global adoption for evaluating its clinical potential in the realm of solid tumors, exhibiting promising efficacy in clinical trials. Nevertheless, numerous challenges persist in engineering TCR-T cells with the requisite affinity and functionality to eliminate tumors and forestall recurrences. These hurdles encompass the selection of target antigens, evasion mechanisms employed by tumors to evade immune responses, concerns regarding off-target effects and safety, T cell unresponsiveness, and the phenomenon of T cell exhaustion. Conquering these challenges stands as a pivotal prerequisite for the realization of clinical success in TCR-T immunotherapy. Furthermore, a landmark achievement was reached in 2022 with the FDA’s approval of Kimmtrak, a bispecific T-cell junction product developed by Immunocore. This regulatory endorsement marks a significant breakthrough in the field of TCR therapy, underscoring its growing prominence in the ongoing battle against cancer. Therefore, despite persistent barriers in TCR-T cell therapy, it is conceivable that ongoing scientific research and technological advancements will usher in novel breakthroughs, further advancing the frontiers of cancer treatment.
(2) Combining antibody-drug conjugates with immunotherapy for malignancies
Antibody-drug conjugates (ADCs) are composed of three units: ① an antibody that is selectively targeted to the tumor microenvironment, ② a linker that connects the antibody to the conjugate, and ③ a drug that exerts anti-tumor activity as a payload. A typical ADC has a small-molecule cytotoxic drug as the payload and a monoclonal antibody as the targeting moiety, which can specifically bind to tumor-associated antigens. This enables the conjugate to attack cancer cells precisely, with minimal harm to normal cells. To date, 15 ADC products have been approved and marketed worldwide, including six for hematological malignancies and nine for solid tumors, directed to CD33, CD30, CD22, CD79b, HER2, Nectin-4, Trop-2, BCMA, EGFR, CD19, and TF. Among them, five have been put on the market in China.As researchers make attempts to use a variety of drugs as the payload in ADCs, the concept of “antibody-everything conjugates” has been put forward. The payloads of ADCs can be generalized to include non-cytotoxic small-molecule drugs, cytokines, enzymes, oligonucleotides, bacterial exotoxins, biopolymers, radionuclides, and photosensitizers. For the antibody unit, the targeting moiety can also be nano-antibodies, bispecific antibodies, etc. in addition to the classical monoclonal antibodies. In recent years, it has been found that classical cytotoxic ADCs can mediate immunogenic cell death, thereby increasing the immunogenicity of tumor tissues and promoting the intratumoral recruitment of immune cells to kill “cold tumors”. Based on this, it is believed that cytotoxic ADCs can be combined with tumor immunotherapies to manage malignancies. Furthermore, when an immunomodulatory drug is used as the payload, such an ADC can directly treat malignancies by modulating the immunity against tumors.
(3) Single-cell spatial transcriptomics technology
With the advent of the era of precision medicine, single-cell multi-omics technology is driving the studies of cancer, developmental biology, microbiology, immunology, and neuroscience, gradually becoming the focus of various disciplines of life sciences. However, during the process of cell dissociation, conventional single-cell sequencing technologies inevitably lose information regarding the spatial organization of cells essential for the functionality of complex tissue organs. This caveat can be overcome by single-cell spatial transcriptomics technology, which not only obtains transcriptome profiles of individual cells, but also locates the three- dimensional ordinates of transcripts and the cells expressing them. This, in turn, allows for the restoration of the architecture of the cell-type distribution and the internal operation of cell-to-cell communication between discrete cell subpopulations in different environments and organ systems. In 2020, single-cell spatial transcriptomics technology was named the technology of the year by Nature Methods, illustrating the enormous potential that this new technology. In the future, single-cell spatial transcriptomics technology will be able to change the understanding of complex tissues in various research fields. In particular, comparative studies of diseased and healthy tissues using single-cell spatial transcriptomics technology will prove useful in improving patient prognosis, optimizing therapeutic strategies, and uncovering potential therapeutic targets.
(4) Chimeric antigen receptor natural killer cell therapy
Chimeric antigen receptor natural killer (CAR-NK) cell therapy embodies an innovative cellular immunotherapy strategy wherein natural killer (NK) cells undergo genetic engineering with chimeric antigen receptor (CAR) genes, conferring upon them the capacity to selectively recognize and target tumor cells. These genetically modified NK cells undergo ex vivo expansion and are subsequently administered to patients to exert therapeutic effects in the context of cancer treatment. The genetic blueprint of the chimeric antigen receptor encompasses extracellular domains, including CARs (or NKR/TCR), facilitating precise tumor cell targeting, transmembrane domains, and intracellular signaling domains. Furthermore, functional components aimed at bolstering cell survival, promoting immune cell infiltration, and conferring resistance to the tumor microenvironment can be integrated into NK cells using viral or non-viral delivery systems. Following rigorous in vitro expansion and cultivation, CAR-NK cells are introduced into patients. CAR-NK cell therapy primarily finds its application in the domain of cancer treatment but extends its utility to clinical therapies for autoimmune conditions, infectious diseases, and age-related ailments. NK cells, recognized as “natural killers” of tumor cells within the body, offer inherent advantages in terms of safety, versatility, and off-the-shelf availability. They originate from diverse sources and offer cost-effective solutions, presenting considerable promise for the treatment of solid tumors. These inherent advantages position CAR-NK cell therapy as a promising avenue for broad application and industrial-scale production, thereby offering an expansive market outlook. A multitude of ongoing clinical trials focused on CAR-NK cell therapy attest to its safety and efficacy. This has garnered the attention of international stakeholders, propelling intensified research and development efforts within the NK cell arena and fostering the sustained expansion of the global market. Presently, immunotherapy encounters challenges in precisely and controllably regulating the in vivo activity of immune cells. Therefore, the application of synthetic biology techniques to CAR-NK cell immunotherapy, encompassing logic circuits, feedback mechanisms, intelligent control systems, and related technologies, facilitates quantitative, controlled, and scalable manipulation of NK cell functions. This groundbreaking approach yields intelligent CAR-NK cell therapeutics, characterized by their “living” nature, manipulability, and intelligence as synthetic immune cell drugs. This scalable and industrialized methodology surmounts existing limitations in immunotherapy effectiveness and holds potential for application in the treatment of major diseases, ultimately shaping the future of immunotherapy.
(5) Application of medical nanorobots in cancer treatment
An injectable medical antitumor nanorobot is a cutting-edge nanoscale functional assembly created by sophisticated nanofabrication techniques and applied for precise tumor localization, diagnosis, and treatment in vivo via intravenous injection. Nanorobots are typically fabricated from nanoscale biological materials that have undergone chemical and biological modifications to enhance their functionality. It can be effectively powered and controlled using various energy sources, including blood flow, chemical energy, magnetic fields, light waves, acoustic energy, and bioenergy. Moreover, nanorobots can sense and respond to pathological and physiological stimuli in the tumor microenvironment, which enables them to activate or deactivate specific functions, such as the controlled release of medications. Medical nanorobots hold vast application potential for cancer treatment, encompassing tumor monitoring, diagnosis, tumor microenvironment regulation, and comprehensive tumor therapy. Currently, the development of medical nanorobots is still in its infancy and faces several challenges. Key technical obstacles that must be addressed for clinical applications include scaling up the production of these nanorobots, ensuring their biosafety once injected into the body, and resolving the complexities of autonomous navigation and precise control within the bloodstream. Despite these technical challenges, medical nanorobots demonstrate immense potential, representing the future of precise drug delivery. They are expected to provide more accurate and personalized treatment for cancer patients, improve therapeutic efficacy, and prolong patient survival. The field is set to evolve in several key aspects: diversifying functionalities, enhancing intelligent driving mechanisms, and incorporating biodegradable materials. Furthermore, the integration of medical nanorobots with emerging technologies such as artificial intelligence and machine learning could further allow autonomous decision-making and self-optimizing treatment plans. As a novel approach to combat tumors, these nanorobots are anticipated to address some critical issues of traditional cancer treatments, such as low response rates, poor prognoses, and/or drug resistance.
(6) Technologies for synthetic immunology
Currently, the effectiveness of immunotherapy in the treatment of most tumors, especially solid tumors, is limited. Therefore, it is crucial to make significant advancements in the associated technologies and approaches. Additionally, traditional immunological engineering has evolved into the synthetic biology stage, giving rise to Synthetic Immunology, a novel discipline that combines basic immunology with modern synthetic biological technologies. Synthetic Immunology focuses on various major diseases such as tumors, autoimmune diseases, viral infections, and organ transplantation. By utilizing functional units/modules of immune molecules, cells, or systems, Synthetic Immunology employs binary systems and logical circuits for calculations. It designs and constructs logical gates, switches, feedback loops, oscillators, and other functional modules, forming synthetic immunological circuits that enable logical calculations to generate intelligent, controllable, more effective, and less toxic immune responses, thereby promoting the safety and efficacy of immunotherapy. The goal of Synthetic Immunology is to develop, scale up, and industrialize immunotherapy strategies against major diseases. It achieves this by renormalizing, redirecting, and reconstituting the host immune system through predictable, quantifiable, regulatable, and programmable rational designs. Advancements in Synthetic Immunology have significantly contributed to the development of modern immunotherapy theories, technologies, and products for the treatment of major diseases. In the future, there will be a demand for improved gene delivery capabilities to facilitate the design and application of synthetic immune cells with more complex gene circuits and enhanced intelligence.
(7) Small nucleic acid drugs
Small nucleic acid drugs refer to drugs can specifically silence the expression of disease genes using small nucleic acid molecules such as antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and microRNAs (miRNAs) to cure specific diseases. These include ASOs, siRNAs, miRNAs, small activating RNAs (saRNAs), messenger RNAs (mRNAs), and RNA aptamers. The biggest challenge in the development of small nucleic acid drugs is to ensure that the drug stays in the body long enough and enters the targeted cells accurately to exert therapeutic effects while minimizing damage to normal cells after injection into patients. These problems can be solved by chemical modification and delivery systems to make nucleic acid drugs effective. The advantage of small nucleic acid drugs is their specificity in targeting multiple genes to treat diseases, thereby interfering with cell proliferation, angiogenesis, metastasis, and chemotherapy resistance. These advantages have led to the development of small nucleic acid drugs for a variety of diseases, including tumors, rare diseases such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, spinal muscular atrophy, viral diseases, kidney diseases, and cardiovascular diseases. The first small nucleic acid drug was launched in 1998, and currently, there are more than ten small nucleic acid drugs on the market worldwide, with about 80% launched after 2015. From the perspective of indications, most small nucleic acid drugs on the market are for genetic diseases. Compared with existing small molecule and antibody drugs, small nucleic acid drugs have the advantages of fast target screening, high R&D success rate, low drug resistance, broader treatment areas, long-lasting effects, and great development potential. In the future, with continuous breakthroughs and innovations in the application and technical fields of small nucleic acid drugs, the market demand and scale will continue to expand, and small nucleic acid drugs will have a broader development space.
(8) Single-molecule protein sequencing
Single-molecule protein sequencing is a technique for measuring the amino acid sequences that make up proteins at the single- molecule level. Protein sequencing presents a greater challenge than nucleic acid sequencing; proteins are complex and composed of 20 natural amino acids (in contrast, DNA molecules are formed from four nucleotides). Some proteins are only a few molecules in the cell, and for proteins, there are no similar techniques, such as nucleic acid amplification, making it difficult to detect low- abundance proteins. Protein sequencing studies are of great significance for the prediction of protein structure, detection of diseases, and development of protein drugs. The realization of single-molecule protein sequencing technology will bring new opportunities to proteomic research, digital biology, disease diagnosis, and medical development. Because proteins can provide profound information about health and disease, research on single-molecule protein sequencing technology has received much interest, and significant advances have been made in single-molecule protein sequencing methods based on fluorosequencing and nanopore technology, such as the optical protein sequencing chip by Quantum-Si and single amino acid identification in nanopores. Technologies with better spatiotemporal resolution, high-throughput sequencing methods, and more accurate and rapid signal analysis algorithms will be key to promoting major breakthroughs in single-molecule protein sequencing. The deep integration of single-molecule protein sequencing and proteomics, medical research, and artificial intelligence will bring new progress for the analysis of protein structure, early diagnosis of diseases, and development of biological drugs, while promoting the development of single-molecule protein sequencing into a convenient and rapid biotechnology with diversified application scenarios in the future.
(9) The application of large language model in digital healthcare
Large language models (LLMs) refer to language models trained on large-scale textual corpora that contain billions of levels (or more) of parameters, aimed at understanding and generating human language. They are trained on a large amount of text data and can perform a wide range of tasks, such as text summarization, translation, and sentiment analysis, among which GPT-4 is the most popular. LLMs include three main steps when processing input data: first, word embedding and converting words into high- dimensional vector representations, and then passing the data through multiple transformer layers. Finally, after being processed by the transformer layer, the model predicts the next most likely word or marker in the sequence based on the context to generate text. LLMs have a powerful ability to understand and generate text, making them stand out in the rapid development of modern medicine. In improving the level of medical diagnosis and treatment, LLMs help clinical doctors standardize the textual data generated during the diagnosis and treatment process and provide more accurate medical diagnosis and personalized treatment suggestions. In the field of medical image-assisted diagnosis, LLMs help radiologists interpret medical images, generate structured descriptive reports with standardized formats and language, and improve the quality and efficiency of image data management and deep mining. In promoting new drug research and development, LLMs assist clinical researchers in developing new treatment methods and drugs, identifying potential drug targets, and predicting drug side effects by achieving the largest global database- level literature review and meta-analysis. In terms of patient health management, LLMs combine individual genes, medical history, lifestyle habits, and other information to intelligently provide personalized medical and health management advice, helping people better prevent and self-manage chronic diseases. It can be foreseen that the future prospects of LLMs in the field of digital healthcare are full of hope. Although there are still challenges in ethical governance, data security, and human-machine collaboration, with the gradual maturity of the digital healthcare ecosystem and the intergenerational leap of artificial intelligence technology, the integration of LLMs and medical professional knowledge will completely change the traditional medical service model, giving new meaning and connotation to intelligent medical analysis and decision-making.
(10) Epigenetic editing technology
Epigenetic editing (EE) is a technology that precisely regulates the expression of target genes by altering epigenetic markers, such as DNA methylation and histone modification, without changing the genome sequence. This technology maintains the high specificity of traditional gene-editing tools while avoiding the potential genetic risks associated with DNA damage. Epigenetic editing technology can also simultaneously edit multiple targets to achieve precise and highly controlled adjustments, making it widely applicable in the treatment of complex polygenic diseases and personalized therapy. With the accumulation of non-clinical research data on epigenetic editing technology and the development and maturation of nucleic acid drug delivery systems, this technology will gradually move into the clinical stage, providing new treatment options for patients with complex diseases such as cancer, diabetes, viral infections, and autoimmune diseases. Based on the broad prospects of epigenetic editing technology in the field of disease treatment, China and developed countries in Europe and the USA attach great importance to research and development investments in this technology. At present, the research and development of epigenetic editing therapeutic drugs have achieved initial results, and many companies have disclosed preclinical research data. The development of high-precision and diverse epigenetic editing toolboxes, safe and efficient nucleic acid drug delivery systems, and large-scale production of nucleic acid drugs will be key to the future clinical transformation of epigenetic editing technology. The deep integration of epigenetic editing technology with epigenomics, organic chemistry, nanoscience, and artificial intelligence technology will provide new ideas for the optimization of epigenetic editing tools and the development of delivery systems, bringing great benefits to the treatment of many diseases.
《2.2 Interpretations for three key engineering development fronts》
2.2 Interpretations for three key engineering development fronts
2.2.1 T cell receptor engineered T cell therapy
T-cell receptor-engineered T-cell therapy (TCR-T) is a new type of cellular immunotherapy. A TCR gene that can specifically recognize tumor antigens is transfected into the patient’s own T cells to express specific exogenous TCRs. The CD3 molecule transmembrane region on the T cell membrane is connected to the transmembrane region of the two chains of TCR through a salt bridge to form a TCR-CD3 complex, which effectively recognizes and binds to pMHC, thereby generating the first signal to activate T cells and forming an immune synapse with the participation of other cooperative signaling molecules to promote T cells. Cell division and differentiation further guide T-cells to kill injured and diseased cells.
TCR-T can recognize extracellular antigens, membrane antigens, and intracellular antigens, thus can recognize over 90% of antigens. Cancer is one of the leading causes of death worldwide and an important public health issue. Data show that there are currently an estimated 18.1 million new cancer cases and 9.6 million cancer deaths. The three traditional treatment methods of surgery, chemotherapy, and radiotherapy (RT) have shortcomings, such as large wounds, drug resistance, and easy tumor recurrence. Immunotherapy, including TCR-T-cell therapy, suppresses tumor formation and growth by enhancing the human immune system. Immunotherapy has become an increasingly reliable cancer treatment method over the past two decades, and is expected to form a new pillar industry. TCR-T-cell immunotherapy is widely used to treat cancer, viral infections, and other diseases. TCR-T cell therapy, currently in the clinical trial stage, is mainly studied for solid tumors.
In 2002, Rosenberg’s team and Cassian Yee’s team discovered that tumor-infiltrating lymphocytes (TIL) isolated from patients with melanoma and antigen-specific CD8+ T cells isolated from the patient’s peripheral blood could be expanded in vitro. After the increase is infused back into the patient, it can specifically kill tumor cells and achieve a certain therapeutic effect. However, these T cells are difficult to obtain, take a long time to expand in vitro, and have weak anti-tumor effects after reinfusion. Therefore, researchers have explored whether known antigen-specific TCR genes could be introduced into normal peripheral blood lymphocytes (PBL). This is the origin of TCR T cells. An article published by Rosenberg’s group in Science in 2006 showed that genetically modified TCR-T cells showed good application prospects in the treatment of melanoma, as 2 of the 17 patients participating in the trial developed an anti-tumor response. This study demonstrated, for the first time, the feasibility of genetically modified TCR for tumor treatment.
The development of TCR-T cell technology has undergone four key iterations, each contributing to its evolving efficacy and applicability. The first generation involved isolating tumor-infiltrating T cell subsets from a patient’s tumor tissue, amplifying them in vitro, and reinfusing them for treatment; however, the scarcity of such T cell clones and individual variability posed challenges to industrial scalability. The second generation advanced this by obtaining antigen-specific wild-type TCR sequences through T cell cloning and transducing them into the patient’s peripheral T cells, thereby enhancing the technology’s industrial potential. The third generation employed affinity-optimized TCR genes to transduce either patient-specific or universal T cells, aiming to improve tumor infiltration, resistance to depletion, and anti-tumor immune suppression, thereby enhancing both the safety and efficacy of TCR-T therapy. The fourth generation builds upon this foundation by incorporating anti-depletion mechanisms, tumor chemotaxis and infiltration pathways, metabolic adaptations to the tumor microenvironment, and universal functions. These advancements not only bolster the anti-tumor capabilities of TCR-T cell immunotherapy but also enhance its safety and efficacy, with the potential to alleviate the economic burden on patients.
Global TCR-T therapy mainly targets the solid tumor market and has become an international research hotspot. Until September 2023, nearly 300 studies using TCR-T therapies are ongoing on Clinical Trials. Indications include metastatic non-small cell lung cancer, hepatocellular carcinoma, multiple myeloma, soft tissue sarcoma, head and neck cancer, melanoma, liposarcoma, and cervical cancer. As a TCR drug targeting pMHC, Kimmtrak, a bispecific T cell adapter developed by Immunocore in 2022 that can redirect T cells in vivo, was the first to obtain FDA approval for TCR therapy, marking a milestone breakthrough in TCR therapy targeting pMHC.
The countries with the largest number of core patent output are the USA, China, and the UK (Table 2.2.1). Cooperation between the USA and China, Germany, Switzerland, and the UK is more frequent (Figure 2.2.1). The top institutions in terms of core patent output are the USA, as represented by the Secretary Department of Health and Human Services, Guangdong Xiangxue Life Sciences Company Limited, and University of Texas (Table 2.2.2). There is no cooperative relationship among these institutions. It can be seen from the core patent-producing countries and the top institutions that China is in the forefront of the world in the R&D and industrial layout of TCR-T therapy.The number of TCR-T clinical applications in China ranks second in the world. Guangdong Xiangxue Life Sciences Company Limited, Beijing Dingcheng Taiyuan Biotechnology Company Limited, Chengdu Exab
《Table 2.2.1》
Table 2.2.1 Countries with the greatest output of core patents on “T cell receptor engineered T cell therapy”
| No. | Country | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations per patent |
| 1 | USA | 372 | 44.66 | 2 560 | 64.63 | 6.88 |
| 2 | China | 278 | 33.37 | 691 | 17.45 | 2.49 |
| 3 | UK | 51 | 6.12 | 262 | 6.61 | 5.14 |
| 4 | Germany | 37 | 4.44 | 196 | 4.95 | 5.3 |
| 5 | Japan | 21 | 2.52 | 40 | 1.01 | 1.9 |
| 6 | Republic of Korea | 16 | 1.92 | 38 | 0.96 | 2.38 |
| 7 | France | 14 | 1.68 | 89 | 2.25 | 6.36 |
| 8 | Switzerland | 13 | 1.56 | 162 | 4.09 | 12.46 |
| 9 | Canada | 12 | 1.44 | 38 | 0.96 | 3.17 |
| 10 | Israel | 10 | 1.2 | 10 | 0.25 | 1 |
Note: The selected publication date is from January 1, 2017 to December 31, 2022, the same below.
《Figure 2.2.1》
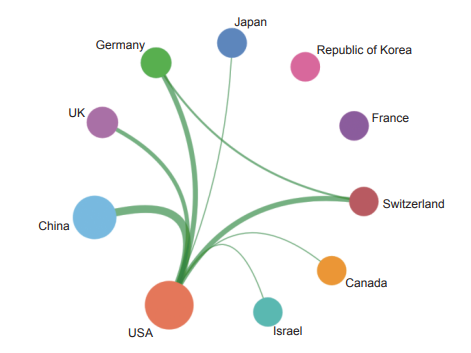
Figure 2.2.1 Collaboration network among major countries in the engineering front of “T cell receptor engineered T cell therapy”
Biotechnology Company Limited, and Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences have become the top institutions in terms of core patent outputs. A team of Chinese scholars from Guangdong Xiangxue Life Sciences Company Limited led to the application of China’s first IND for TCR-T TAEST16001. The objective response rate (ORR) for soft tissue sarcoma in Phase I clinical trials was 41.7%.
Although TCR-T cells therapy has great potential in treating tumors, how to make it widely used in clinical practice and exert its optimal efficacy is the urgent focus of TCR-T cell therapy, mainly including: how to reduce manufacturing cost and complexity, how to improve T cell persistence, how to hostile TME, and how to promote epitope spreading in order to address tumor cell escape. With other sophisticated engineering technologies such as artificial intelligence (AI), CRISPR-Cas9, high throughput screening and single cell sequencing, TCR-T cell therapy has made significant progress in antigen/target prediction, optimal affinity of TCR screening and the stability of T cell function. Therefore, the establishment of antigen target prediction model based on AI, the construction of new generation of TCR-T cells with the ability to change the TME in order to eliminate tumors, and the construction of universal TCR-T product will further deepen the application of TCR-T cells therapy in clinical practice. Figure 2.2.2 shows the roadmap of the engineering development front of “T cell receptor engineered T cell therapy”.
《Table 2.2.2》
Table 2.2.2 Institutions with the greatest output of core patents on “T cell receptor engineered T cell therapy”
| No. | Institution | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations per patent |
| 1 | United States Department of Health and Human Services | 23 | 2.76 | 162 | 4.09 | 7.04 |
| 2 | Guangdong Xiangxue Life Sciences Company Limited | 22 | 2.64 | 91 | 2.3 | 4.14 |
| 3 | University of Texas | 21 | 2.52 | 90 | 2.27 | 4.29 |
| 4 | Beijing Dingcheng Taiyuan Biotechnology Company Limited | 19 | 2.28 | 3 | 0.08 | 0.16 |
| 5 | TCR2 Therapeutics Incorporated | 18 | 2.16 | 118 | 2.98 | 6.56 |
| 6 | Chengdu Exab Biotechnology Company Limited | 16 | 1.92 | 65 | 1.64 | 4.06 |
| 7 | University of Pennsylvania | 13 | 1.56 | 97 | 2.45 | 7.46 |
| 8 | University of California | 12 | 1.44 | 54 | 1.36 | 4.5 |
| 9 | Guangzhou Institute of Biomedicine and Health,Chinese Academy of Sciences | 12 | 1.44 | 32 | 0.81 | 2.67 |
| 10 | Adaptimmune Company Limited | 10 | 1.2 | 86 | 2.17 | 8.6 |
《Figure 2.2.2》

Figure 2.2.2 Roadmap of the engineering development front of “T cell receptor engineered T cell therapy”
2.2.2 Combining antibody-drug conjugates with immunotherapy for malignancies
The extent and duration of clinical benefits of using ADC alone remain suboptimal due to resistance mechanisms and patient differences. However, some ADCs have demonstrated potential for tumor immunotherapy in immunologically normal preclinical animal models, with potential mechanisms by which ADCs mediate immunogenic cell death, enhance immune infiltration, and promote PD-L1 or MHC expression to increase the sensitivity of immunotherapy. The combination of ADCs with immunotherapy is an emerging strategy, and extensive randomized clinical trials are needed to validate its superiority over standard treatments.
Combination with anti-PD-1/PD-L1 or anti-CTLA-4 antibody. Exploratory research has been conducted on HER2-targeted ADCs, such as trastuzumab emtansine (linked to DM1), trastuzumab deruxtecan (linked to Dxd), and disitamab vedotin (linked to MMAE), in combination with immune checkpoint inhibitors (ICIs), and synergistic inhibitory effects on tumor progression have been demonstrated. In an open, randomized clinical trial of ADC in combination with ICI (Study KATE2) comparing the efficacy of trastuzumab emtansine plus atezolizumab (an anti-PD-L1 antibody) and trastuzumab emtansine plus a placebo in patients with previously treated HER2+ breast cancer, the combination regimen was shown to be beneficial to the PD-L1-positive population. In models of patients with ICI-refractory melanoma and non-small cell lung cancer, enapotamab vedotin, an AXL-specific ADC, induced T cell infiltration and enhanced antigen presentation, promoting the efficacy of anti-PD-1 antibody and effectively exerting anti-tumor effects.
Combination with other immunotherapy. Polatuzumab vedotin (directed to CD79b, linked to MMAE) enhances CD20 abundance in tumors through the AKT and ERK signaling pathways, leading to a combinatorial effect with anti-CD20 antibody and CD20/CD3 bispecific antibody therapies. Belantamab mafodotin (directed to BCMA, linked to MMAF) can be combined with OX40 agonists to increase the activation of tumor-infiltrating T cells and dendritic cells for synergistic anti-tumor effects. This drug has been actively undergoing combination studies in clinical settings, such as in the DREAMM-5 project, to explore its value in combination with anti- ICOS antibodies, OX40 agonists, and anti-PD-1 antibodies.
Immune-stimulating antibody conjugates, composed of an immunostimulatory small-molecule compound linked to an antibody, an immune-stimulating antibody conjugate (ISAC), are designed to trigger the activation of tumor-associated myeloid cells and thus induce anti-tumor immune responses in a comprehensive manner. Although ISACs have the same structure as traditional ADCs, that is, the conjugate of an antibody and a small molecule, they often incorporate TLR agonists or Sting activators as small molecules, which differs from the common use of cytotoxic drugs in traditional ADCs. Moreover, the antibodies in ISACs can be targeted to tumor-associated myeloid cell antigens in addition to tumor-specific antigens, whereas those in traditional ADCs are mostly targeted only to the latter.
By utilizing antibodies, ISACs can mediate the targeted delivery of small-molecule immune agonists to the tumor microenvironment and release the payload locally, providing a solution to the immunotoxicity of small-molecule agonists in systemic administration. In terms of the mechanism of action, ISACs can activate tumor-associated myeloid cells through antibody-mediated endocytosis and increase their antigen-presenting function to further activate T cells, ultimately killing tumor cells by driving both innate immunity and adaptive immunity, and inducing long-term immune memory. Moreover, ISACs can successfully convert cold tumors into hot tumors, solving the problem of the low response rate to single immune checkpoint inhibitors.
Currently, leading companies engaged in ISACs include Bolt Biotherapeutics, Silverback Therapeutics, Tallac Therapeutics, Mersana Therapeutics, Takeda, and ImmuneSensor Therapeutics. Additionally, Jacobio, Chinook Therapeutics, Novartis, Sutro Biopharma, ALX Oncology, Seven and Eight Biopharmaceuticals, and Chinese companies such as Hengrui Pharmaceuticals, BeiGene, Innovent Bio, Shanghai Institute of Materia Medica, and Shenzhen University have also made plans for this field. Among them, Bolt Biotherapeutics, Silverback Therapeutics, and Tallac Therapeutics focus on TLR agonist-based ISACs, and Mersana Therapeutics, Takeda, and ImmuneSensor Therapeutics focus on Sting agonist-based ISACs. The most popular targets of these antibodies are HER2, NECTIN4, CCR2, CD22, PD-L1, SIRPA, ASGR1, TROP-2, and CD73. Despite the above, ISACs still need to be explored and optimized because of their low response rates and difficulties in dose escalation in clinical settings.
In addition to the small-molecule immune agonist-antibody conjugate, immune-stimulating cytokine-antibody conjugates have also been extensively investigated in preclinical and clinical settings. Commonly used cytokines include IL-2, IFN-γ, and IL-15.
Currently, the countries with a notable number of core patent output in this front are the USA, China, and Switzerland (Table 2.2.3). As shown in the cooperation network among major countries, there is a cooperative relationship between Germany and the Netherlands, and between the USA and China, Switzerland, and other countries (Figure 2.2.3). Regeneron Pharmaceuticals Inc. (US), Immunomedics Inc. (US), and Sapreme Technologies (Netherlands) have the highest number of core patent (Table 2.2.4). There are collaborations between Sapreme Technologies (Netherlands) and Charité–Universitätsmedizin Berlin (Germany), and between The University of Texas System and Immunomedics Inc. (US) (Figure 2.2.4).
At present, the global pipelines of ADCs under development focus on the five major diseases: non-small cell lung cancer, gastric cancer, ovarian cancer, colorectal cancer, and breast cancer. The pipelines in China are mainly distributed in five major tumors: non-small cell lung cancer, gastric cancer, gastroesophageal junction cancer, breast cancer, and urothelial cancer. The most popular target of the underdeveloped ADC pipelines is HER2. Based on the development and application of the project of “combining antibody-drug conjugates with immunotherapy for malignancies”, in terms of combination therapy, it is necessary to screen out optimal combinations of ADCs with tumor immunotherapy, gradually determine the combination strategy and mechanism that can synergistically combat malignant tumors, and improve the clinical response rate and efficacy of ADCs and tumor immunotherapy. From the perspective of ISAC development, it is necessary to explore the combination of antibody targets and immunostimulatory payload targets, and optimize or upgrade the types of antibodies and linkers, conjugation strategies, payload activity, etc., to maximize the therapeutic window of ADC products and achieve a balance between pharmacological activity and safety. Extensive efforts are underway in academia and industry to establish novel ADCs or combination therapies that modulate the tumor microenvironment, gradually understand their pharmacology and related predictive biomarker combinations, and conduct preclinical evaluation in well-characterized patient-derived xenograft models to select the most promising ADC-based combinations or ISACs, providing new clinical options for precision treatment of malignancies (Figure 2.2.5).
《Table 2.2.3》
Table 2.2.3 Countries with the greatest output of core patents on “combining antibody-drug conjugates with immunotherapy for malignancies”
| No. | Country | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations per patent |
| 1 | USA | 194 | 58.08 | 1 235 | 68.04 | 6.37 |
| 2 | China | 61 | 18.26 | 240 | 13.22 | 3.93 |
| 3 | Switzerland | 19 | 5.69 | 124 | 6.83 | 6.53 |
| 4 | Germany | 18 | 5.39 | 85 | 4.68 | 4.72 |
| 5 | Republic of Korea | 13 | 3.89 | 58 | 3.2 | 4.46 |
| 6 | Netherlands | 12 | 3.59 | 80 | 4.41 | 6.67 |
| 7 | UK | 12 | 3.59 | 67 | 3.69 | 5.58 |
| 8 | France | 12 | 3.59 | 36 | 1.98 | 3 |
| 9 | India | 7 | 2.1 | 52 | 2.87 | 7.43 |
| 10 | Canada | 5 | 1.5 | 3 | 0.17 | 0.6 |
《Figure 2.2.3》

Figure 2.2.3 Collaboration network among major countries in the engineering front of “combining antibody-drug conjugates with immunotherapy for malignancies”
《Table 2.2.4》
Table 2.2.4 Institutions with the greatest output of core patents on “combining antibody-drug conjugates with immunotherapy for malignancies”
| No. | Institution | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations per patent |
| 1 | Regeneron Pharmaceuticals Incorporated | 14 | 4.19 | 126 | 6.94 | 9 |
| 2 | Immunomedics Incorporated | 11 | 3.29 | 99 | 5.45 | 9 |
| 3 | Sapreme Technologies | 11 | 3.29 | 32 | 1.76 | 2.91 |
| 4 | Charité–Universitätsmedizin Berlin | 9 | 2.69 | 31 | 1.71 | 3.44 |
| 5 | Seagen Incorporated | 9 | 2.69 | 29 | 1.6 | 3.22 |
| 6 | Novartis AG | 8 | 2.4 | 62 | 3.42 | 7.75 |
| 7 | The University of Texas System | 7 | 2.1 | 25 | 1.38 | 3.57 |
| 8 | OSE Immunotherapeutics | 7 | 2.1 | 22 | 1.21 | 3.14 |
| 9 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | 7 | 2.1 | 16 | 0.88 | 2.29 |
| 10 | AstraZeneca | 6 | 1.8 | 38 | 2.09 | 6.33 |
《Figure 2.2.4》
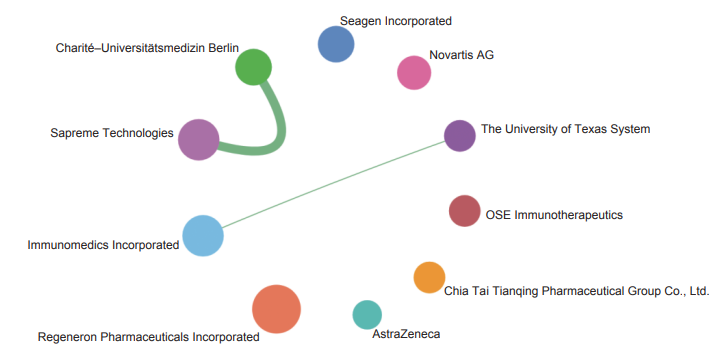
Figure 2.2.4 Collaboration network among institutions in the engineering development front of “combining antibody-drug conjugates with immunotherapy for malignancies”
《Figure 2.2.5》
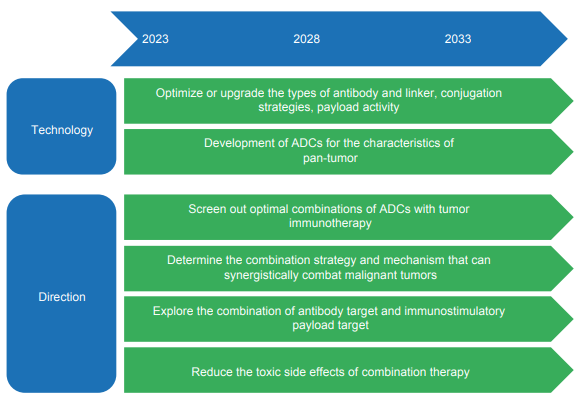
Figure 2.2.5 Roadmap of the engineering development front of “combining antibody-drug conjugates with immunotherapy for malignancies”
2.2.3 Single-cell spatial transcriptomics technology
Organ systems are composed of various cell subpopulations, and the spatial arrangement of these cell subpopulations is closely related to their functions within established tissues. Single-cell RNA sequencing (scRNA-seq) characterizes the transcriptomes of individual cells and can reveal cell subpopulations in specific organs. However, in the cell dissociation step of scRNA-seq protocols, the spatial positioning information of individuals in the native tissue is usually destroyed. By recording and reconstructing the spatial information of cells in complex tissues, single-cell spatial transcriptomics can locate single cells with transcriptional features in their native tissue environment. Integrating scRNA-seq and spatial transcriptomic data may increase our understanding of the roles of specific cell subpopulations and their interactions in development, homeostasis, and disease.
Single-cell spatial transcriptomics integrates data from single-cell transcriptome sequencing and spatial information records. Data analysis consists of two steps. First, the scRNA-seq data are used for dimensionality reduction and clustering, and subsequent deconvolution of cell type profiles within tissues and organs. Second, spatial information records are employed to map each scRNA-seq-annotated cell type to a specific niche or region of a tissue. Such analyses can provide a spatial context for the reliable evaluation of putative ligand-receptor interactions to predict intercellular signaling. Therefore, it is possible to extend insights from single-cell transcriptome-based cell typing to cell signaling research that drives phenotype changes at the protein interaction level.
Currently, spatial transcriptomic techniques are largely focused on measuring mRNA transcripts through next-generation sequencing (spatial barcoding) or fluorescent markers (HPRI). In addition to improving deconvolution and mapping algorithms, a focus that warrants attention is the development of novel deep learning models to help distinguish which features of spatial transcriptomes are the most biologically relevant. Furthermore, defining 3D spatial transcriptomes and real-time cell tracking offer additional frontiers for future technological development. These new directions will use 3D single-molecule fluorescence in situ hybridization data for computational reconstruction or inference of the location of scRNA-seq cells.
An even deeper understanding of tissue function can be achieved by extending the resolution of the spatiotemporal transcriptome by spatially resolving all biomolecules integral to the central dogma of molecular biology. For example, DBiT-seq can spatially resolve protein and mRNA transcripts within the same tissue. In situ 3D imaging of the genome, subcellular resolution of RNA, or simultaneous imaging of the nucleoli and RNA can all be performed at the single-cell level. They hold great promise in advancing our understanding of how the machinery of the central dogma functions in the 3D context of a cell to reveal the inner workings of developmental trajectories and disease.
In the future, the application of single-cell spatial transcriptomics technology in the clinical analysis of patient single cells or patient-derived in vitro models will help explore the molecular mechanisms of diseases and define the spatial location of rare cell types and cell subpopulations during disease progression. Furthermore, single-cell spatial transcriptomic technologies will contribute to the discovery of new therapeutic targets. The efficacy of the newly discovered drugs will then be tested in patient-derived in vitro models and monitored using single-cell technologies to define the cell-type- specific responses of the patient to treatment, which can then be used to specify the best therapeutic strategy for the individual patient.
The countries with the largest number of core patent output are the USA, China, and Germany (Table 2.2.5). As shown in the cooperation network among major countries, a strong cooperative relationship is most notable between the USA and the UK (Figure 2.2.6). The top institutions in terms of core patent output are the Broad Institute Incorporation, Massachusetts Institute of Technology, and University of California (Table 2.2.6). There are cooperative relationships between the Broad Institute Incorporation, MIT, Harvard University, and the University of California (Figure 2.2.7).
As more spatial transcriptomic analyses are performed, it will be increasingly challenging to disentangle definitive, disease-relevant cell types and their gene modules. With increasingly detailed annotation and mapping of cell types, tools such as Seurat Integration, Harmony, and LIGER may evolve to integrate data across different experimental assays to determine whether specific cell types are consistently observed in each tissue. Integrative single-cell spatial transcriptomic technology is rapidly developing. Depending on the biological questions being asked, the experimental methodology can be designed to integrate any spatial transcriptomic approach with other scRNA-seq technologies. In addition to developing enhanced methods, careful selection of algorithms for integrating such data is of paramount importance because methods that spatially resolve tissue organization at the resolution of conventional scRNA-seq technologies or at the depth of whole- transcriptome coverage do not yet exist. Integrating the analysis of multiple approaches is beginning to shed light on the spatial maps of specific cell subpopulations in development and disease and to reveal the mechanisms whereby such cell populations collaboratively shape tissue phenotypes (Figure 2.2.8).
《Table 2.2.5》
Table 2.2.5 Countries with the greatest output of core patents on “single-cell spatial transcriptomics technology”
| No. | Country | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations per patent |
| 1 | USA | 83 | 51.23 | 743 | 78.87 | 8.95 |
| 2 | China | 61 | 37.65 | 190 | 20.17 | 3.11 |
| 3 | Germany | 4 | 2.47 | 1 | 0.11 | 0.25 |
| 4 | UK | 2 | 1.23 | 4 | 0.42 | 2 |
| 5 | Netherlands | 2 | 1.23 | 3 | 0.32 | 1.5 |
| 6 | Japan | 2 | 1.23 | 1 | 0.11 | 0.5 |
| 7 | Belgium | 2 | 1.23 | 0 | 0 | 0 |
| 8 | Switzerland | 1 | 0.62 | 0 | 0 | 0 |
| 9 | Finland | 1 | 0.62 | 0 | 0 | 0 |
| 10 | France | 1 | 0.62 | 0 | 0 | 0 |
《Figure 2.2.6》

Figure 2.2.6 Collaboration network among major countries in the engineering development front of “single-cell spatial transcriptomics technology”
《Table 2.2.6》
Table 2.2.6 Institutions with the greatest output of core patents on “single-cell spatial transcriptomics technology”
| No. | Institution | Published patents | Percentage of published patents/% | Citations | Percentage of citations/% | Citations per patent |
| 1 | The Broad Institute Incorporation | 17 | 10.49 | 173 | 18.37 | 10.18 |
| 2 | Massachusetts Institute of Technology | 15 | 9.26 | 203 | 21.55 | 13.53 |
| 3 | University of California | 7 | 4.32 | 43 | 4.56 | 6.14 |
| 4 | Stanford University | 7 | 4.32 | 22 | 2.34 | 3.14 |
| 5 | Bio-Rad Laboratories Incorporated | 6 | 3.7 | 50 | 5.31 | 8.33 |
| 6 | Harvard University | 5 | 3.09 | 107 | 11.36 | 21.4 |
| 7 | 10x Genomics Incorporated | 5 | 3.09 | 86 | 9.13 | 17.2 |
| 8 | University of Washington | 4 | 2.47 | 19 | 2.02 | 4.75 |
| 9 | Southeast University | 4 | 2.47 | 12 | 1.27 | 3 |
| 10 | Sigma-Aldrich Company Limited | 4 | 2.47 | 11 | 1.17 | 2.75 |
《Figure 2.2.7》

Figure 2.2.7 Collaboration network among major institutions in the engineering development front of “single-cell spatial transcriptomics technology”
《Figure 2.2.8》
Figure 2.2.8 Roadmap of the engineering development front of “single-cell spatial transcriptomics technology”
Participants of the Field Group
Leaders CHEN Saijuan, ZHANG Boli
Academicians and Experts
GAO Tianming, XIA Ningshao, YANG Jianhua, ZHOU Hongwei, ZHAO Xiaoyang, XIA Laixin, ZHENG Lei,
XU Binghe, JI Xunming, HU Yu, ZHANG Luxia, JIN Yang, ZENG Chunyu, NIE Yongzhan, LIU Jie, ZHANG Aihua,
YE Jing, WU Chuanjie, DONG Jiahong, XIA Qiang, DONG Nianguo, JIANG Tao, WAN Jun, LI Chengquan,
CHEN Zhiyu, LIANG Xiao, NING Guochen, ZHAO Yimin, SHI Songtao, DENG Xuliang, BAI Yuxing, YANG Chi,
YE Ling, ZHANG Yufeng, CHEN Faming, WU Tangchun, HU Zhibin, YU Hongjie, LIU Wei, LI Ying, CHEN Rui,
ZHENG Jusheng, ZHENG Yan, HE Yonghan, HAN Tianshu, WANG Chaolong, TIAN Jinzhou, CHEN Jiaxu,
ZHANG Junhua, LIU Cunzhi, YANG Hongjun, JIA Zhenhua, NI Jingnian, XIAO Wei, GUO Dean, DUAN Jinao,
CAO Peng, WU Zhisheng, SONG Xiaoting, ZHANG Lei, CAO Gang
Secretary Group
DING Ning, ZHAO Xilu, XI Xiaodong, YAN Xiaoyu, CHEN Yinyin, XI Wenda, YIN Wei, ZHANG Jianan,
WU Huiyi, CHU Jingshen
Data Support Group
QIU Xiaochun, DENG Peiwen, WU Hui, FAN Rong, KOU Jiande, LIU Jie, TAO Lei, JIANG Hongbo,
CHEN Daming, LU Jiao, MAO Kaiyun, YUAN Yinchi, FAN Yuelei, ZHANG Yang
Report Writers
SHAO Zhimin, JIANG Yizhou, WANG Jianwei, TANG Xiaoli, ZHENG Jusheng, LIU Guanghui,
SONG Moshi, LI Jingyi, ZHAO Yong, LI Pilong, YU Yingyan, JIANG Hong, LI Yi, LIU Qi,
GONG Likun, LIU Feng, TIAN Zhigang, NIE Guangjun, ZHANG Yinlong, BI Jiacheng,
FENG Jiandong, WANG Xingpeng, HE Ping, LI Zeyu, PENG Wenbo














 京公网安备 11010502051620号
京公网安备 11010502051620号




