《1.Introduction》
1.Introduction
Considering environmental problems and the exhausting supply of global energy, it is urgently necessary to exploit sustainable energy systems. Solar energy, as a clean, renewable, and abundant energy, is one of the most promising candidates to substitute for fossil fuels. With a specific energy density of 120 kJ·g−1 and only water (H2O) as a byproduct when consumed, hydrogen (H2) is considered to be an efficient energy carrier as well as the cleanest option [1–3]. Moreover, H2 can be stored and carried over a long distance as a stable energy supply [2–4]. Photocatalytic water splitting, which stores solar energy in H—H (H2) bonds, is a simple and green solar-energy-conversion approach [1], and has therefore attracted global research interest during the past decades. Many materials have been investigated for solar water splitting with the hope of achieving a commercially feasible solar-to-hydrogen (STH) efficiency of over 10% [5–10]. To date, some material systems can achieve overall water splitting, but most of these photocatalysts can only be active under ultraviolet (UV) light irradiation (which accounts for only 4% of solar energy) [11–14]. Only limited material systems, such as In1–xNixTaO4 (x = 0–0.2) [15], (Ga1–xZnx)(N1–xOx) [16], (Zn1+xGe)(N2Ox)[17], TaON [18], C3N4 [19,20], and LaMgxTa1–xO1+3xN2–3x (x = 0–2/3) [21],are able to achieve visible-light-responsible water splitting. However, the STH efficiency of these photocatalysts is still too low for practical application. Many metal dichalcogenides (e.g., CdS, CdSe, and CuInS2), which can absorb visible light, show highly efficient photocatalytic H2 evolution from water with sacrificial agents [22–24]. However, most suffer from photocorrosion, which damages the long-term stability of these photocatalysts. Therefore, the question of how to better design and develop low-cost and stable photocatalysts for efficient water splitting is of great importance.
As many excellent reviews for photocatalytic water splitting have been published [6,7,11,12,25–35], we provide only a brief introduction to the photocatalytic water-splitting mechanism here. As shown in Fig. 1, the major steps in a photocatalytic water-splitting reaction are as follows.
《Fig. 1》

Fig. 1. Main scheme of photocatalytic water splitting. CB: conduction band; VB: valence band.
(1)Photon absorption and electron-hole pair generation. During this process, only photons with energy higher than the bandgap energy of the semiconductor are effective for excitation. Once excited, electrons from valence bands (VBs) jump to conduction bands (CBs), and holes are left in the VBs. The photo-excited electrons and holes lead to redox reactions on H2O, electrons for water reduction, and holes for water oxidation. As illustrated in Fig. 2, the energy potential of the CB minimum and VB maximum are key points for the photocatalytic reaction. The CB minimum should be more negative than the H2 production potential (0 V vs. normal hydrogen electrode (NHE) while the VB maximum should be more positive than the oxygen production potential (1.23 V). Otherwise, the photocatalysts can only conduct a half reaction with the assistance of sacrificial agents, which will increase the cost of the hydrogen produced.
《Fig. 2》
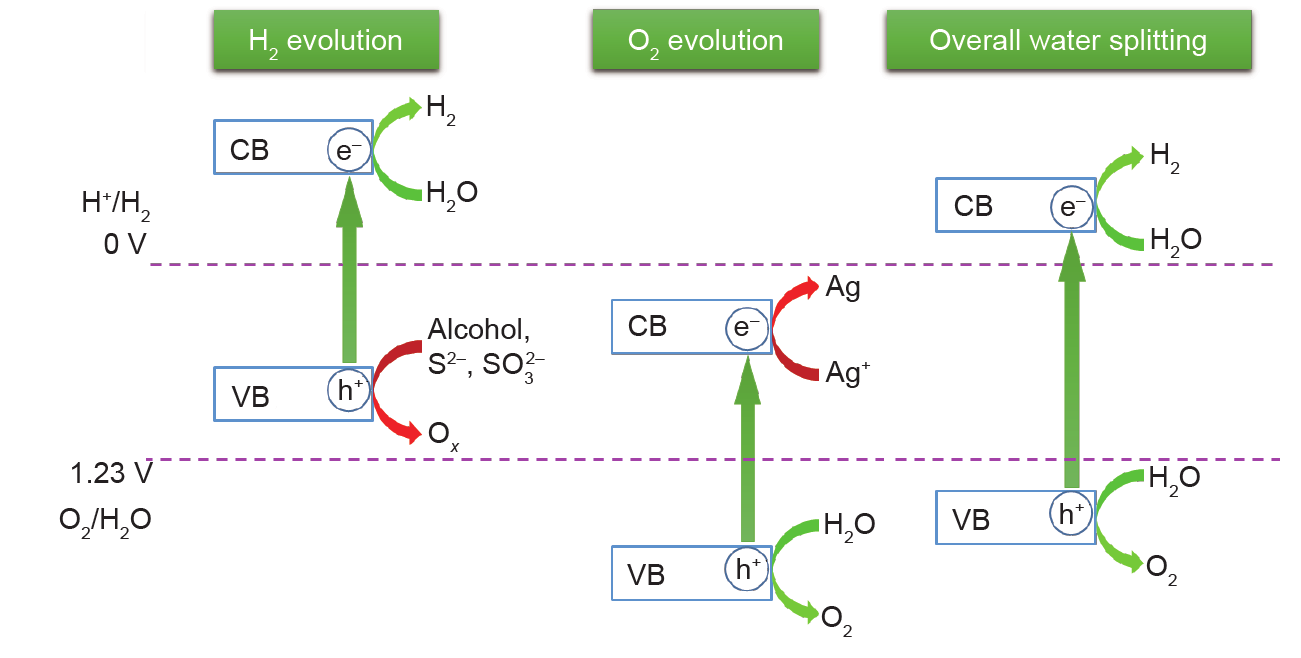
Fig. 2. Scheme of water splitting using semiconductors.
(2)Separation and transfer of electrons. Photo-generated electronhole pairs separate and migrate to the surface to take part in the water redox reaction. During this process, charge carriers easily meet each other and recombination occurs, which is undesirable for photocatalysis.
(3)Surface chemical reactions. In this step, the photo-excited charge carriers on the surface active sites react with water.
Due to the 10% STH conversion efficiency that is required for commercial application, the development of highly efficient photocatalysts that can maximize the efficiency of each of the three steps mentioned above is extremely important [1,36–38]. The theoretical maximum STH efficiency depends on the bandgap of the photocatalysts, because only light with energy higher than the bandgap of the photocatalysts can be absorbed and used for the water redox reaction. Theoretically, the bandgap should be a minimum of 1.23 eV. However, due to the thermodynamic energy (~0.4 eV) and kinetic loss (~0.4 eV), a bandgap of 2 eV is usually necessary for overall photocatalytic water splitting [11]. For photocatalysts with a bandgap energy ranging from 2.0 eV to 2.5 eV, the theoretical STH efficiency ranges from 10% to 16% under simulated sunlight irradiation (air mass 1.5 global, AM 1.5G) [39]. Considering the energy loss in practical applications, a narrow bandgap is a key criterion for photocatalysts to achieve high STH efficiency. Here, we define narrow bandgap photocatalysts as photocatalysts with a bandgap no greater than 2.5 eV (corresponding to ~500 nm light absorption).
Tantalum (oxy)nitride-based photocatalysts, which have bandgaps varying from 1.9 eV to 2.5 eV and bandgap positions that are suitable for water splitting, are considered to be a group of potential photocatalysts for efficient water splitting [39]. Over the past years, great development and encouraging progress have been achieved in tantalum (oxy)nitride-based photocatalysts. However, the STH efficiency of these photocatalysts is still less than 1% [40–45]. One major problem is the asymmetric capability of these materials for water reduction and oxidation. Tantalum (oxy)nitride-based photocatalysts have shown a comparably much higher water oxidation capability than water reduction capability [40,46–48]. Because overall water splitting is a synergistic reaction system between water reduction and water oxidation, a relatively weaker water reduction capability will inevitably impede the overall water-splitting reaction. Thus, it is of great importance to improve the water reduction efficiency of tantalum (oxy)nitride-based photocatalysts. In addition, both surface defects and self-oxidation damage the long-term stability of tantalum (oxy)nitride-based photocatalysts. This review provides an up-to-date critical review on narrow bandgap tantalum (oxy)nitride-based photocatalysts for water splitting. In particular, recent progress in the development of effective strategies for enhancing their water reduction capability is discussed. An outlook perspective for further development of tantalum (oxy)nitride-based photocatalysts is also proposed.
《2.Tantalum-based narrow bandgap photocatalysts》
2.Tantalum-based narrow bandgap photocatalysts
Tantalum (oxy)nitride-based photocatalysts are a large family; the main research focus is on Ta3N5, TaON, LaTaON2, and ATaO2N (A= Ca, Sr, Ba), which possess narrow bandgaps and suitable band positions for the water redox reaction [49]. Among these, Ta3N5 and TaON have been intensely studied, and over 10% quantum efficiency (QE) for water oxidation and 9.5% QE for water reduction have been achieved [43,47,48]. In addition, BaTaO2N has shown H2 evolution from water without sacrificial agents by forming a solid solution with BaZrO3, and TaON was demonstrated to show overall water-splitting activity with surface modification and appropriate co-catalysts [18,50]. Moreover, very recent progress showed that LaMgxTa1–xO1+3xN2–3x and CaTaO2N could achieve overall water splitting when a Rh-Cr bimetallic oxide co-catalyst was loaded on the surface [51]. All these findings strongly indicate the great potential of tantalum (oxy)nitride-based narrow bandgap photocatalysts for achieving highly efficient solar water splitting.
《2.1. Basic properties of tantalum (oxy)nitrides》
2.1. Basic properties of tantalum (oxy)nitrides
Many reviews have introduced and discussed the fundamental parameters, optical properties, and electronic properties of tantalum (oxy)nitride-based materials, both theoretically and experimentally [14,30,41,42,52,53]. Here, we briefly introduce the basic properties that are relevant to photocatalytic performance. Table 1 [49] presents typical tantalum (oxy)nitride-based photocatalysts. Ta3N5 and TaON show anosoviteand baddelyite-type crystal structures, respectively, while the others are perovskite-type crystals. The bandgap ranges from 1.9 eV to 2.4 eV, and corresponds to as long as 660 nm light absorption. The absolute position of the CB minimum and of the VB maximum is one of the key factors that influences the water reduction and water oxidation capability of photocatalysts. Domen et al. [54] experimentally identified the CB and VB positions of Ta3N5 and TaON by combining UV photoelectron spectroscopy (UPS) with electrochemical analysis. As shown in Fig. 3 [54], the CB minimums of Ta3N5 and TaON are ~−0.3 eV and ~−0.5 eV, respectively, versus NHE at pH = 0, while the VB maximums of Ta3N5 and TaON are ~1.6 eV and ~2.1 eV, respectively. The bandgap positions indicate that both Ta3N5 and TaON can be used for the photocatalytic water redox reaction. Balaz et al. [55] investigated the bandgap positions of tantalum oxynitride perovskite (ATaO2N, A = Ca, Sr, Ba) by combining depth-resolve cathodoluminescence spectroscopy (DRCLS), ultraviolet-visible (UV-Vis) spectroscopy, Kelvin probe force microscopy (KPFM), and X-ray photoemission spectroscopy (XPS). As shown in Fig. 4 [55], all of the VB maximums except that of PrTaO2N are more positive than the oxygen evolution potential of water, and the CB minimums are more negative than the hydrogen evolution potential. Based on the investigation of band structure and the study of photocatalytic water-splitting activity, it is anticipated that tantalum (oxy)nitride-based materials can be designed as highly efficient catalysts for solar water splitting.
《Table 1》
Table 1 Representative tantalum (oxy)nitride-based photocatalysts [49].

《Fig. 3》
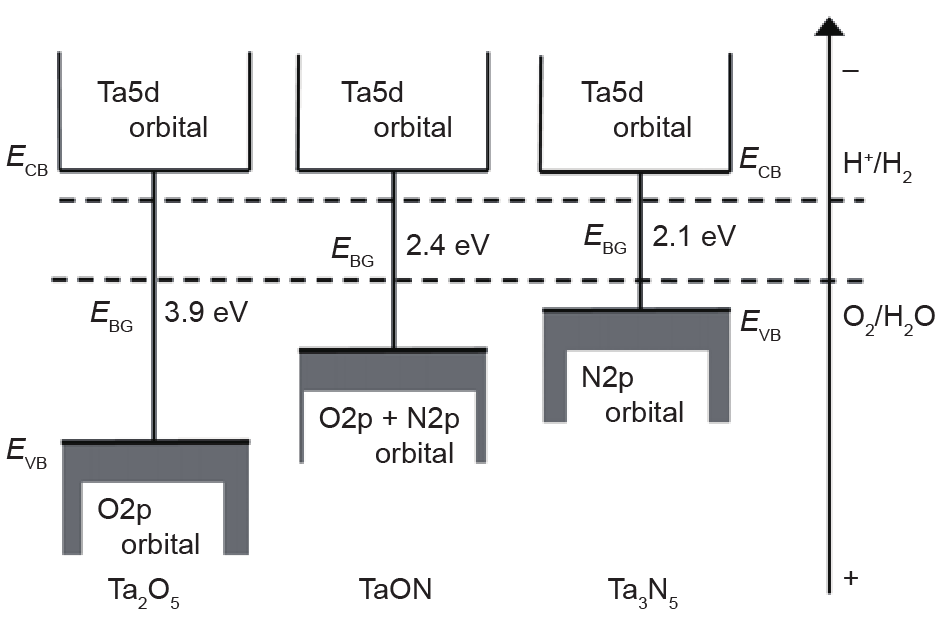
Fig. 3. Schematic band structures of Ta2O5, TaON, and Ta3N5 [54]. (Copyright 2003, American Chemical Society)
《Fig. 4》

Fig. 4. Bandgap diagrams of tantalum-based oxynitride perovskite [55]. EF: Femi lever; EVAC: vacuum lever. (Copyright 2013, American Chemical Society)
《2.2. Synthesis of tantalum (oxy)nitrides》
2.2. Synthesis of tantalum (oxy)nitrides
In general, tantalum (oxy)nitride-based materials are synthesized from tantalum-based oxide precursors via nitridation at high temperature with ammonia (NH3) gas as the nitrogen source [14]. For Ta3N5 and TaON, many preparation methods have been reported to synthesize Ta2O5 precursors, including reverse homogeneous precipitation reactive sputtering, atomic layer deposition, and vapor phase hydrothermal processes [56–61]. For the tantalum-based oxynitride perovskite, a solid-state reaction is normally applied to synthesize the oxide precursors. The key step to transform the precursors into (oxy)nitride is high-temperature thermal treatment in NH3 gas. The nitridation conditions, such as temperature, pressure, and precursor, are important to the final photocatalytic performance [62–64].
The nitridation temperature, duration, and NH3 gas flow determine the final products. During the nitridation process, the oxygen (O) atoms are substituted by nitrogen (N) atoms. When using Ta2O5 as the precursor, low temperature, short time, and low NH3 gas flow lead to a slow reaction and to the partial substitution of O atoms by N atoms, resulting in TaON as the final product. To obtain oxygen-free Ta3N5, higher temperature (> 850 °C), longer treatment (>110 h), and higher NH3 gas flow (> 20 mL·min) are necessary. The influence of nitridation temperature on the photocatalytic performance of Ta3N5 has been systematically studied [62]. During the nitridation process, the duration and NH3 gas flow were fixed, with the only variation being that of temperature. Once the temperature was above 850 °C, Ta3N5 could be obtained. Further increasing the temperature to 950 °C did not influence the structural and optical properties of Ta3N5. However, increasing the temperature to 1000 °C led to the formation of a tantalum (Ta) reduced species, which was confirmed by the sub-bandgap absorption that was observed in the UV-Vis light absorption spectrum (Fig. 5) [62]. The Ta reduced species acted as a recombination center, and samples with such defects showed lower photocatalytic performance. Thus, suitable nitridation temperature is important to obtain Ta3N5 with good crystallinity and pure phase.
《Fig. 5》

Fig. 5. UV-Vis light absorption spectra of Ta3N5 prepared at 850 °C, 900 °C, 950 °C, and 1000 °C in NH3 gas collected in different mode: (a) transmission mode and (b) integrating sphere. Pictures inset in (b) show the gray tint in the sample treated at 950 °C and 1000 °C [62]. (Copyright 2014, American Chemical Society)
Pressure is another factor that influences the final performance of tantalum (oxy)nitride photocatalysts [65]. Kishida and Watanabe[64] studied the effect of pressure on the final photocatalytic H2 generation reaction of Ta3N5. It was demonstrated that Ta3N5 obtained under high-pressure ammonothermal treatment (> 50 MPa) showed improved photocatalytic H2 evolution efficiency. The improved performance was attributed to the decrease of surface defects, which reduced surface recombination and improved the transfer of electrons to the surface active sites (platinum (Pt) co-catalyst or surface H+). A higher density of defects was expected to cause stronger absorption at a wavelength longer than the absorption edge of the semiconductors [46,66–68]. Ta3N5 treated with an ammonia pressure of 50 MPa showed the lowest absorption intensity and the highest photocatalytic activity (Fig. 6) [64]. Thus, high-pressure ammonothermal treatment should be an effective way to suppress surface defects and enhance the photocatalytic activity of tantalum (oxy)nitridebased photocatalysts.
《Fig. 6》

Fig. 6. UV-Vis light diffuse reflectance spectra of Ta3N5. (a) Pristine Ta3N5; (b–e) samples treated at 823 K for 5 h with (b) 10 MPa of N2, (c) 10 MPa of NH3, (d) 50 MPa of NH3, and (e) 100 MPa of NH3 [64]. (Copyright 2012, Elsevier)
In addition to pressure, flux-assisted nitridation was adopted to synthesize the Ta3N5 photocatalysts with high crystallinity. Takata et al. [69] conducted a systematic study of flux-assisted nitridation for Ta3N5 preparation. Zinc (Zn), NaCl, and Na2CO3 were used as flux during the nitridation of Ta2O5 or TaCl5 into Ta3N5. It was found that the introduction of NaCl and Na2CO3 as flux during the nitridation process led to the formation of morphologically defined particles. When TaCl5 was nitrided along with NaCl, well-crystallized Ta3N5 nanoparticles were obtained (Fig. 7) [69]. In addition, the particle size could be controlled by varying the temperature. It was explained that nitridation and dissolution might happen simultaneously during flux-assisted nitridation, which would more effectively promote the diffusion of atoms in the materials. This result demonstrates that the morphology, dispersity, crystallinity, and surface states of tantalum (oxy)nitride can be controlled by varying the nitridation conditions, including temperature, ammonia pressure, precursor, and flux.
《Fig. 7》

Fig. 7. Scanning electron microscope (SEM) images of Ta3N5 prepared by various fluxassisted nitridation methods. (a) Ta2O5-NaCl (850 °C for 10 h); (b) Ta3N5-NaCl (850 °C for 10 h); (c) TaCl5-NaCl (800 °C for 10 h); (d) TaCl5-Zn (800 °C for 10 h) [69]. (Copyright 2011, American Chemical Society)
《2.3. Strategies for improving water-splitting activity》
2.3. Strategies for improving water-splitting activity
The three main steps in photocatalytic water splitting, namely, light absorption, charge carrier migration, and surface redox reaction, have a synergistic effect on the activity of photocatalytic water splitting. Based on these three steps, many strategies including doping, morphological control, surface modification, co-catalyst design, and heterostructure design have been intensively explored in order to enhance the water-splitting activity of tantalum (oxy)nitridebased photocatalysts.
2.3.1. Doping
Heteroatom doping is an important strategy to tune the basic properties of photocatalysts, such as bandgap structures, electronic conductivity, and surface structure. Doping is usually used to narrow the bandgap, increase the electronic conductivity, or make the surface active, thus promoting light harvesting, charge transfer reactions, and surface reactions. Through doping, the water-splitting performance of photocatalysts is significantly improved [70–75]. Foreign-ion-doped tantalum (oxy)nitride photoanodes or photocatalysts for O2 evolution have been prepared and investigated intensively [76–80], but limited research attention has been paid to the H2 evolution of doped tantalum (oxy)nitride-based photocatalysts. Seo et al. [81] systematically investigated magnesium (Mg)and zirconium (Zr)-doped Ta3N5 photocatalysts for H2 evolution. As shown in Fig. 8(a) [81], single substitution with Mg or Zr enhanced H2 evolution by several times, while the activity of Ta3N5 : Mg + Zr (doping concentration: 25 at%) for H2 evolution was increased drastically, to approximately 15 times the performance of pure Ta3N5. According to the band structure investigation, the enhanced H2 evolution activity was attributed to the negative shift of bandgap (Fig. 8(b)) [81]. The Mg + Zr co-doped Ta3N5 had a more negative CB and thus possessed a higher reduction potential, which favored water reduction.
《Fig. 8》

Fig. 8. (a) Time courses for photocatalytic H2 production on Ta3N5 variants separately replaced with different amounts of Mg, Zr, or Mg + Zr: (i) undoped; (ii) 25 at% Mg; (iii) 40 at% Mg; (iv) 25 at% Zr; (v) 40 at% Zr; (vi) 25 at% Mg + Zr; (vii) 40 at% Mg + Zr. (b) Band structure diagram of Ta3N5 and Ta3N5 : Mg + Zr [81]. Efb: flat band energy. (Copyright 2015, American Chemical Society)
When Mg ions were introduced into LaTaON2, the resulting solid solution of LaMgxTa1–xO1+3xN2–3x (x = 0–2/3) acted as a photocatalyst for water splitting at light absorptions up to 600 nm [21]. Further investigation revealed that the introduction of Mg ions enabled fine-tuning of the bandgap energy and positions, resulting from a co-substitution of Mg2+ for Ta5+ and O2– for N3–. As shown in Fig. 9(a) [82], the bandgap energy of LaMgxTa1–xO1+3xN2–3x could easily be tuned by varying the Mg/(Mg + Ta) ratio. The absolute bandgap position of LaMg0.33Ta0.67O2N was revealed by combining photoelectron spectroscopy in air (PESA) with theoretical calculations. Both methods confirmed the negative shift of the CB and positive shift of the VB for LaMg0.33Ta0.67O2N compared with LaTaON2 (Fig. 9(b)) [82]. An increased redox potential can enhance the water reduction and oxidation capability of photocatalysts, resulting in overall water splitting.
《Fig. 9》

Fig. 9. (a) (F(R ∞ )hv) 1/2versus the energy curve of LaMgxTa1–xO1+3xN2–3x derived from UV-Vis light diffuse reflectance spectra. (b) Band levels of LaMgxTa1–xO1+3xN2–3x (x = 0 and 0.33) obtained by PESA and theoretical calculations (CAL) [82]. (Copyright 2016, The Royal Society of Chemistry)
2.3.2. Morphological control
The morphology and particle size of the photocatalysts play important roles in charge separation and migration, as well as in surface reactions [12,83]. Smaller particle size means a shorter migration distance and a higher specific surface area. More photogenerated charge carriers can move to the surface and take part in the water redox reaction. Ta3N5 and TaON are typically obtained by the nitridation of Ta2O5 precursor. Various methods have been adopted to reduce the size of particles and control the morphology of the Ta2O5 precursor. However, phase transition occurs during high-temperature nitridation, which may cause the breakdown of nanostructures. Hisatomi et al. [84] solved this problem by coating the surface of the mesoporous Ta2O5 precursor with a silica layer before nitridation. The silica layer, which was obtained by chemical vapor deposition of tetramethyl orthosilicate, was used to support the mesoporous structure of Ta2O5 during high-temperature nitridation. As shown in Fig. 10(a) [84], ordered mesoporous Ta3N5 was obtained. The photocatalytic H2 evolution efficiency was two times higher than that of conventional bulk Ta3N5 , as a result of the thinwall morphology. This ordered mesoporous structure had a wall thickness of 2 nm, a pore size of 4 nm, and a surface area as large as 100 m2·g−1. Shorter charge migration distance and increased surface active sites contributed to the enhanced efficiency. The same group also reported a precursor route for preparing Ta3N5 nanoparticles (Fig. 10(b)) [85]. Ta2O5 nanoparticles, as a precursor for Ta3N5, were prepared using a precipitation method. With the particle size decreasing to 30–50 nm, the nanoparticulate Ta3N5 showed three times higher H2 evolution activity than normal Ta3N5 (particle size 300–500 nm). The template-assisted method is another method of preparing nanostructured materials. The template not only acts as a model to control the morphology, but also acts as a support to prevent the Ta2O5 nanostructures from collapse during nitridation. By adopting mesoporous C3N4 as a template, Ta3N5 nanoparticles as small as 20 nm were obtained (Fig. 10(c)) [86]. Decreased particle size and increased surface area shortened the diffusion length of photo-excited electrons and holes, and further improved the charge carrier separation, making the resulting activity a magnitude higher than that of bulk Ta3N5. Similar to the silica nanosphere templates mentioned above, polystyrene (PS) spheres are also used as templates to synthesize microporous Ta3N5 (Fig. 10(d)) [87]. The PS sphere templates can easily be removed during calcination. The microporous structure and photonic behavior of the microporous Ta3N5 contributed to enhanced photon absorption and better performance [88,89].
《Fig. 10》

Fig. 10. (a) Transmission electron microscope (TEM) image of mesoporous Ta3N5 [84] (Copyright 2010, American Chemical Society); (b) SEM image of Ta3N5 nanoparticles prepared using the reverse homogeneous precipitation (RHP) method [85] (Copyright 2009, Elsevier); (c) TEM image of Ta3N5 particles prepared by adopting mesoporous C3N4 as the template [86] (Copyright 2010, The Royal Society of Chemistry); (d) SEM image of macroporous Ta3N5 [87] (Copyright 2012, Wiley-VCH).
In addition to nanoparticle and mesoporous structures, the construction of hierarchical nanostructures can effectively promote light harvesting and charge transfer. Hierarchical metastable γ-TaON structures have been reported as efficient H2 evolution photocatalysts (Fig. 11) [90]. First, hierarchical Ta2O5 was prepared using an in situ self-assembly wet-chemical method. Next, a high-temperature nitridation process was used to transform Ta2O5 into TaON and Ta3N5. The hierarchical hollow structure of nano-needles that was assembled not only showed a large specific surface area but also led to multiple light reflections within the chamber. The obtained singlephase γ-TaON hierarchical structures exhibited a H2 evolution rate of 381.6 µmol·h–1 and the apparent QE reached 9.5% (420 nm, ~47.5 times higher than that of traditional TaON). The converted Ta3N5 also showed improved photocatalytic H2 evolution activity, reaching as high as 127.5 µmol·h–1 with an apparent QE of 3.1% (420 nm). The high efficiency is attributed to the synergistic effect of the hierarchical morphology and the crystal and electronic structures.
《Fig. 11》
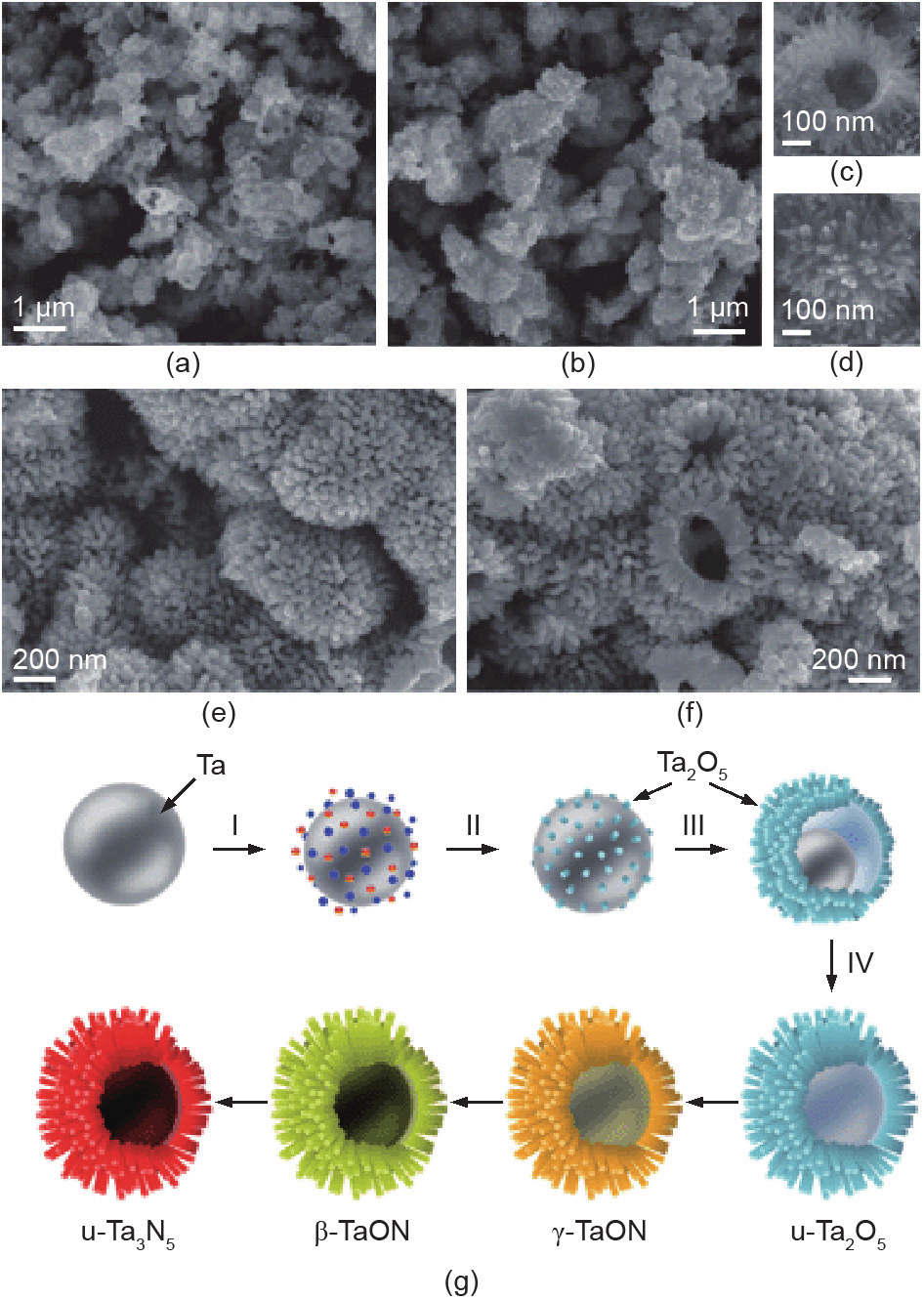
Fig. 11. SEM images of: (a–d) u-Ta2O5, (e) γ/β-TaON(u), and (f) u-Ta3N5. (g) Scheme of the formation of hollow urchin-like u-Ta2O5 hierarchical nanostructures and the subsequent thermal nitridation, successively forming γ-TaON, β-TaON, and u-Ta3N5 [90]. (Copyright 2013, The Royal Society of Chemistry)
On the other hand, use of a nanostructured Ta2O5 precursor can decrease the nitridation temperature and duration, which contributes to the maintenance of the nanostructure and decreases the amount of the Ta reduced species on the surface. Ordered porous Ta2O5 nanostructures were obtained from an ordered porous C3N4 template, which was prepared by using close-stacked silica nanospheres as templates [91]. It is important to note that this ordered porous structured Ta2O5 lowered the nitridation temperature, which resulted in a reduced extent of defect sites. Thus, the enhanced photocatalytic activity of the Ta3N5 photocatalyst obtained by this method was attributed to decreased defect sites, increased surface area, and efficient light harvesting. Similarly, the use of amorphous and shape-controlled Ta2O5 can decrease the nitridation time of Ta3N5, leading to fewer surface defects. Amorphous Ta2O5 nanoplates and octahedra were synthesized via aerosol-assisted molten salt syntheses (AMSS). The obtained porous Ta3N5 nanoplates and octahedra showed enhanced photocatalytic H2 evolution rate, which could be attributed to suppression of the reduced Ta5+ surface states and increased surface area (Fig. 12) [92].
《Fig. 12》
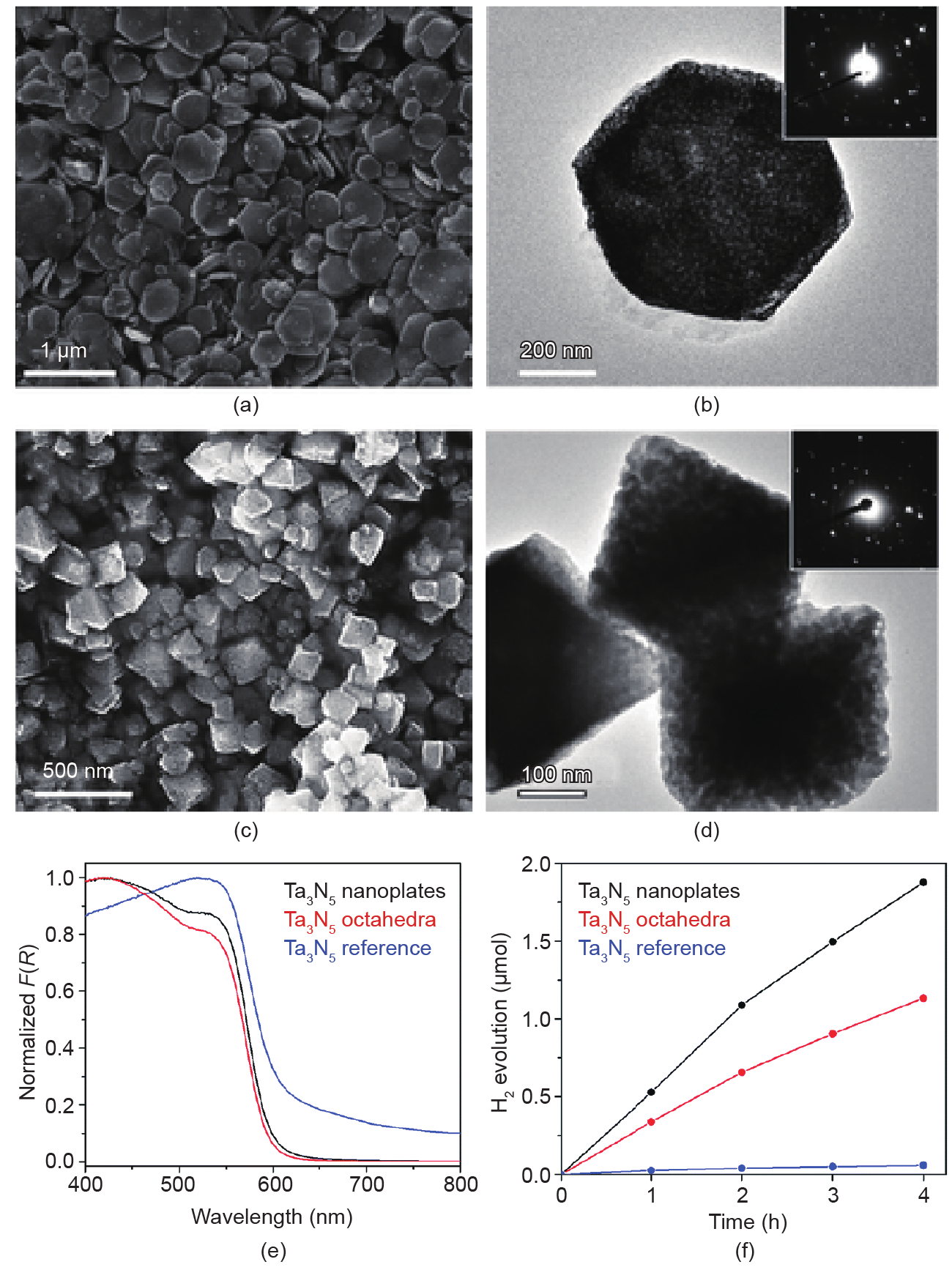
Fig. 12. (a, c) SEM and (b, d) TEM images (top right insets: electron diffraction) of (a, b) Ta3N5 nanoplates and (c, d) Ta3N5 octahedra. (e) Diffuse reflectance spectra and (f) timedependent H2 evolution reaction of different Ta3N5 samples [92]. (Copyright 2016, The Royal Society of Chemistry)
2.3.3. Surface modification
Nanostructures can decrease the distance migrated by the charge carrier and reduce body recombination. However, surface defects normally play the role of recombination centers for photo-excited charge carriers. Therefore, surface charge recombination is one of the major hazards for the photocatalytic performance of tantalum (oxy)nitride-based photocatalysts. However, reducing surface defects has been a challenge for tantalum (oxy)nitride-based photocatalysts [12]. ZrO2 was reported to be an effective surface-passivated layer for TaON and Ta3N5 [93–96]. It was found that a solid solution formed between ZrO2 and tantalum (oxy)nitride during the nitridation process. The solid solution on the surface could suppress the formation of reduced Ta species. Due to the decrease in reduced Ta species, ZrO2-modified TaON and Ta3N5 photocatalysts showed an enhanced photocatalytic H2 evolution rate. Another report about BaTaO2N may further elucidate the positive effect of surface modification [50]. Through the modification of a BaTaOx precursor with a Ba-Zr oxide species, a BaZrO3-BaTaO2N solid solution formed after nitridation and showed sixto nine-fold enhancement of H2 evolution without a sacrificial agent. This improved performance resulted from the enlarged bandgap and reduced defect density compared with bare BaTaO2N, which led to an enhanced driving force for the water redox reaction and reduced charge recombination [85].
Self-oxidation and the reverse reaction are the main problems that impede the water-splitting activity of tantalum (oxy)nitridebased photocatalysts. A perovskite-type CaTaO2N photocatalyst was recently produced via appropriate surface modification, and was reported to exhibit overall photocatalytic water splitting (Fig. 13) [51]. CaTaO2N has a narrow bandgap and enough bandgap potential for water-splitting reactions (Fig. 13(a)) [51]. However, surface selfoxidation and the release of N2 make this photocatalyst unstable. The surface of the CaTaO2N was modified with a thin layer of titanium oxyhydroxide through photodepostion, which effectively suppressed the surface self-oxidation and the reverse reaction. After loading the co-catalysts for the redox reactions, the modified CaTaO2N achieved stable overall water splitting (Fig. 13(b)) [51].
《Fig. 13》

Fig. 13. (a) UV-Vis diffuse reflectance spectra (inset: Tauc plot) and (b) band position of CaTaO2N as determined by calculation. Time courses of gas evolution during water splitting on titanium-oxyhydroxide-deposited RhCrOy/CaTaO2N under (c) UV + Visible light (λ ≥ 300 nm) and (d) visible light (λ ≥ 420 nm) [51]. (Copyright 2015, The Royal Society of Chemistry)
Depositing co-catalysts on the surface of photocatalysts is a general strategy to improve the water-splitting performance. However, surface states often result in the formation of an interface barrier between the co-catalyst and the photocatalyst, which is unfavorable for interfacial charge transfer. Chen et al. [97] reported that introducing a magnesia nanolayer between Pt nanoparticles and Ta3N5 could enhance the photocatalytic H2 evolution efficiency significantly. The Pt/MgO-Ta3N5 photocatalysts reached 22.4 µmol·h for H2 evolution, which is about 17 times higher than the activity of the unmodified sample. It was found that the Pt nanoparticles deposited on MgO-Ta3N5 had a relatively small particle size and uniform dispersion, which resulted in more active sites and a faster surface reaction (Fig. 14(a, b)) [97]. This finding was attributed to the passivation of surface defects by MgO and the improvement of surface charge transfer from Ta3N5 to Pt. The same group further modified the surface of Ta3N5 with barium (Ba) to promote the photocatalytic H2 evolution rate, as shown in Fig. 14(c) [98]. It was found that BaTaO2N formed on the surface of Ta3N5 after modification with Ba. BaTaO2N not only passivated the surface defects but also formed a heterojunction with Ta3N5 (Fig. 14(d)) [98]. The improved photocatalytic H2 evolution rate should be attributed to the synergetic effect of increased charge separation and decreased surface recombination.
《Fig. 14》
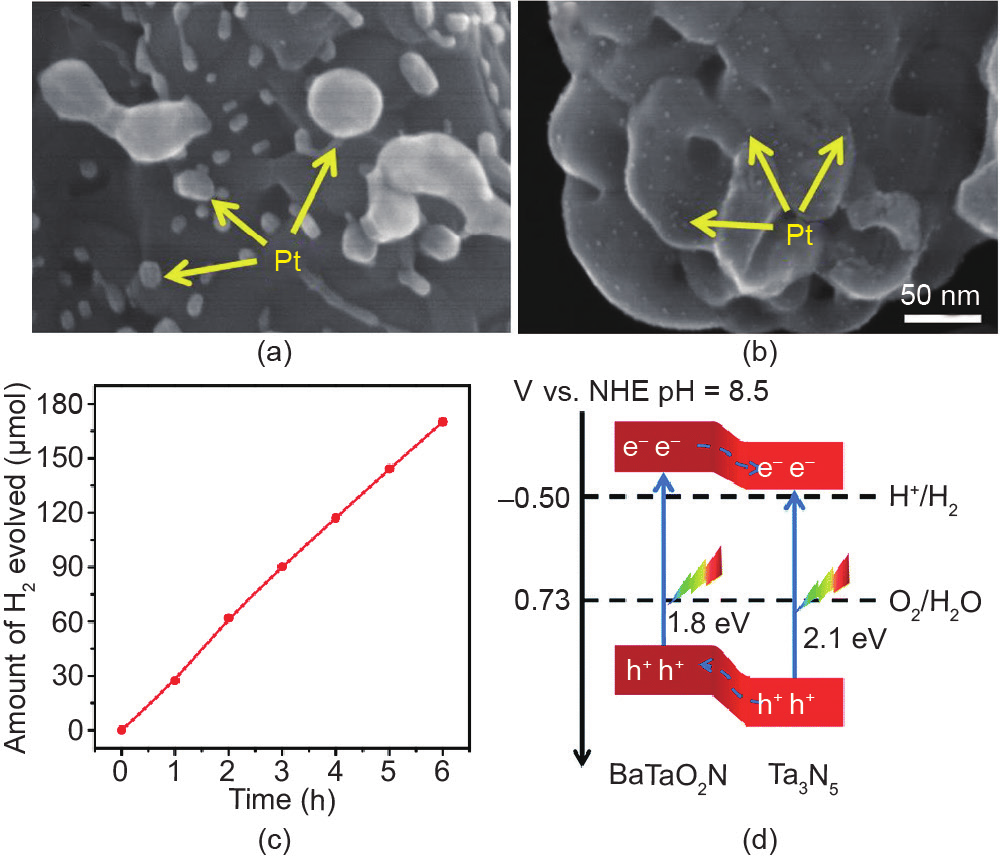
Fig. 14. Field emission scanning electron microscope (FESEM) images of (a) Pt/Ta3N5 and (b) Pt/MgO(in)-Ta3N5 [97] (Copyright 2016, Elsevier); (c) Time course of visiblelight H2 evolution with 0.5 wt% Pt/Ba(0.3)-Ta3N5; (d) relative band positions of the Ta3N5/BaTaO2N heterostructure [98] (Copyright 2017, The Royal Society of Chemistry).
2.3.4. Co-catalysts and heterostructures
Separating the photo-generated electrons and holes in different regions or sites to react with water by loading co-catalysts is an efficient way to increase photocatalytic efficiency [20,99–105]. Cocatalysts, which extract charge carriers from photocatalysts, show high catalytic activity for the water redox reaction. Faster extraction of charge carriers not only reduces bulk recombination but also suppresses the self-oxidation resulting from hole accumulation in the bulk, thereby improving the stability of tantalum (oxy)nitride-based photocatalysts. For example, Ta3N5 hollow spheres decorated with spatially separated co-catalysts were prepared with the assistance of silica spheres, and showed improved stability and enhanced H2 evolution activity (Fig. 15) [40]. Pt nanoparticles were placed on the inner Ta3N5 shell surface as electron collectors, and IrO2 or CoOx nanoparticles were loaded on the outer shell surfaces as hole collectors (Fig. 15(a)) [40]. This hollow structure with separated co-catalysts has two advantages: ① It has an increased surface area and more active sites than bulk Ta3N5; and ② separated co-catalysts promote the migration and separation of photo-excited electrons and holes toward the inner and outer surfaces, respectively, thus suppressing recombination and reverse reactions. This system showed significant enhancement for photocatalytic water reduction (Fig. 15(d)) [40]. Moreover, no N2 gas was detected during the H2 evolution process, indicating improved stability.
《Fig. 15》

Fig. 15. (a) Scheme illustration of two separated co-catalysts used to decorate Ta3N5 hollow spheres to create an effective photocatalyst for water splitting. (b,c) SEM images of (b) Ta3N5/Pt/SiO2 spheres and (c) Ta3N5/Pt hollow spheres. The scale bar is 500 nm. (d) Time course of H2 evolution on Ta3N5 photocatalysts with and without spatially separated co-catalysts [40]. TPS: Ta3N5/Pt/SiO2; TS: Ta3N5/SiO2. (Copyright 2013, Wiley-VCH)
As mentioned in Section 2.3.3, surface modification with ZrO2 can suppress reduced Ta defects and enhance the water reduction performance of TaON. Therefore, ZrO2/TaON was furthered modified with appropriate nanoparticulate co-catalysts for H2 and O2 evolutions, resulting in redox reagent-free overall water splitting (Fig. 16) [18]. As shown in Fig. 16(a) [18], the overall water-splitting reaction is affected by the synergistic combination of RuOx, Cr2O3, and IrO2. RuOx loaded on a ZrO2/TaON composite promoted both water reduction and oxidation activity [106]. Due to H2-O2 recombination to form H2O and to the photoreduction of O2 on the surface of RuOx, a Cr2O3 shell that was permeable for protons and hydrogen, but not oxygen, was loaded onto the surface of RuOx to prohibit the reverse reactions and make the overall water splitting achievable [107–110]. The function of IrO2 is to improve the stability of the photocatalyst. The authors attributed the improved stability to the promotion of water oxidation by IrO2, which is known to be the most effective catalyst for O2 evolution [111–113]. Thus, the overall water-splitting reaction on the composite photocatalyst IrO2/Cr2O3/RuOx/ZrO2/TaON can provide some useful insight into the reasonable design of a functional co-catalysts/photocatalyst system.
《Fig. 16》
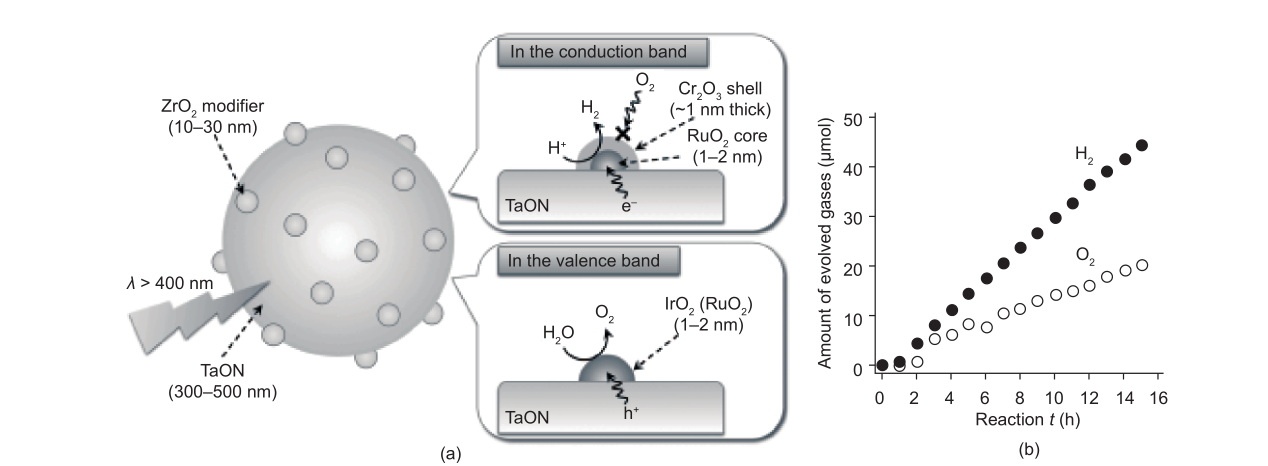
Fig. 16. (a) Scheme of the mechanism of overall water splitting on an IrO2/Cr2O3/RuOx/ZrO2/TaON photocatalyst. (b) Time course of gas evolution using IrO2/Cr2O3/RuOx/TaON under visible light (λ > 400 nm) [18]. (Copyright 2013, Wiley-VCH)
Combining different materials to form heterostructured photocatalysts is another effective strategy to improve water-splitting performance, due to the improvement of photon absorption and charge separation [5,11]. Numerous composite photocatalysts, including Cu2O/TiO2, TiO2/CdS, and CdS/WO3, were combined and showed remarkably improved photocatalytic performance compared with single-component photocatalysts [114–117]. Increasing efforts have been made to construct tantalum (oxy)nitride-based heterostructured photocatalysts. Liu et al. [118] reported a nano Au/ Ta3N5 composite as a photocatalyst for H2 generation under visiblelight irradiation. The nano Au/Ta3N5 composite was prepared through nitridation of Au/Ta2O5, which was synthesized via a simple Pechini-type sol-gel process. Gold (Au) nanoparticles with a diameter of 15–20 nm were embedded in the Ta2O5 host (Fig. 17(a,b)) [119]. The prepared nano Au/Ta3N5 heterostructures showed remarkably improved H2 evolution performance under visible-light irradiation compared with pure Ta3N5 nanoparticles. The authors attributed the enhanced H2 evolution activity to the synergetic effect of a near-field electromagnetic effect induced by the surface plasmon resonance of Au nanoparticles (Fig. 17(c)) and charge transfer in the Au/Ta3N5 composite. Fig. 17(d) [119] shows the time course of H2 evolution for the Au/ Ta3N5 composite photocatalysts. A certain amount of Au nanoparticles can enhance the photocatalytic H2 evolution performance, whereas excessive Au nanoparticles may act as recombination centers and impede the photocatalytic H2 evolution activity [119].
《Fig. 17》

Fig. 17. (a) A TEM image of Au nanoparticles; (b) high-angle annular dark-field scanning transmission electron microscope (STEM) image of nano Au/Ta3N5; (c) the UV-Vis diffuse reflectance spectra of nano Au/Ta3N5; (d) the time course of H2 evolution for the nano Au/Ta3N5 composites [119]. (Copyright 2014, The Royal Society of Chemistry)
Heterojunctions formed between two photocatalysts with suitable bandgaps and matched band positions have been demonstrated to be effective for improving photocatalytic performance [120]. Adhikari et al. [121] reported the composites of Ta3N5 or TaON with Bi2O3 to have enhanced visible-light-driven H2 evolution activity. They ascribed the increased visible-light activity for Ta3N5 or TaON to the trapping of holes by Bi2O3. Under visible-light irradiation, both Ta3N5 and Bi2O3 were excited to generate electrons and holes. The electrons from Ta3N5 or TaON took part in the H2 evolution activity and holes were recombined with the electrons from Bi2O3, similar to the Z-scheme mechanism shown in Fig. 18 [121]. In other words, the enhanced H2 evolution activity of Bi2O3/Ta3N5 or Bi2O3/TaON composites could be attributed to improved charge separation [121].
《Fig. 18》

Fig. 18. Relative bandgap positions and charge transfer mechanism (Z-scheme) in Bi2O3 and Ta3N5 under visible-light irradiation [121]. (Copyright 2015, The Royal Society of Chemistry)
As the bandgaps of most tantalum (oxy)nitride-based photocatalysts straddle water redox potentials, tantalum (oxy)nitride-based photocatalysts can act as both H2 evolution photocatalysts (HEPs) and as O2 evolution photocatalysts (OEPs) in a Z-scheme system. For example, Higashi et al. [122] designed a Z-scheme system with TaON photocatalysts to achieve visible-light overall water splitting. As shown in Fig. 19(a) [122], the TaON acted as both HEP and OEP after the loading of appropriate co-catalysts. With the assistance of an IO-3 /I- redox mediator, the Pt/TaON (HEP) and RuO2/TaON (OEP) mixtures reacted with water to produce H2 and O2 under visible-light irradiation. Later, Tabata et al. [123] further decorated the surface of Ta3N5 with rutile titania (R-TiO2), which acted as an OEP in a two-step water-splitting system (Fig. 19(b)). After the loading of iridium (Ir) nanoparticles, Ir/R-TiO2/Ta3N5 achieved visible-light overall water splitting along with Pt/ZrO2/TaON as a HEP, assisted by an IO-3 /I- redox mediator. It was believed that the decorated R-TiO2 hindered the adsorption of I– on Ta3N5 , while the Ir nanoparticles played the role of active sites to reduce IO-3 to I- , thereby allowing Ta3N5 to evolve O2 in the two-step water-splitting system.
《Fig. 19》

Fig. 19. (a) Scheme of the Z-scheme overall water splitting on a RuO2/TaON and Pt/ TaON mixture with an IO–3/I– redox mediator [122] (Copyright 2008, The CSJ Journals); (b) Scheme of two-step water splitting on a Pt/ZrO2/TaON and Ir/R-TiO2/Ta3N5 mixture with an IO–3/I– redox mediator [123] (Copyright 2010, American Chemical Society).
《2.4. Performance of tantalum (oxy)nitride-based photocatalysts》
2.4. Performance of tantalum (oxy)nitride-based photocatalysts
To improve the water-splitting efficiency, great effort has been focused on each step of the photocatalytic process, including photon absorption, charge separation and migration, and surface reaction. The main strategies—doping, morphological control, surface modification, co-catalysts, and heterostructures—are discussed above. High activity and stability for photocatalysts are based on the synergistic effect of these steps. Photocatalytic overall water splitting has been achieved on modified TaON, CaTaO2N, and LaMgxTa1–xO1+3xN2–3x. Table 2 [18,21,40,43,51,63,65,81,84,87,90,92,96–99,118,119] summarizes and compares relevant studies on this topic. It is clear that nanostructures show relatively higher H2 evolution performance due to the increased number of active sites and the short charge transfer distance. In addition, heteroatoms-doping that is based on bandgap engineering is an effective strategy to enhance the H2 evolution performance of tantalum (oxy)nitride-based photocatalysts. However, a greater surface area means more surface defects, which not only act as recombination centers but also impede the photo-excited charge transfer between the photocatalyst and the co-catalyst. Also, the heteroatom dopant may act as a recombination center. Thus, surface modification seems to be a more powerful strategy for the improvement of the H2 evolution performance of tantalum (oxy) nitride photocatalysts.
《Table 2》
Table 2 Tantalum (oxy)nitride-based photocatalysts for water splitting.

《3.Conclusions and outlook》
3.Conclusions and outlook
Tantalum (oxy)nitride-based photocatalysts, which have a narrow bandgap for visible-light absorption and suitable bandgap edges for the water redox reaction, are regarded as a class of promising candidates for water splitting. Because they have much lower water reduction capability than water oxidation capability, considerable attention has been devoted to the improvement of the photocatalytic H2-generation efficiency of tantalum (oxy)nitride-based photocatalysts. Surface states are considered to be a major factor influencing photocatalytic water-splitting activity. Surface defects caused by nitrogen vacancies and Ta reduced species act as recombination centers and impede the surface charge transfer and reaction. In addition, self-oxidation and the reverse reaction during a photocatalytic reaction make these photocatalysts unstable. In order to improve the water-splitting performance of tantalum (oxy)nitride-based photocatalysts, many strategies have been adopted, including doping, morphology control, surface modification, co-catalysts, and heterostructures design. These strategies work by improving charge separation, charge migration, and surface reactions. Photocatalytic overall water splitting has been achieved on some tantalum (oxy)nitridebased photocatalysts. In addition, the stability of tantalum (oxy)nitridebased photocatalysts has been significantly improved with appropriate co-catalyst design and surface modification.
To further improve the photocatalytic overall water-splitting efficiency of tantalum (oxy)nitride-based photocatalysts, increased attention should be paid to better designing and utilizing the synergistic effect of various strategies. The following key points should be considered in future research.
(1)Defects control. It is believed that most defects on the surface or in the bulk will act as recombination centers for photo-excited electrons and holes. Moreover, surface defects may generate interfacial barriers between the photocatalysts and the co-catalysts, and impede charge transfer. It is very important but quite challenging to reduce the defects because of the harsh nitridation conditions. It is easy to introduce nitrogen vacancies and Ta reduced species during the high-temperature nitridation process in a NH3 atmosphere. The exploration of new precursors and synthesis methods under mild conditions is necessary in order to reduce the defects of tantalum (oxy)nitride-based photocatalysts.
(2)Morphology control. The morphology of photocatalysts is a key factor that influences light absorption, charge migration, and surface reaction. Nanotechnology has been intensively used to design various photocatalysts, and for the most important aspect of morphology control. Reducing the particle size to the nanoscale results in a shorter migration distance that makes more charge carriers move to the surface before recombination. Furthermore, nanostructures decrease the temperature and duration of nitridation, which reduces defects such as nitrogen vacancies and Ta reduced species. In addition, ordered porous nanostructures increase light absorption because of the photonic effect. An ordered hierarchical structure will provide room for improving the water-splitting activity of tantalum (oxy)nitride-based photocatalysts.
(3)Co-catalyst design. Loading co-catalysts is an effective approach to improve photocatalytic performance. Tantalum (oxy)nitride-based photocatalysts suffer from self-oxidation during the photocatalytic process, as a result of the poor extraction of photo-generated holes. The loading of a co-catalyst for water oxidation can promote the extraction of holes and improve the stability of photocatalysts. Precise control of the particle size and dispersion of co-catalysts should show a positive effect on the stability and performance of the photocatalyst. Thus far, Pt is the most-used H2 evolution co-catalyst for Ta3N5 based photocatalysts. However, due to its rarity and high cost, Pt is not favorable for scale-up applications. Therefore, the development of other earth-abundant co-catalysts for H2 evolution is greatly welcome.
In conclusion, much effort has been made to improve the water-splitting efficiency of tantalum (oxy)nitride-based photocatalysts, and great progress has been achieved over the past years. Due to the synergetic effect of light harvesting, charge separation and migration, and surface reaction during the photocatalytic water-splitting process, more research attention should be paid to combining strategies into multi-strategies. Persistent efforts in this area are expected to realize highly efficient overall water splitting on these photocatalysts. Furthermore, the knowledge and understanding of mechanisms that have been acquired from studying these materials will shed light on the development of other new, efficient, and sustainable photocatalysts. For the future development of photocatalytic water splitting, unified evaluation standards and criteria for overall photocatalyst performance, including efficiency and stability, are urgently required to allow this research community to achieve its ambition of long-term sustainable solar fuel production.
《Acknowledgements》
Acknowledgements
The authors would like to acknowledge financial support from the Australian Research Council through its DP and FF programs. Mu Xiao acknowledges support from the Australian Government Research Training Program Scholarship. Financial support from the National Natural Science Foundation of China (513228201) is also highly appreciated.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Mu Xiao, Songcan Wang, Supphasin Thaweesak, Bin Luo, and Lianzhou Wang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




