《1. Introduction》
1. Introduction
Infectious diseases caused by bacteria affect millions of people throughout the world. The discovery of antibiotics had a profound impact on human health. Unfortunately, with the frequent emergence of drug-resistant bacterial strains, traditional antibiotics are becoming increasingly ineffective [1]. This trend prompts an urgent need for a new class of antibiotics with novel mechanisms of action.
The nuclear localization sequence (NLS, PKKKRKV) of SV40 T large antigen, an extensively studied peptide, can facilitate the nuclear transport of heterologous molecules [2−4]. Interestingly, the NLS was reported to show antimicrobial activity against both gram-negative and gram-positive bacteria, although its mechanism of action remains obscure and its antimicrobial activity is relatively low [5].
Acridine (Acr), a heteroaromatic polycyclic molecule, is well known for its DNA intercalating abilities and pharmacological properties [6]. A considerable number of acridine derivatives have been synthesized and used as antimicrobial, antimalarial, and anticancer drugs [7−10]. The first clinical use of acridines as antimicrobial agents occurred in 1917 [10]. The emergence of penicillins with greater therapeutic efficacy eclipsed acridines in antimicrobial therapy. However, the serious problem of antibiotic resistance has once again directed considerable attention toward acridines [10].
Attaching small molecule drugs to peptides has been proved to be an effective strategy offering a variety of benefits, such as increasing aqueous solubility, improving pharmacokinetics, and overcoming drug resistance [11,12]. In this study, we attempt to design a novel type of effective antimicrobial agent by conjugating acridines to an NLS. Thus Acr3-NLS was designed and synthesized by attaching three Lys(acridine) to the N-terminus of the NLS in this study. Dimerization has been reported to be a useful strategy to enhance the antimicrobial potency and resistance to proteases of antimicrobial peptides [13,14]. Therefore, to improve the antimicrobial activity of our agent, we also synthesized the dimeric (Acr3-NLS)2 by joining two Acr3-NLS monomers together at the C-terminus via a disulfide linker, which has been extensively used for drug conjugation [15]. Figure 1 presents these conjugates. We subsequently evaluated the antimicrobial activity and studied the action mechanisms of Acr3-NLS and (Acr3-NLS)2.
《Fig. 1》
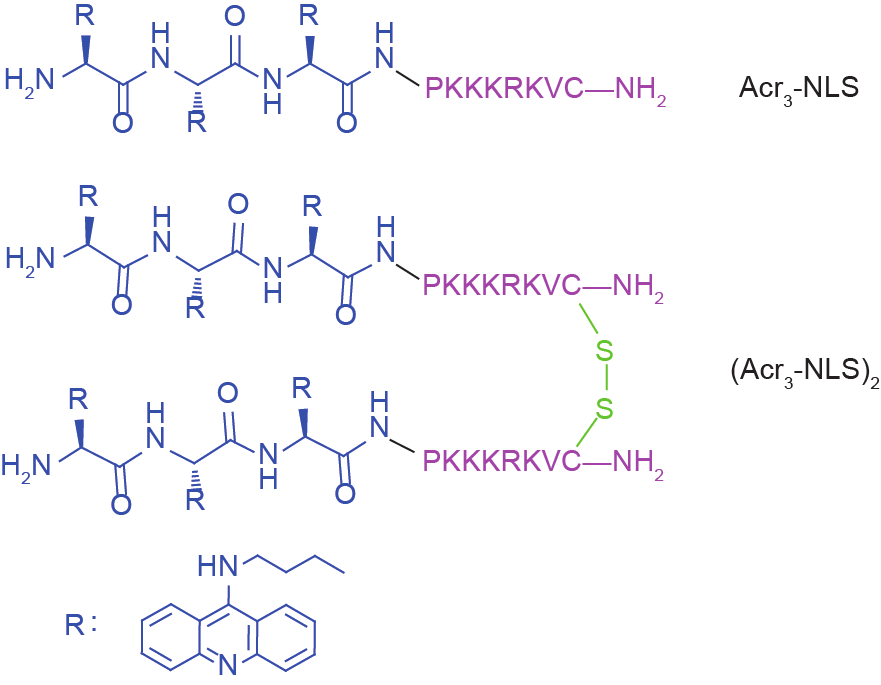
Fig.1 The structures of Acr3-NLS and (Acr3-NLS)2.
《2. Materials and methods》
2. Materials and methods
《2.1. Peptide synthesis and purification》
2.1. Peptide synthesis and purification
The NLS and Acr3-NLS were synthesized on a p-methylbenzhydrylamine (MBHA) resin using the standard Fmoc-chemistry-based strategy. Following a procedure reported in previous literature [16], Fmoc-Lys(acridine) was synthesized and coupled as an amino acid. The synthesis of (Acr3-NLS)2 was performed according to our previously reported procedure [17]. All crude peptides were purified by reversed-phase high-performance liquid chromatography (RP-HPLC) on a C18 column and then characterized by electrospray ionization mass spectrometry (ESI-MS). Purity analysis was checked by analytical RP-HPLC, and peptides were eluted using a liner gradient of 5%−95% acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 1 mL·min−1 within 30 min. The retention time ( tR) of each peptide was determined when the peak was at its maximum height.
《2.2. Antimicrobial activity assay》
2.2. Antimicrobial activity assay
Gram-negative ( Escherichia coli, ATCC 25922; Pseudomonas aeruginosa, ATCC 27853) and gram-positive ( Bacillus subtilis, ATCC 23857; Staphylococcus aureus, ATCC 25923) bacteria were cultured in Mueller-Hinton (MH) broth at 37 °C. The antimicrobial activities of the agents against gram-negative and gram-positive bacteria were measured using a standard serial dilution method. Briefly, 100 μL of 2-fold serial diluted aliquots of the agents in a range from 1 μmol·L−1 to 128 μmol·L−1 in MH broth were added to 96-well plates containing 100 μL of diluted bacterial cells (106 CFU·mL−1, CFU is short for colony forming unit). After incubation for 18−20 h, the minimum inhibitory concentration (MIC) was detected. Controls were performed in the absence of agents. The experiment was conducted in triplicate.
《2.3. Time-killing assay》
2.3. Time-killing assay
The killing kinetic of the conjugates against E. coli was analyzed by a time-killing assay. E. coli was grown to log phase and diluted to 106 CFU·mL−1 in MH broth. The conjugates were added to the bacterial suspensions at concentrations of 0.5-fold, 1-fold, and 2-fold of the MIC. At 0, 15 min, 30 min, 60 min, 90 min, and 120 min, 100 μL of cell suspension was collected and diluted into growth medium, then plated onto MH agar plates. Colonies were counted after peptide treatment for 24 h. The experiment was conducted in triplicate.
《2.4. Propidium iodide uptake assay》
2.4. Propidium iodide uptake assay
To verify the membrane-lytic activity of the conjugates, we used a propidium iodide (PI) uptake assay. E. coli was grown to log phase and diluted to 106 CFU·mL−1 in MH broth. The medium, with 1 mL of phosphate-buffered saline (PBS) containing E. coli with a density of 1 × 106 CFU·mL−1, was added to the dish, and E. coli cells were treated with 1-fold MIC of the conjugates at 37 °C. After incubation at different time intervals, PI was added and further incubated for 10 min, and PI uptake was observed using a laser confocal scanning microscope.
《2.5. Scanning electron microscopy》
2.5. Scanning electron microscopy
For scanning electron microscopy (SEM) sample preparation, E. coli cells were washed and re-suspended at 5 × 108 CFU·mL−1 in PBS and incubated with the conjugates (1-fold MIC) at 37 °C for 1 h. Subsequently, the cells were centrifuged for 5 min at 10 000 r·min−1 and fixed with 3% glutaraldehyde at 4 °C. All fixed cells were impregnated in 2.5% tannic acid (Sigma) for 2 d, and counter-fixed with 2% osmium tetroxide (Sigma) for 2 h, followed by dehydration in ethanol and drying in a critical point dryer (Ion Tech, Teddington). After coating with gold, E. coli cells were observed using a scanning electron microscope (JSM-6380Lv).
《2.6. Hemolysis assay》
2.6. Hemolysis assay
Freshly collected mice blood was centrifuged to remove the buffy coat, and the obtained erythrocytes were washed three times with PBS, centrifuged for 10 min at 1000 g ( g = 9.8 m·s−2), and re-suspended in PBS to 4% (V/V). 100 μL of the conjugates solution at various concentrations was added to a 96-well plate containing 100 μL of erythrocyte suspension. Cells treated with PBS and 0.1% Triton X-100 were as 0 and 100% hemolysis, respectively. After treatment for 1 h, the plates were centrifuged at 1000 g for 10 min, and 100 μL of supernatants was transferred to a 96-well plate. The release of hemoglobin was determined using a microplate reader at 450 nm.
《2.7. DNA binding assay》
2.7. DNA binding assay
Gel retardation experiments were performed to evaluate the DNA binding ability of the conjugates. E. coli ATCC 25922 genomic DNA was extracted using a TIANamp Bacteria DNA Kit. 10 μL of genomic DNA (approximately 400 ng) was dissolved in TE buffer and mixed with an equal volume of varying concentrations of the conjugates. The reaction mixtures were incubated at room temperature for 30 min. The mixtures and native loading buffer were then subjected to gel electrophoresis on a 1% agarose gel. The migration of DNA was detected by the fluorescence of ethidium bromide.
《2.8. Cell uptake of conjugates》
2.8. Cell uptake of conjugates
E. coli cells were incubated with the conjugates at 0.5-fold MIC for 1 h. The cells were then washed three times with PBS. Images were taken with an inverted Zeiss LSM 710 confocal microscope. Excitation was performed by a 405 nm line, and a fluorescence emission maximum at 487 nm was observed. Differential interference contrast (DIC) images were taken along with both fluorescence channels, and no bleedthrough was observed during co-localization studies with these parameters.
《3. Results and discussion》
3. Results and discussion
《3.1. Antimicrobial activity of conjugates》
3.1. Antimicrobial activity of conjugates
The emergence of multidrug-resistant bacteria creates an urgent need for alternative antibiotics with new mechanisms of action. In this study, we synthesized a novel type of antimicrobial agent by conjugating acridines to the N-terminus of an NLS. The antimicrobial activity of all agents was determined against two gram-negative bacteria ( E. coli and P. aeruginosa), and two gram-positive bacteria ( S. aureus and B. subtilis). Table 1 summarizes the MIC of all agents. Although the NLS was reported to exhibit antimicrobial activity [5], our results showed that it did not kill bacteria at the concentrations tested in this study. As expected, Acr3-NLS and dimeric (Acr3-NLS)2 displayed significant antimicrobial activity against gram-negative and gram-positive bacteria compared to that of the NLS. Importantly, the antimicrobial activity of dimeric (Acr3-NLS)2 was significantly higher than that of Acr3-NLS. We subsequently investigated the time-killing activity of Acr3-NLS and (Acr3-NLS)2 against E. coli. As shown in Figure 2, our results showed that both Acr3-NLS and (Acr3-NLS)2 can kill bacteria within a short time, especially at higher concentrations. In addition, the killing activity of dimeric (Acr3-NLS)2 was significantly faster than that of Acr3-NLS at the same MIC. Based on their rapid bacteria-killing activity and physicochemical properties, we speculate that Acr3-NLS and (Acr3-NLS)2 can kill bacteria by disrupting their membranes, like most antimicrobial peptides [18,19].
《Tab.1》
Tab.1 Amino acid sequence, physicochemical properties, and antimicrobial activity of NLS and its analogues containing acridine.

Notes: * Calculated monoisotopic molar masses. ** Observed monoisotopic masses. $ Hydrophobicity represented as tR was measured by RP-HPLC.
《Fig. 2》

Fig.2 Time-killing activity of (a) Acr3-NLS and (b) (Acr3-NLS)2 at different concentrations against E. coli. Representative of triplicate experiments.
《3.2. Membrane-lytic activity of conjugates》
3.2. Membrane-lytic activity of conjugates
Antimicrobial peptides are cationic short peptides with an overall content of 50% hydrophobic residues, which exhibit potent broad-spectrum antimicrobial properties [19]. Unlike traditional antibiotics that have specific molecular targets, antimicrobial peptides bind selectively and rapidly disrupt negatively charged bacterial membranes, rather than neutral mammalian cell membranes, through a series of physical processes [18].
Firstly, we adopted the PI uptake assay, a typical method of studying membrane disruption by antimicrobial peptides, in order to study the change in the integrity of the cell membranes after Acr3-NLS or (Acr3-NLS)2 treatment. PI is a fluorescent molecule that can be excluded by the membranes of viable cells but that can pass through damaged plasma membranes. As shown in Figure 3, our results showed that PI could rapidly enter E. coli cells after Acr3-NLS or (Acr3-NLS)2 treatment, indicating that the E. coli cell membranes were damaged. We observed the subtle morphological changes of E. coli membranes after Acr3-NLS or (Acr3-NLS)2 treatment using SEM. As shown in Figure 4, E. coli treated with conjugates appeared to display the formation of blebs on the cell surface, which is likely to represent pore formation, while untreated E. coli showed a normal smooth surface. The result derived from SEM was in agreement with that derived from the PI uptake assay, confirming that Acr3-NLS and (Acr3-NLS)2 can kill bacteria via membrane disruption, just like many naturally occurring antimicrobial peptides. Membrane-lytic activity may endow Acr3-NLS and (Acr3-NLS)2 with the following advantages: ① Unlike traditional antibiotics, they can be used to kill drug-resistant bacteria with a new mechanism of membrane damage; ② it will be very difficult for bacteria to develop resistance to them, because reconstruction of the membrane system is impossible.
《Fig. 3》

Fig.3 The PI uptake in E.coli after Acr3-NLS and (Acr3-NLS)2 treatment at the concentration of 1 × MIC for different times.
《Fig. 4》

Fig.4 Scanning electron micrographs of E.coli. (a) Control; (b) Acr3-NLS treatment; (c) (Acr3-NLS)2 treatment. The bacteria were treated with peptide at the concentration of 1 × MIC for 1 h. The control was done without peptides. Scale bar= 1 μm.
Cationicity and hydrophobicity play an important role in the membrane-lytic activity of antimicrobial peptides [20−22]. Positive charges give antimicrobial peptides an electrostatic affinity to the outer leaflet of bacterial membranes, whereas hydrophobicity can force the insertion of antimicrobial peptides into the hydrophobic bacterial membranes [23]. An appropriate increase in positive charge and hydrophobicity will contribute to improving the membrane-lytic activity and bacteria-killing activity of antimicrobial peptides [19,21,24]. The NLS in this study is a cationic peptide containing five basic amino acids (four lysines and one arginine), but only one hydrophobic amino acid. Consequently, it is very hard for the NLS to enter a bacterial membrane and form stable pores because of its low hydrophobicity [25]. In our previous study, we found that the attachment of hydrophobic camptothecin can turn the hydrophilic cell-penetrating peptide Tat into a membrane-lytic peptide [26]. In this study, acridine might act as an ideal hydrophobic anchor to promote the membrane insertion and subsequent membrane disruption of the conjugates, resulting in increased antimicrobial activity. To evaluate the effect of acridine on the hydrophobicity of peptides, we determined the retention time ( tR) of peptides using RP-HPLC, which is a particularly good method to represent apparent peptide hydrophobicity [24]. As shown in Table 1, the retention time of Acr3-NLS was significantly prolonged compared to that of the NLS, indicating that Acr3-NLS is more hydrophobic than the NLS. Therefore, the increased hydrophobicity of Acr3-NLS by conjugation with the hydrophobic end tag Lys(acridine) could account for its enhanced membrane-lytic activity. Likewise, dimeric (Acr3-NLS)2, which has increased hydrophobicity, displayed higher antimicrobial activity than monomeric Acr3-NLS.
《3.3. Hemolytic activity of conjugates》
3.3. Hemolytic activity of conjugates
Low toxicity against host cells is very important for the clinical development of potent antimicrobial peptides. Hemolysis assay is the most commonly used procedure to assess the toxicity of antimicrobial peptides [27]. A high level of hydrophobicity generally enhances the interaction of antimicrobial peptides with the neutral membranes of red blood cells, resulting in an increase in hemolytic activity [24]. Despite having more hydrophobicity than the NLS, Acr3-NLS did not display virtual hemolysis even up to 200 μmol·L−1, which is far higher than its MIC (Figure 5). Although dimerization has been reported to increase the hemolysis of antimicrobial peptides [28], the dimeric (Acr3-NLS)2 did not show significantly increased hemolysis compared to monomeric Acr3-NLS. Overall, our results demonstrated that Acr3-NLS and (Acr3-NLS)2 displayed low toxicity to normal cells, which means that higher drug concentrations can be used in infection therapy.
《Fig. 5》

Fig.5 The hemolytic activities of NLS, Acr3-NLS, and (Acr3-NLS)2. Cells were treated with melittin at 10 μmol·L−1. Representative of triplicate experiments.
《3.4. DNA binding activity of conjugates》
3.4. DNA binding activity of conjugates
Recently, more and more studies have revealed that membrane disruption is not the only mechanism of action of antimicrobial peptides. Some antimicrobial peptides have been reported to kill bacteria by interaction with one or more intracellular targets, such as nucleic acids and enzymes [29,30]. Compared to other intracellular targets, cationic antimicrobial peptides preferentially attack the anionic nucleic acids, resulting in bacterial death [30]. In our previous study, NK-18, a truncated peptide derived from NK-lysin, was found to be able to kill bacteria not only by disrupting the bacterial membrane but also by binding to DNA [31]. Because nucleic acids are the established sites of action of acridine-derived antimicrobial agents in bacteria [10], we speculated that Acr3-NLS and (Acr3-NLS)2, because they contain acridines, could also kill bacteria by binding to DNA and interfering with the synthesis of DNA.
Firstly, gel retardation assay was used to evaluate the nucleic acid binding ability of the peptides. As shown in Figure 6, Acr3-NLS and especially (Acr3-NLS)2 caused significant retardation of bacterial DNA mobility compared to the NLS, indicating that the presence of acridine remarkably enhanced the DNA binding ability of Acr3-NLS and (Acr3-NLS)2. However, in order to interact with DNA, Acr3-NLS and (Acr3-NLS)2 must first translocate across the bacterial membranes. Interestingly, more and more studies demonstrate that many antimicrobial peptides can translocate across cell membranes, much like cell-penetrating peptides, because of their similarities in charge, structure, and initial membrane interactions [32]. Although the NLS was reported to overcome the nuclear membrane barrier and to promote nuclear translocation, there are few reports on its translocation across the cell membrane. In this study, we observed the cell uptake of Acr3-NLS and (Acr3-NLS)2 in E. coli using confocal microscopy. As shown in Figure 7, both Acr3-NLS and (Acr3-NLS)2 could penetrate and accumulate inside the E. coli cells, like many cell-penetrating peptides. According to the above results, we can infer that Acr3-NLS and (Acr3-NLS)2 can kill bacteria by disrupting the cell membrane and by interfering with the synthesis of DNA. The double targets—the cell membrane and intracellular DNA—minimize the chances of bacterial resistance to Acr3-NLS and (Acr3-NLS)2, as resistance would require the complete alteration of the cell membrane or the bypassing of several biochemical pathways.
《Fig. 6》
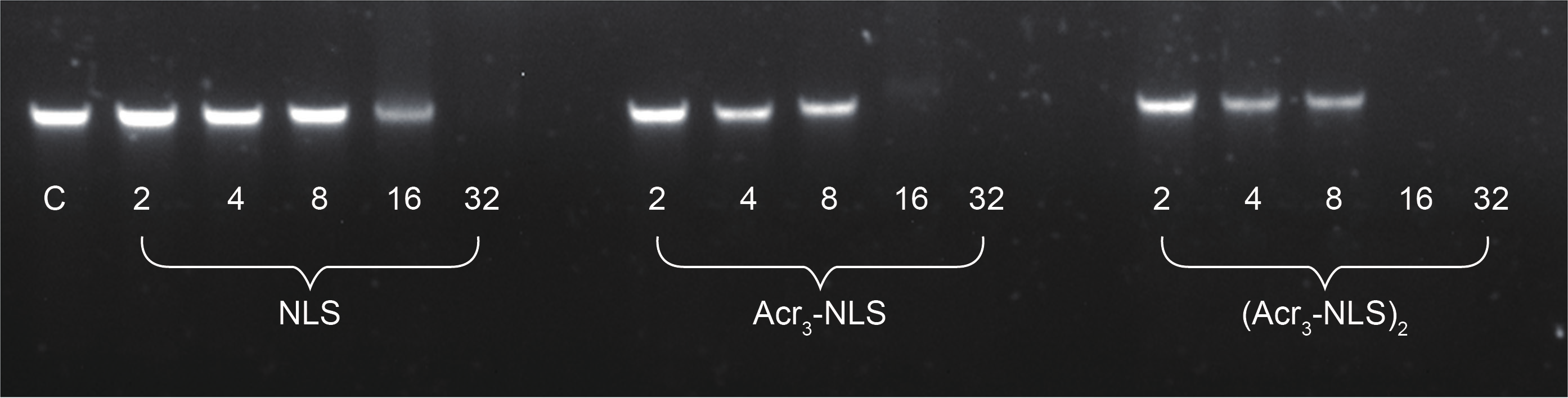
Fig.6 The DNA-interacting effect of NLS, Acr3-NLS, and (Acr3-NLS)2. DNA binding ability assayed by determining the retardation of DNA migration.
《Fig. 7》
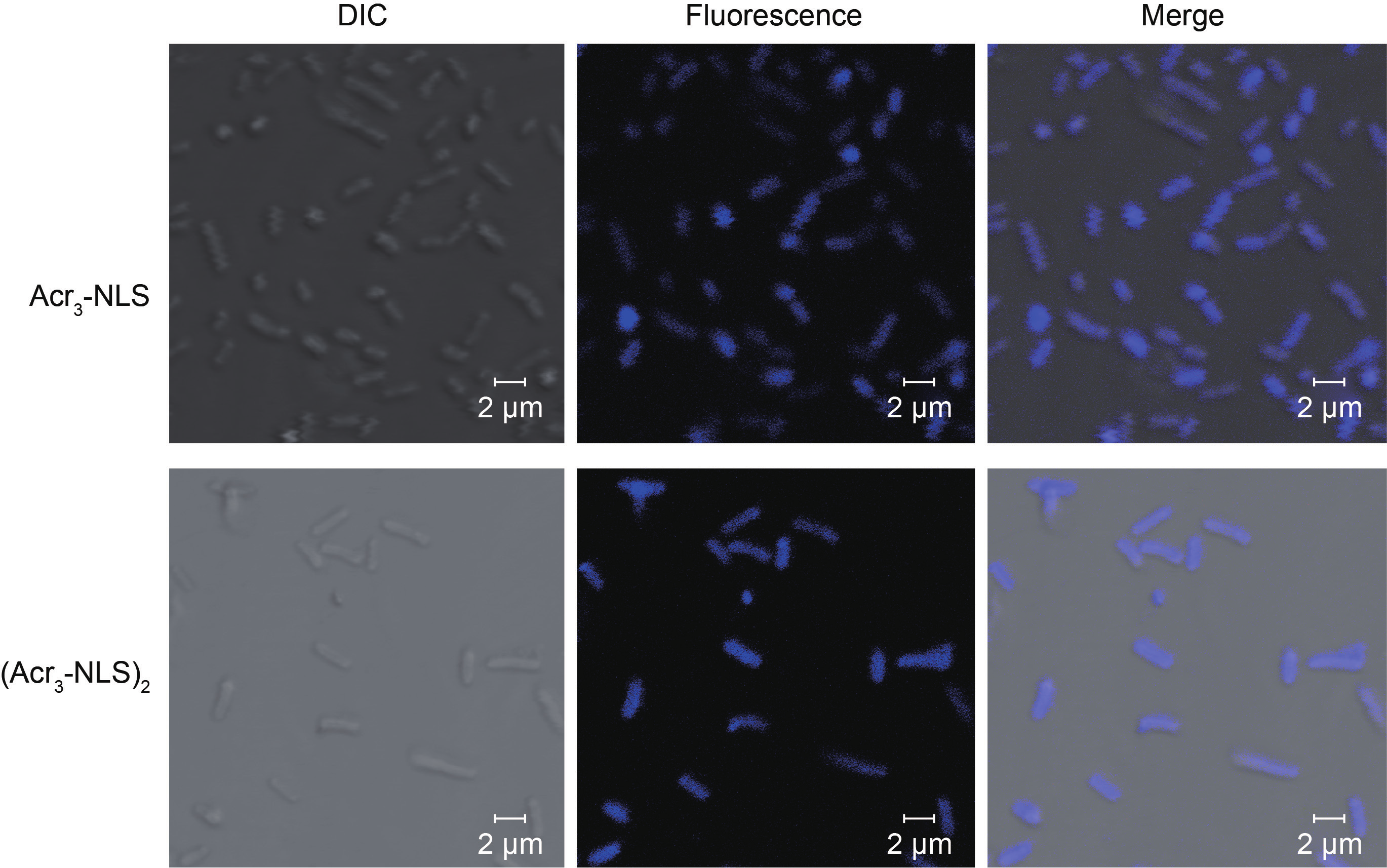
Fig.7 The cell uptake of Acr3-NLS and (Acr3-NLS)2 in E. coli after treatment at the concentration of 0.5 × MIC for 1 h.
《4. Conclusions》
4. Conclusions
In this study, we developed a new type of antimicrobial peptide by attaching acridines to an NLS. Our new agents can kill bacteria through a double action mechanism: Acridine can act as a membrane anchor to endow Acr3-NLS and (Acr3-NLS)2 with membrane-lytic activity, and acridine can also enhance the DNA binding ability and subsequent bacteria-killing activity of Acr3-NLS and (Acr3-NLS)2. Therefore, Acr3-NLS and (Acr3-NLS)2 can effectively kill bacteria in a manner that is not easily affected by known bacterial resistance capabilities. Although much further work is needed to obtain a systematic evaluation of the future application of these agents, this study opens a new avenue to design a new type of antimicrobial peptide with multiple mechanisms of action.
《Acknowledgements》
Acknowledgements
We are grateful for the grants from the National Natural Science Foundation of China (81402776 and 81202400), the Key National S&T Program “Major New Drug Development” of the Ministry of Science and Technology of China (2012ZX09504-001-003), the Fundamental Research Funds for the Central Universities (lzujbky-2014-142 and lzujbky-2015-169), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20130211130005), and China Postdoctoral Science Foundation (2013T60896).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Wei Zhang, Xiaoli Yang, Jingjing Song, Xin Zheng, Jianbo Chen, Panpan Ma, Bangzhi Zhang, and Rui Wang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




