《1. Introduction》
1. Introduction
Cancer is now the leading cause of death in the world [1]. China constitutes approximately 20% of the world’s population, and comprises 29.42% of new cancer cases and 27% of cancer deaths worldwide. In 2012, the cancer incidence rate in China was 1.74%, and the cancer mortality rate was 1.22%. In China, cancers of the stomach and liver have traditionally occurred with the highest frequency; however, the incidence of lung cancer has increased as the leading cause of cancer in recent years [2]. Despite the fact that huge advances in diagnostic technologies have led to an explosion of knowledge in cancer research, only a minute number of cancer patients are diagnosed at early stages due to the poor selectivity and sensitivity of conventional diagnostic techniques.
Traditional imaging technologies reflect mostly anatomical changes that differentiate pathological from normal tissue rather than measuring the biological processes responsible for disease. Molecular imaging (MI) is a rapidly emerging biomedical research method that enables visual representation, characterization, and quantification of biological processes at the cellular and/or molecular level within living organisms [3−5]. Several MI modalities are currently available, including fluorescence and bioluminescence imaging, targeted ultrasound (US), molecular magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), single-photon-emission computed tomography (SPECT), and positron emission tomography (PET). Probes or beacons, which can accumulate at the site of interest and allow for imaging, are required for MI. However, the limitations of some MI techniques include poor spatial resolution, low sensitivity, or poor signal penetration through tissues. For example, optical imaging is restricted in its depth-penetration capability, leading to poorer resolution, whereas MRI can image deep tissues but is much less sensitive (Table 1).
《Tab.1》
Tab.1 Molecular imaging modalities.

Nanoparticles (NPs) are emerging as a new class of MI agent to overcome these major hurdles in detecting human disease. NPs have great potential for accurate cancer diagnosis via passive accumulation and/or active-targeting approaches [6]. Combining their large payload-carrying capacity, high signal intensity, and stability, NPs can deliver high concentrations of imaging agents to the target region. NPs are typically smaller than cells [7], and are comparable in size to molecules and proteins [8].
Historically, many of the developed NPs are considered to accumulate in tumors based on the enhanced permeability and retention (EPR) effect, which in nanomedicine research has been considered a universal feature of solid malignant tumors that can serve as the basis for passive tumor-targeting by therapeutic and diagnostic NPs [9−17]. The EPR effect was first reported as a concept of the delivery of macromolecular drugs to tumors [18]. However, the heterogeneity of the EPR effect provoked a debate about the real value of this effect. Many experimental scientists, pharmacologists, and nanotechnology engineers hold to the premise that solid tumors consist of uniform tissue; that is, that tumors are homogeneous [19]. However, as this is not the case, new strategies such as active targeted NPs have been pursued. Targeted NPs can perform with both high sensitivity and specificity to achieve high accumulation at the tumor site [20−23]. Active targeting requires the therapeutic agent to be guided to the target by conjugating the therapeutic agent or carrier system to a tissue or cell-specific ligand. In addition, using targeted NPs with a variety of moieties to reach multiple binding sites can provide higher binding efficiencies for targeting specific tumor sites.
A number of articles review the current applications of nanotechnologies for MI [24]. This paper presents a brief overview of recent developments in nanotechnology for MI, and will also address the drawbacks and future challenges of current nanoplatforms for the clinical translation of imaging.
《2. Nanoplatforms》
2. Nanoplatforms
A wealth of NP-based systems exploits nanoplatforms for many imaging modalities. These NPs come in a wide variety of compositions, sizes, shapes, and structures from a variety of materials [25,26]. These different materials and shapes range from spheres, rods, and cubes to resembling snowflakes, flowers, thorns, hemispheres, worms, discoids, and chains−a wide variety that enables many imaging modalities (e.g., optical imaging, MRI, US, and/or nuclear imaging) for combining disease diagnosis and therapy into so-called “theranostic” (therapy plus diagnostic) applications. As mentioned earlier, NPs have been known to target tumors via passive- and/or active-targeting pathways. Due to abnormally leaky vasculature and the lack of an effective lymphatic drainage system in tumor tissues, NP platforms can passively accumulate in tumor tissues. These unique phenomena are jointly referred to as the EPR effect. Moreover, NPs can recognize, bind to, and internalize into tumor cells via receptor-mediated endocytosis when modified with tumor-targeting moieties such as antibodies, nucleic acids, proteins, or other ligands. In this section, we will describe exemplary theranostic nanoplatforms along with their applications regarding cancer.
《2.1. Optical nanoparticles》
2.1. Optical nanoparticles
Optical imaging offers high sensitivity, cost-effectiveness, non-ionizing radiation, and great potential for small-animal studies. However, the penetration depth of light prohibits deep-tissue imaging and is the major disadvantage for in vivo imaging in humans; thus, optical imaging is typically not quantitative for living-subject imaging studies. An abundance of probes, including synthetic fluorophores, semiconductor fluorescent crystals, and probes based on lanthanide rare-earth ions, have been developed for small-animal imaging [27−29].
Quantum dots (QDs) are the most widely studied NPs for preclinical optical imaging applications. Compared with organic fluorophores, QDs possess many superior properties for biological imaging, such as a strong resistance to photobleaching and chemical degradation, high quantum yields, continuous absorption spectra from ultraviolet (UV) to near-infrared (NIR), and large effective Stokes shifts [30−33]. Arginine-glycine-aspartic acid (RGD)-QDs could selectively bind to luminal endothelium in mouse tumor neovasculature [34]. However, because of their small size, QDs demonstrated poor retention inside the tumor, easily washing back out into the bloodstream [33] (Fig. 1). Nevertheless, the major drawbacks for the clinical utility of QDs are their potential toxicity and lack of deep-tissue imaging and quantification ability [35]. With the help of small-animal PET, radiolabeled QDs were found to have rapid uptake into the reticuloendothelial system (RES), liver, and spleen [36,37]. In order to reduce the uptake of QDs into the RES, human serum albumin (HSA) was explored as a coating for QD800-mercaptopropionic acid (MPA) NPs. The resulting QD800-MPA-HSA NPs show reduced localization in mononuclear phagocytic system-related organs over QD800-MPA, plausibly due to the low uptake of QD800-MPA-HSA in macrophage cells. QD800-MPA-HSA may have great potential for in vivo fluorescence imaging [38]. Affibody-modified QDs also showed high specificity for targeting human epidermal growth factor receptor 2 (HER2)-expressing cells and tumors [39].
《Fig. 1》
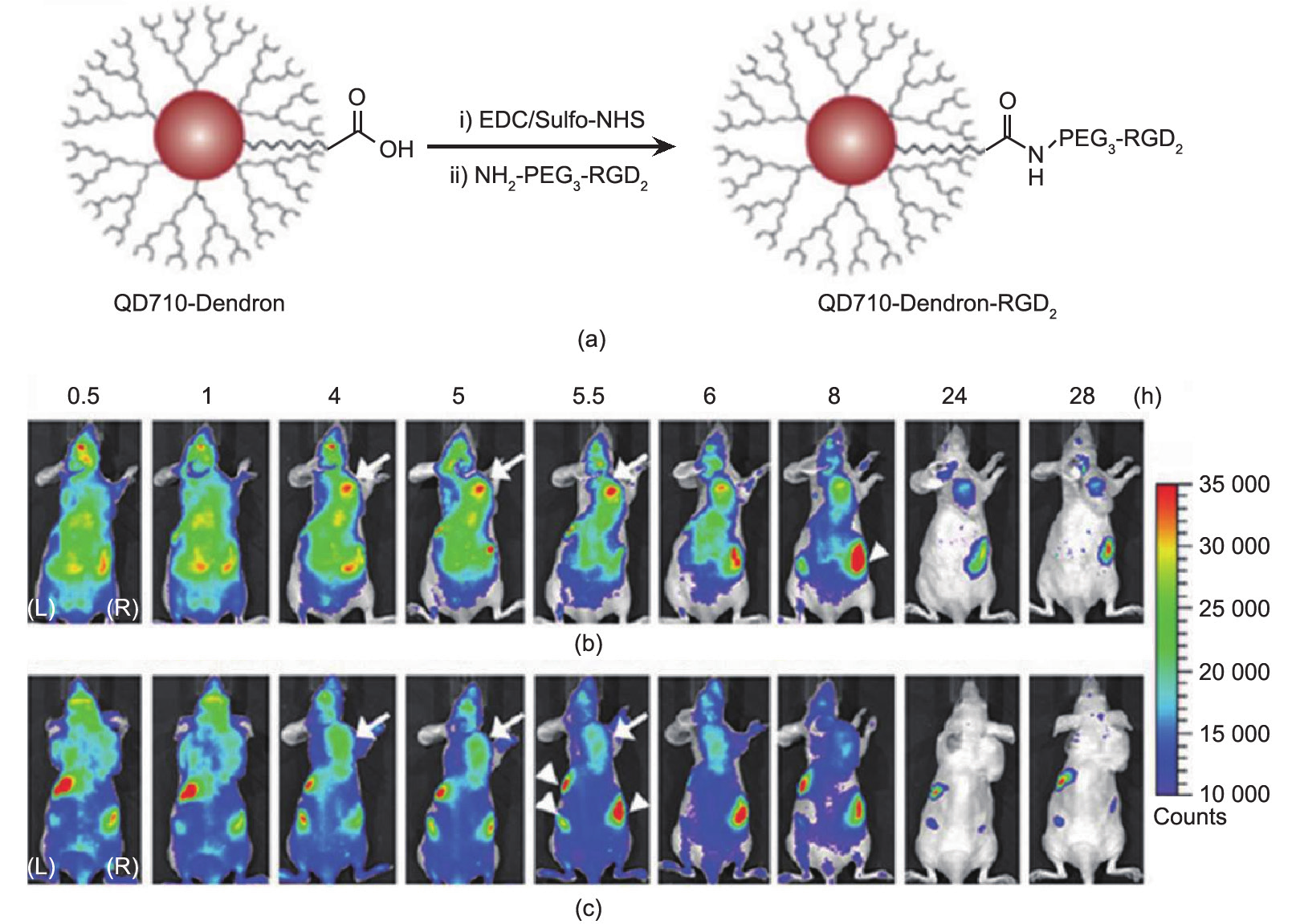
Fig.1 (a) Structure and synthesis of QD710-Dendron-RGD2 conjugate. QD710-Dendron with carboxylate terminal group was conjugated with RGD dimer by carbodiimide coupling. In vivo near-infrared fluorescence imaging. The dorsal images of SKOV3 tumor-bearing (arrows) mice (L, left side; R, right side) injected with (b) QD710-Dendorn-RGD2 (200 pmol) and (c) QD710-Dendron (200 pmol) at 0.5 h, 1 h, 4 h, 5 h, 5.5 h, 6 h, 8 h, 24 h, and 28 h, respectively. The incidental high fluorescent signals in other body parts (arrowheads) might have originated from regular rodent food in stomach and feces in intestine. Adapted with permission from Gao et al. [33], © 2012 American Chemical Society.
Dyes that can be incorporated or encapsulated in polymeric NPs include indocyanine green (ICG) [40], NIR region fluorescent cyanine 7 (Cy7) [41], and dialkylcarbocyanine fluorophores [42], which have been approved by the United States Food and Drug Administration (FDA). A nanoplatform strategy is a promising method to enable optical imaging to overcome the drawbacks of free and small dyes [43,44]. Various nanocarriers have been investigated for the delivery of NIR dye to tumor sites, including liposomes, silica, polymersomes, and targeted rare-earth nanocrystals [45].
To overcome the light penetration issue and realize deep-tissue imaging, radiation-luminescence-excited fluorophores such as QDs can be used for in vivo multiplexed optical imaging by mimicking a natural bioluminescence resonance energy transfer (BRET) process, in which chemical energy is converted into photons to excite the QDs [46]. Photocaged upconversion nanoparticles (UCNPs) take advantage of the photon upconversion of NIR light to UV light to trigger the uncaging of D-luciferin from D-luciferin-conjugated UCNPs. The released D-luciferin effectively confers enhanced fluorescence and bioluminescence signals in vitro and in vivo, with deep light penetration and low cellular damage [47].
Cerenkov luminescence imaging is an emerging imaging modality, similar to bioluminescence imaging, which captures visible photons emitted by Cerenkov radiation [48]. Radioluminescent nanophosphors (RLNPs) have been proposed as MI probes in the development of combined X-ray/optical imaging modalities, such as X-ray luminescence computed tomography (XLCT) [49−51]. PEGylated Eu3+-doped nanophosphors under biological conditions were shown to emit luminescence under excitation by either18F radioisotope or X-rays. This capability provides the potential to enable novel imaging modalities, such as XLCT or deep-tissue Cerenkov luminescence imaging [52].
Raman spectroscopy is based on the light-scattering phenomenon, as opposed to the absorption/emission in fluorescence, and Raman active molecules are more photostable than fluorophores, which are rapidly photobleached. Surface-enhanced Raman spectroscopy (SERS), active NPs, and single-wall nanotubes (SWNTs) can be used for noninvasive imaging in small living subjects by using Raman spectroscopy [53,54]. Affibody-functionalized gold silica NPs performed Raman MI of the epidermal growth factor receptor in a colon cancer mouse model [55].
Photoacoustic imaging (PAI) is a new hybrid biomedical imaging modality related to optical imaging, which has the potential for both functional and anatomic imaging [56]. The generated ultrasonic waves are detected by ultrasonic transducers and then analyzed to produce images. However, exogenous contrast agents are needed for MI, due to the differences in the absorption spectra of different tissues [57]. Non-plasmonic or plasmonic NPs, such as gold nanoshells, nanorods, or nanocages, can promote light absorption, improving the contrast of PAI signals [58]. Perylene-diimide (PDI)-based NIR-absorptive organic NPs (micelle-enveloped PDI) are an efficient contrast agent for the PAI of deep brain tumors in living mice. By encapsulating them into amphiphilic molecules, water-soluble PDI NPs were easily synthesized and exhibited excellent PAI properties [59]. PAI of targeted SWNTs may contribute to the noninvasive cancer imaging and monitoring of nanotherapeutics in living subjects [60−63]. Melanin nanoparticle (MNP) is another biopolymer with good biocompatibility and biodegradability, intrinsic photoacoustic (PA) properties, and binding ability to drugs, as an efficient endogenous nanosystem for imaging-guided chemotherapy [64,65]. Sorafenib (SRF) is chosen for constructing PEGylated-melanin-drug system. SRF-PEG-MNPs were labeled with 64Cu and used to perform a PET/PAI dual modalities imaging-guided therapy [66]. By embedding ultrasmall MNPs into the cavities of apoferritin (APF), nanomaterials apoferritin-melanin-Fe (AMF) NPs with core-shell structures can easily load 64Cu2+ and Fe3+ to achieve trimodal (PET/MRI/PAI) imaging [67] (Fig. 2).
《Fig. 2》
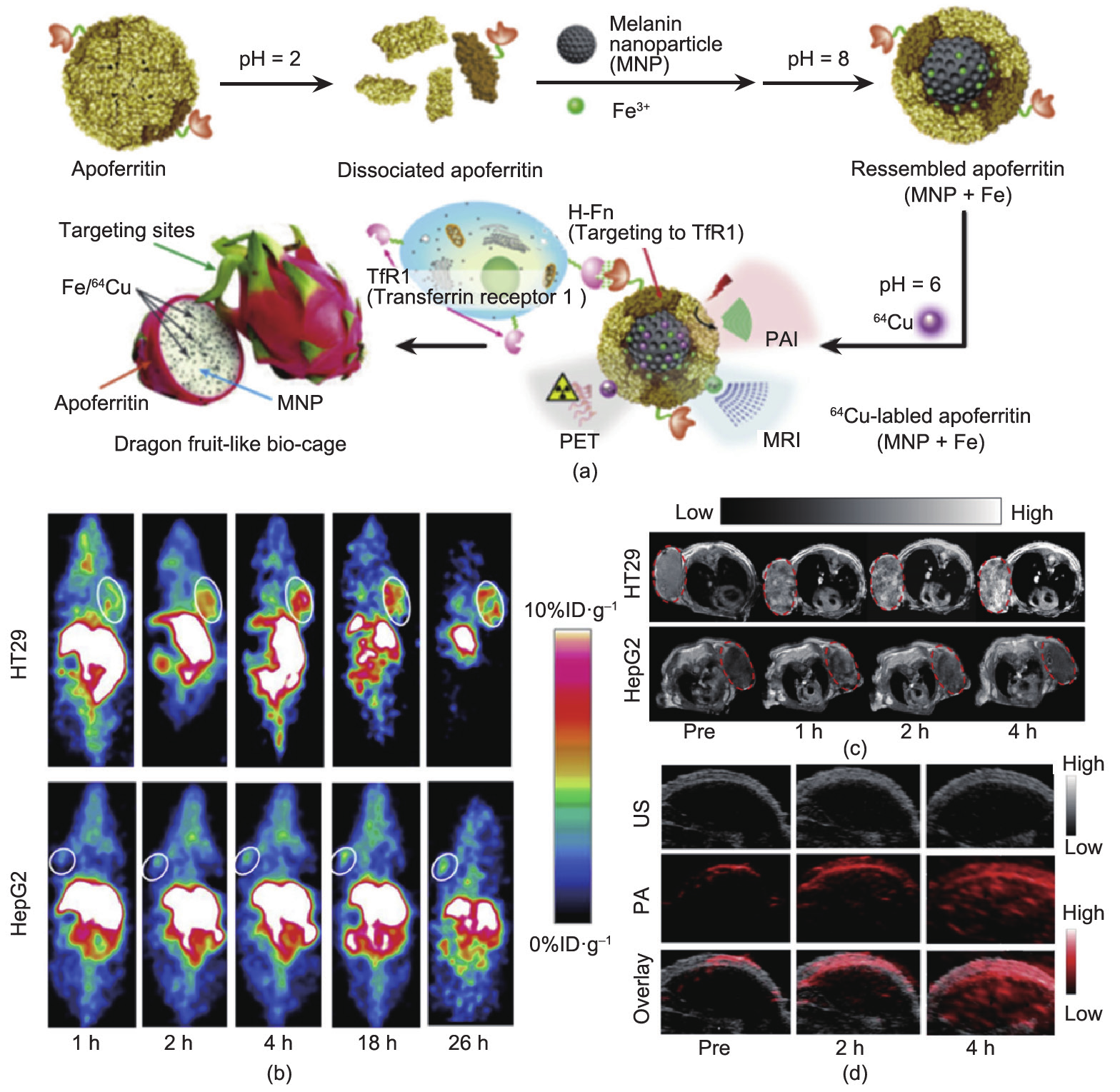
Fig.2 (a) Schematic illustration of AMF nanocage synthesis. (b) Representative coronal small animal PET images of HT29 (top) and HepG2 (bottom) tumor-bearing mice (n= 4) at 1 h, 2 h, 4 h, 18 h, and 26 h after administration of64Cu-AMF. White circles indicate tumor location. (c) T1 magnetic resonance images of HT29 (upper) and HepG2 (lower) tumor model after administration of AMF, red circles indicate the area of tumor. (d) The ultrasound (grey, top), photoacoustic (red, middle) and overlayed coronal sections (bottom) of HT29 tumor models before and after tail-vein injection of AMF nanocages. Adapted with permission from Yang et al. [67], © 2015, Elsevier Ltd.
《2.2. Ultrasound agents》
2.2. Ultrasound agents
US has excellent spatial resolution and contrast sensitivity. Due to its low cost and wide availability, US is the most commonly used clinical imaging modality [68]. Its use is limited in the imaging of some organs, such as lungs. Nanoscale US agents have been reported in the past decade [69,70]. A strategy to improve the in vivo half-life of US agents has been explored by targeting vascular markers of tumor angiogenesis [71]. While there have been recent examples of nanoscale US agents, due to the short in vivo half-life of US agents, the vast majority of targeted agent development has focused on seeking vascular markers of tumor angiogenesis. Anderson et al. [72] modified the surface of microbubbles (MBs) with cRGD ligand and then showed that these exhibit five-fold higher adhesion to immobilized recombinant αvβ3 integrin, compared with control groups. Yan et al. [73] synthesized iRGD-lipopeptides into an MB membrane. The binding specificity of iRGD-MBs for endothelial cells was found to be significantly stronger than that of control MBs under in vitro static and dynamic conditions. Furthermore, many peptides have been developed as ligands to direct MBs to integrins. Willmann et al. [74] designed MBs with engineered cystine knot (knottin) peptides, a new class of targeting ligands to αvβ3 integrin, for targeted contrast-enhanced US imaging of tumor angiogenesis. MB-KnottinIntegrin attached significantly more to αvβ3 integrin positive cells than to control cells. In addition to integrins, the expression of vascular endothelial growth factor receptor 2 (VEGFR2) is another target of interest for imaging and monitoring during treatment. Contrast agent MBs for dual-targeting imaging can be modified to attach to both VEGFR2 and αvβ3 integrin [75]. As a result of the increased avidity that comes from the multi-ligand approach, the dual-targeting US MBs exhibited significantly higher signal compared with the single-targeting counterparts.
《2.3. Magnetic nanoparticles》
2.3. Magnetic nanoparticles
MRI is a noninvasive diagnostic technique providing superb image resolution and exquisite soft-tissue contrast for anatomical details. A considerable amount of work has been done in the development of MRI NPs, and especially with iron oxide NPs (IONPs). This is an important class of nanoplatform with the advantages of high sensitivity, shortened T1 and T2 relaxation times in a dose-dependent manner, and the versatility to present a wide variety of ligands for cellular and molecular imaging. There are more than 20 current clinical trials of superparamagnetic iron oxide (SPIO) or ultrasmall superparamagnetic iron oxide (USPIO). The iron oxide NPs are usually taken up by the RES [76], which also permits liver [77−79], spleen [80], and lymph node imaging. The accumulation of these NPs at the tumor site is based on the EPR effect due to the presence of leaky vasculature as well as uptake by macrophages.
IONPs such as SPIO NPs have been extensively studied for tumor-targeting probes due to their excellent biocompatibility [10]. The synthesis process for these particles ranges from traditional wet chemistry solution-based methods to more exotic techniques [81,82]. The field of magnetic NP probe technology has recently been promoted by efforts devoted to developing its potential as an important tool for efficient cross-application MI [83]. Hybrid nanotrimers performed dual T1- and T2-weighted MRI with high accuracy and reliability, as revealed by a PET imaging study, and displayed favorable biodistribution and suitability for in vivo imaging [84] (Fig. 3).
《Fig. 3》
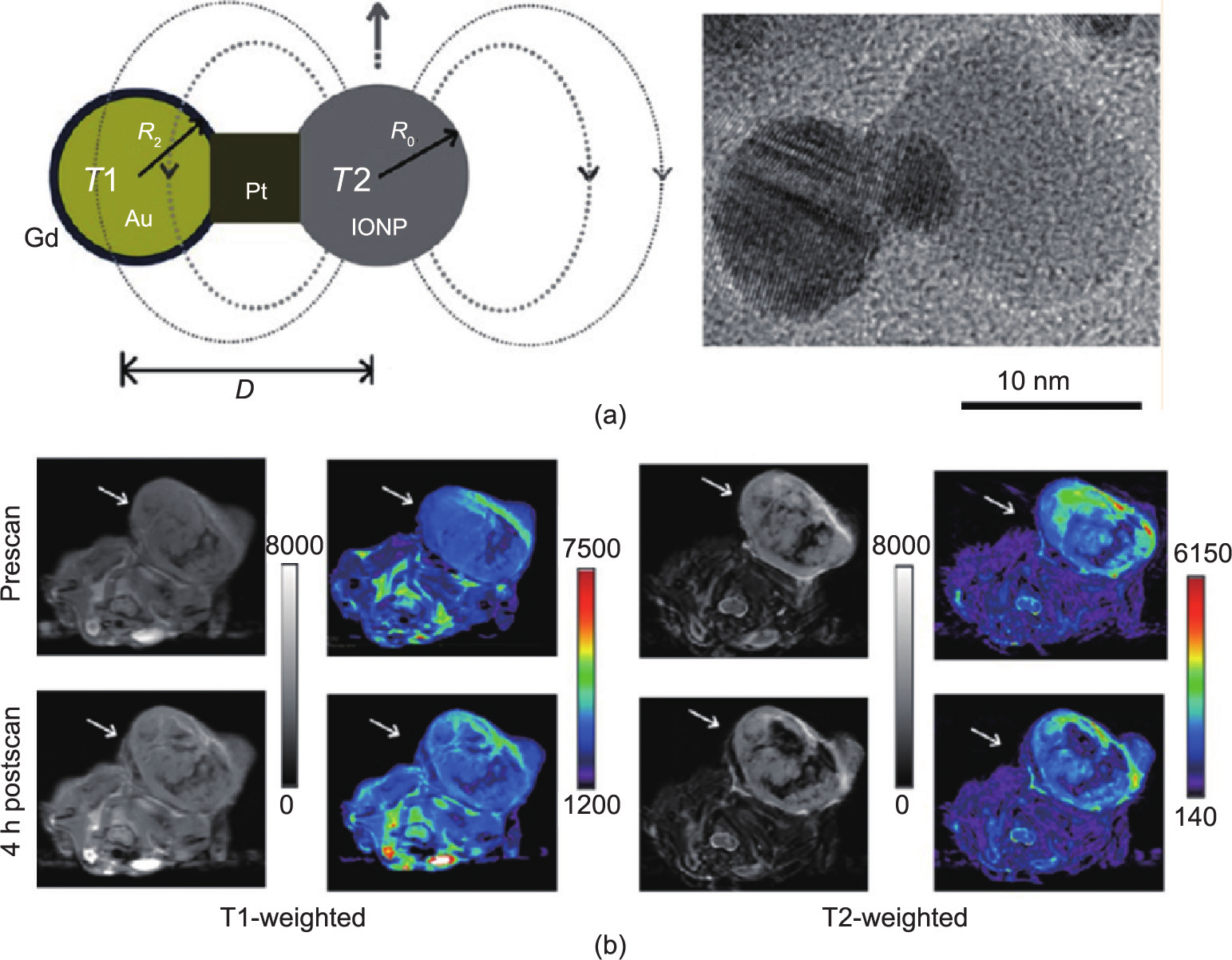
Fig.3 (a) Engineering the heterogeneous nanostructures for magnetic coupling of T1 and T2 contrast agents (left) and transmission electron microscope (TEM) image of dumbbell structure (right). (b) T1- and T2-weighted magnetic resonance images of HT29 tumor bearing mice (n= 3) before and after intravenous injection of Gd-DB-HNTs. The left ones show gray scale images, and the right ones show the pseudocolored images. Adapted with permission from Cheng et al. [84], © 2014 American Chemical Society.
Research with SPIO NPs has already demonstrated their utility as an important tool for enhancing magnetic resonance (MR) contrast [85]. It is desirable for the NPs used for this application to have high magnetization values and a size smaller than 100 nm, with a narrow particle-size distribution [86]. Magnetic NPs offer a number of advantages, including laser-induced thermal therapy, the ability to target specific sites, and relatively low toxicity [87]. Biological applications of magnetic NPs also require magnetic particles to have a special surface coating that must be nontoxic and biocompatible, and that must permit targetable delivery with particle localization in a specific area. Such magnetic NPs can be easily conjugated to drugs, proteins, enzymes, antibodies, or nucleotides and can be directed to an organ, tissue, or tumor using an external magnetic field.
The specific structure of dendrimers makes them an attractive NP platform for gadolinium (Gd)-loading due to their narrow size distribution (5−10 nm); branched structure, which facilitates the presentation of multiple ligands or contrast agents; and associated renal clearance. For example, Gd-loaded dendrimers targeting the folate receptor displayed enhanced MR signal in an animal model of a κB-tumor. When designing Gd-based agents, several factors need to be considered, since signal intensity at the target site is not linearly related to the concentration of the imaging moiety. For example, the intracellular accumulation of Gd can markedly impact signal intensity. Furthermore, the presence of Gd atoms in an NP structure also impacts the efficiency of NP MR probes.
《2.4. Nanoparticles for nuclear imaging》
2.4. Nanoparticles for nuclear imaging
With the development of the micro scanner contributing to small-animal imaging, nuclear imaging can be performed with low mass amounts to measure the function of biological processes [88]. With high contrast sensitivity, PET imaging plays an important role in MI, albeit with the disadvantage of low resolution. Based on existing knowledge of nuclear imaging with small molecules, NP-based nuclear imaging agents have been rapidly developed. During the past decade, there have been various reports on PET imaging with targeted NPs. The most common way to integrate PET imaging capability into NPs is to attach a radiometal (e.g.,64Cu) via a metal chelator, such as 1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) or 1,4,7-triazacyclononane- N,N′,N′′-triacetic acid (NOTA). After purification, NPs radiolabeled with 64Cu can reach greater than 95% radiochemical purity [89−91].
Since no single modality is perfect and sufficient to obtain all necessary information, several other NP platforms have been investigated for the development of multimodal contrast agents, such as for use with combined PET and optical imaging, or PET and MRI. Multimodal imaging enables the use of PET for treatment planning with whole-body imaging, and the use of optical techniques during therapeutic intervention. Cai et al. [92] developed a near-infrared fluorescence (NIRF) imaging and PET imaging agent based on QD. RGD peptides were modified on the surface of QDs, allowing them to target the αvβ3 integrin. At the same time, DOTA was also conjugated for 64Cu labeling. This dual-modality probe sufficiently improves tumor contrast while decreasing the dose required for in vivo NIRF imaging, thus leading to a significant reduction in the potential toxicity of QDs [93,94]. Similarly, Chen et al. [95] synthesized a64Cu-DOTA-QD-VEGF nanoprobe to primarily target the vasculature through a VEGF-VEGFR interaction. Nahrendorf et al. [96,97] synthesized a dextran-coated SPIO NP co-labeled with 64Cu and an NIR fluorophore (VivoTag 680), which can perform both fluorescence molecular tomography (FMT) and PET imaging of tumor-associated macrophages. Yang et al. [98] modified a multimodal RGD-targeted SPIO. After labeling with 64Cu, the NPs can be used for monitoring drug delivery to solid tumors through PET/MR dual-modal imaging [98]. As a photosensitizer, porphyrin not only provides photodynamic ability but also serves as a promising dye for fluorescent imaging. Liu et al. [99] developed a 64Cu-porphorysome nanoplatform presenting the ability to detect small bone metastases in lower limbs through optical-PET dual-modal imaging. Chen et al. [100] recently investigated targeted hollow mesoporous silica NPs (HMSNs) for PET/optical imaging of tumor angiogenesis by using CD105. They demonstrated a three-fold higher accumulation compared with their non-targeted group [100] (Fig. 4). Liu et al. [61] investigated integrin-targeted SWNTs, which performed remarkable dual Raman and PET tumor-targeting imaging. Zirconium-89 (89Zr) is a new and promising PET radionuclide for in vivo studies [101−105]. Ruggiero et al. [106] developed antibody-labeled 89Zr-SWNT, which targeted the monomeric vascular endothelial-cadherin (VE-cad) epitope with monitoring by PET and NIRF imaging. Using this nanoplatform, the tumor angiogenesis can be estimated. Lee et al. [107] monodispersely synthesized polymer-coated UCNPs and dimeric RGD-conjugated UCNPs. This NP platform can be used for optical imaging, MRI, and PET, with highly selective targeting to αvβ3 integrin [107]. Lijowski et al. [108] designed T1-based αvβ3-targeted 99mTc-Gd NPs, which produced high sensitivity and specific localization of tumor angiogenesis by SPECT-MR imaging [108]. Hu et al. [109] investigated 111In-perfluorocarbon modified by αvβ3 integrin, producing a multimodal PET-MR agent. Radiolabeling Ac-Cys-ZEGFR:1907-modified Au-IONP (NOTA-Au-IONP-affibody) with PET radionuclide, 64Cu, resulted in an epidermal growth factor receptor (EGFR)-targeted PET/optical/MR imaging probe, 64Cu-NOTA-Au-IONP-affibody, targeted toward EGFR-positive tumors [110].
《Fig. 4》
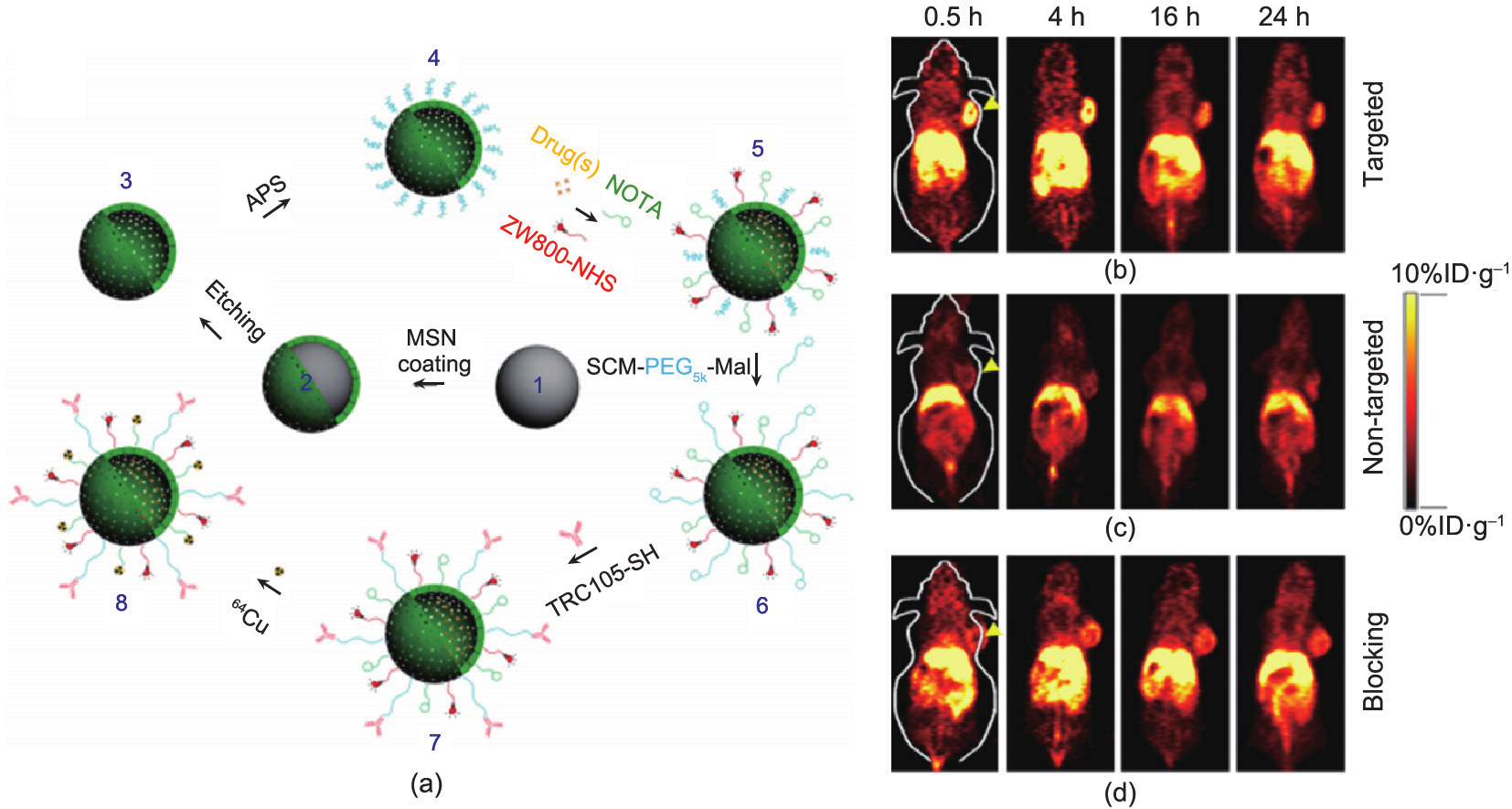
Fig.4 (a) Surface engineering of HMSN. Uniform dense silica (dSiO2, 1) was firstly synthesized and coated with a shell of MSN, forming dSiO2@MSN (2). Tightly-controlled Na2CO3 etching step was introduced to selectively etch dSiO2 away, leaving uniform HMSN (3). As-synthesized HMSN was then surface modified with (3-aminopropyl)triethoxysilane (APS) to form amino groups conjugated HMSN-NH2 (4) before further bio-conjugations. Anticancer drugs (i.e., doxorubicin, DOX) were then loaded, followed by NIR dye (i.e., ZW800) and 64Cu chelator (i.e., NOTA) conjugations, forming NOTA-HMSN(DOX)-ZW800 (5). Afterward, nano-conjugate was PEGylated with SCM-PEG5k-Mal to render its stability in biological buffers (e.g., phosphate buffered saline, PBS), forming NOTAHMSN(DOX)-ZW800-PEG-Mal (6). Then, thiolated anti-CD105 antibody (i.e., TRC105-SH) was conjugated to the NP to obtain NOTAHMSN(DOX)-ZW800-PEG-TRC105 (7). Lastly, PET isotope 64Cu ( = 12.7 h) was used to label the NP, forming 64Cu-NOTAHMSN(DOX)-ZW800-PEG-TRC105 (8). In vivo tumor targeted PET imaging. Serial coronal PET images of 4T1 tumor-bearing mice at different time points post-injection of (b) targeted group: 64Cu-HMSN-ZW800-TRC105, (c) non-targeted group: 64Cu-HMSN-ZW800, or (d) blocking group: 64Cu-HMSN-ZW800-TRC105 with a blocking dose (1 mg per mouse) of free TRC105. Tumors were indicated by yellow arrowheads. Adapted with permission from Chen et al. [100], © 2014 Nature Publishing Group.
= 12.7 h) was used to label the NP, forming 64Cu-NOTAHMSN(DOX)-ZW800-PEG-TRC105 (8). In vivo tumor targeted PET imaging. Serial coronal PET images of 4T1 tumor-bearing mice at different time points post-injection of (b) targeted group: 64Cu-HMSN-ZW800-TRC105, (c) non-targeted group: 64Cu-HMSN-ZW800, or (d) blocking group: 64Cu-HMSN-ZW800-TRC105 with a blocking dose (1 mg per mouse) of free TRC105. Tumors were indicated by yellow arrowheads. Adapted with permission from Chen et al. [100], © 2014 Nature Publishing Group.
《3. Challenges and future perspectives》
3. Challenges and future perspectives
Some fundamental hurdles hamper the approval of NPs for clinical use; these hurdles correlate to various materials characteristics including size, shape, composition, single crystallinity, and magnetism. The first hurdle is that of delivery obstacles. In particular, the RES can phagocytose the NPs, rapidly sweeping them out of blood circulation and accumulating them in the liver, spleen, and bone marrow. This phenomenon gives cause to further toxicity concerns. The toxicity of NPs is another very important hurdle. Toxicity can result not only from concentration in the bone marrow and other organs but also from the composition of the NPs themselves, such as the Cd used in QDs and other coating materials. Additional hurdles include the inability to overcome certain biological barriers, such as the blood-brain barrier (BBB), as well as the lack of control over the distribution of NPs following administration. The fate of NPs after their intravenous administration is highly variable and dependent on their size, morphology, charge, and surface chemistry. An NP size of below 100 nm is often used in order to increase effectiveness and blood-circulation time, for adequate exposure to the tumor and drug release.
Given these existing hurdles, next-generation platforms will likely emphasize the development of small-size NPs, their high colloidal composition, and bio-stability. Significant improvements in sensitivity and target specificity are expected, which will bring huge advancements in current cancer-diagnostic capabilities. Additional regulatory and development considerations will arise when future generic nanomedicines are presented for health authority (FDA) approval with claims of equivalence to the innovator drug. In addition, in the face of the natural biological barriers to the delivery of nanomedicines, tissues with aberrant pathology, such as tumors, are often very efficient in harnessing active biological mechanisms for high nutrient supply and rapid growth. Elucidation of these active transport mechanisms and the ability to harness them with nanomedicines could provide a step forward in the treatment of cancer and other diseases.
《4. Conclusions》
4. Conclusions
Over the last decade, the field of nanotechnology has experienced tremendous growth and advancement. With substantial efforts by both researchers and the biopharmaceutical industry, a few nanomedicines have already been successfully approved for preclinical and clinical studies. However, the field of nanomedicine is still in its early stages due to unfamiliar types of risk in safety and efficacy that require further discussion and cooperation among researchers and governmental agencies. The challenges in developing NPs for use in MI may be overcome in the near future.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Zhen Cheng, Xuefeng Yan, Xilin Sun, Baozhong Shen, and Sanjiv Sam Gambhir declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




