《1.Introduction》
1.Introduction
Since its invention in the 1980s, techniques in three-dimensional (3D) printing, which is more formally referred to as additive manufacturing (AM), have been developed, matured, and applied in various applications by a large number of researchers and industrial companies worldwide. In its early years, 3D printing was primarily a rapid prototyping technique; today, it is revolutionizing manufacturing and many other industries with new processes, materials, and applications. In addition to plastic prototypes, complex engine components, houses, food, and even human organs can now be 3D printed. The 3D printing industry is experiencing rapid growth: Worldwide revenues of the industry grew by 17.4% in 2016 and are worth over $6 billion [1].
One major market for 3D printing is the medical field. For this important application area, 3D printing has provided effective solutions and shown great potential for personalized medicine and care. Current widely practiced medical uses of 3D printing include custommade dentures, hearing aid shells, surgical and medical models, orthotic and prosthetic components, and artificial hip and knee implants [2–7]. One unique use of 3D printing technology is for the fabrication of “phantoms,” or mock-ups of body parts, to allow doctors or surgeons to visualize body parts when preparing, planning, or optimizing complex medical operations or procedures [5,6]. Such phantoms can also be effective tools for surgical training and patient education purposes.
Since the early 2000s, 3D bioprinting technology has been developed and investigated by a number of research groups and biotech companies [8]. 3D bioprinting involves depositing layers of living cells onto gel media to build up 3D bio-functional structures. The ultimate goal is to use the 3D printing technology for tissue engineering (TE) applications in order to build organs and body parts [9,10].
With the rapid advancement of 3D printing and 3D bioprinting technologies, a huge body of research and practical applications exists for these technologies. This paper reviews the applications of 3D printing and 3D bioprinting technologies, with a focus on fabrication of functional materials and structures for medical applications. This review specifically discusses the state of the art and trends for 3D-printed functional structures and bio-structures for medical phantoms and for regenerated tissue and organ applications.
《2. 3D printing-enabled medical phantoms and structures》
2. 3D printing-enabled medical phantoms and structures
《2.1. Need for physical medical phantoms》
2.1. Need for physical medical phantoms
Medical imaging technologies have advanced dramatically in the past decade. With the evolution of imaging techniques such as multidetector computed tomography (MD-CT) and magnetic resonance imaging (MRI), radiological diagnosis has become less invasive and more informative [11,12]. High-resolution 3D image data can be acquired in a short time. Image processing plays an increasingly important role in presenting human organs and structures with high fidelity and providing indispensable support in the diagnosis and treatment of many diseases and medical conditions [13–16]. Today’s image-guided surgeries illustrate how radiologists have been integrated into therapeutic teams together with other surgical specialists. 3D visualization, multi-planar reformation, and image navigation help radiology to be pivotal in many clinical disciplines [17]. However, there is an unmet need to render digital imaging and communications in medicine (DICOM) images. Digital models are limited by the use of flat screens for the visualization of 3D imaging data. In addition to surgical planning applications, tangible medical phantoms are very useful for medical computational models validation, as well as for medical training and patient education. Therefore, there is a great need for high-fidelity physical medical phantoms for clinical practice and educational purposes.
《2.2. Fabrication of medical phantoms》
2.2. Fabrication of medical phantoms
Physical medical phantoms have traditionally been produced by means of conventional manufacturing processes such as casting and molding. Such fabrication processes involve time-consuming and often expensive tooling preparation steps. In addition, it is not economical to fabricate individual, patient-specific medical phantoms due to the high tooling cost. Therefore, most of these phantoms are mass-produced, population-averaged, idealized models for general planning and educational purposes.
2.2.1. Tissue-mimicking medical phantoms
In medical imaging, phantoms are commonly used for developing and characterizing imaging systems or algorithms, as they provide imaging specimens with known geometric and material compositions. Tissue-mimicking medical phantoms can imitate the properties of biological tissue, and can therefore provide a more clinically realistic imaging environment [18]. In the past, casting or injecting molding processes have been used to fabricate tissue-mimicking medical phantoms. Applications of such phantoms can be found in the development and validation of medical imaging modalities such as ultrasound [19,20], MRI [21–24], computed tomography (CT) [25], and others [26]. With the increasing needs of biomedical research, other applications of tissue-mimicking medical phantoms, such as simulation of the electromagnetic properties of tissues [27], mechanical properties mimicking [28], and focused ultrasound ablation [29], have also been demonstrated. In those applications, phantoms were fabricated as population-averaged, idealized models, and the individual differences among patients were overlooked.
2.2.2. 3D printing of medical phantoms
3D printing technologies overcome the drawback of traditional manufacturing processes and are an effective tool for rapidly producing patient-specific, high-fidelity, medical phantoms at low cost, as the need for tooling is eliminated. 3D-printed medical models and phantoms fabricated from CT, MRI, or echocardiography data provide the advantage of tactile feedback, direct manipulation, and comprehensive understanding of a patient’s anatomy and underlying pathologies. In many cases, 3D-printed medical phantoms can assist and facilitate surgeries and shorten the cycle times of medical procedures [30–33]. For example, an orthopedic surgery trainee used CT scan images to create printable copies of a patient’s bones. He then had them printed and used these custom models to plan the patient’s surgery [34]. 3D models have also been used for surgical planning by neurosurgeons [4,6]. Such 3D-printed neuroanatomical models can provide physical representations of some of the most complicated structures in the human body. These detailed highfidelity phantoms can help neurosurgeons discover and visualize the intricate, sometimes obscured relationships between cranial nerves, vessels, cerebral structures, and skull architecture that are difficult to interpret based solely on two dimensional (2D) radiographic images [35]. This can reduce errors and avoid potentially devastating consequences in surgery.
《2.3. Recent progress and future trends in functional medical phantoms》
2.3. Recent progress and future trends in functional medical phantoms
2.3.1. 3D printing of tissue-mimicking medical phantoms
Recent advances in computer-aided design (CAD), medical imaging, and 3D printing technologies have provided a rapid and costefficient method of generating patient-specific, tissue-mimicking medical phantoms from computational models that are reconstructed from the CT or MRI results of individuals [36]. Those patientspecific phantoms have unparalleled advantages in many biomedical applications, such as computational model validation, medical device testing, surgery planning, medical education, and doctorpatient interaction. Biglino et al. [37] demonstrated the fabrication of compliant arterial phantoms with PolyJetTM technology by Stratasys Ltd. (Eden Prairie, MN), an AM technique that deposits a liquid photopolymer layer by layer through orifice jetting and then solidifies it by UV exposure. A rubber-like material named TangoPlus was used in this study for its mechanical properties, which are close to those of real tissue. Cloonan et al. [36] conducted a comparative study on the use of common tissue-mimicking materials and 3D printing materials, including TangoPlus, for abdominal aortic aneurysm phantoms. Their results suggested that TangoPlus was a suitable material for modeling arteries in terms of dispensability, and that its uniaxial tensile properties outperformed those of poly(dimethylsiloxane) (PDMS) SYLGARD elastomers, which are commonly used in the investment casting process.
2.3.2. Radiologically relevant medical phantoms
3D printing technologies have been used to fabricate radiologyrealistic phantoms that have regions with different attenuations [38]. In this study, the multi-material PolyJetTM printing technique from Stratasys Ltd. was used to construct liver and brain phantoms with realistic pathologies, anatomic structures, and heterogeneous backgrounds. The liver and head CT images of patients were segmented into tissue, vessels, liver lesions, white and gray matter, and cerebrospinal fluid. Printing materials that had different CT numbers were assigned to these objects after test scans. Finally, 3D-printed phantoms were scanned on a CT scanner and the images were evaluated. It was found that for the liver, the patient and phantom images had a similar texture. CT images of the brain phantom showed that the CT number differences of objects of interest were similar to those in the patient images. These phantoms have heterogeneous backgrounds and realistic pathology that is similar to that of the real tissue, and could potentially be used for image-quality assessment, radiation dose reduction, and other research and educational activities.
2.3.3. Physiological medical phantoms
Patient-specific and tissue-mimicking medical phantoms contain individual information, and hold great potential for many biomedical applications and clinical benefits, such as computational model validation, medical device testing, surgery planning, medical education, and doctor-patient interaction. As described previously, the 3D printing technology has proven to be an effective manufacturing method of fabricating such phantoms. However, the existing technologies are still inadequate in fully mimicking human organs and tissues. For example, many human organ structures, such as heart valves, have anisotropic mechanical properties due to directional tissue structures; however, the regular 3D-printed phantoms do not possess the same special anisotropic mechanical properties. Therefore, most 3D-printed medical phantoms, even those with patientspecific and tissue-mimicking features, are only anatomically—not physiologically—close to human organ structures.
Most medical phantoms are made using polymeric materials. Although the uniaxial tensile properties of phantom materials can be close to those of soft tissues within the small strain (< 3%) range, the creep tendency, which is an inherent characteristic of polymers, makes them behave quite differently than soft tissues under larger deformation. For tissue-mimicking medical phantoms, the strain range of interest is normally the working strain range of the tissue. As shown in Fig. 1 [39], soft tissues typically exhibit a strain-stiffening behavior initially, which is represented by a convex stress-strain curve in the beginning. As the strain increases, the curve changes from convex to concave, which indicates yielding of the material [40]. In contrast, polymeric materials typically have a concave stressstrain curve at the beginning, indicating strain softening. Even though the initial Young’s modulus of a polymeric phantom can be designed to match the Young’s modulus of real tissues, the mechanical behavior of the phantom will deviate from that of real tissue at higher strain levels.
《Fig. 1》
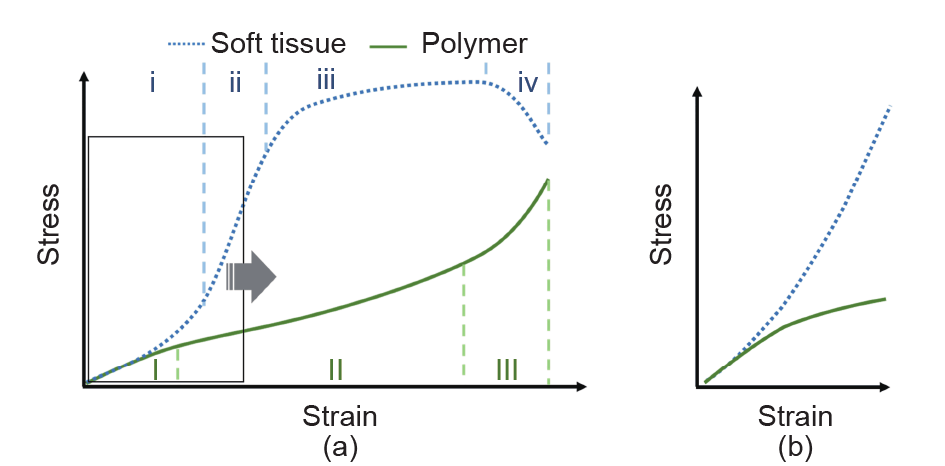
Fig. 1. Comparison of the mechanical behaviors of soft tissue and polymer. (a) Typical stress-strain curves of soft tissue (dotted line) and polymer (solid line). Soft tissue: i— toe region, ii—elastic region, iii—plastic region, iv—failure region; polymer: I—primary creep, II—secondary creep, III—tertiary creep. (b) Magnified view of the curves in the strain range of interest for most tissue-mimicking medical phantoms [39].
Wang et al. [39,41] demonstrated an integrated metamaterial design and multi-material 3D printing approach to fabricate medical phantoms that possess the properties of real soft tissues. Metamaterials are artificially structured materials for achieving and manipulating certain physical properties and/or phenomena. The properties of metamaterials are derived both from the inherent properties of their constituent materials and from the geometrical arrangement of those materials [42,43]. In the context of tissue-mimicking medical phantoms, the key value of the “metamaterial” concept is the idea of constructing artificial models of tissue with heterogeneous microstructures that, although difficult to construct by conventional means, can easily be rendered using 3D printing. With multimaterial 3D printing technology, the feasibility of designing the mechanical properties of metamaterials has been proven [39,41]. These studies investigated the feasibility of mimicking the strain-stiffening behavior of soft tissues using dual-material 3D-printed metamaterials with microstructured reinforcement embedded in a soft polymeric matrix. Three types of metamaterials were designed and tested: sinusoidal wave, double helix, and interlocking chain designs (Fig. 2) [39]. Even though the two base materials were strainsoftening polymers, both finite element analysis and uniaxial tension tests indicated that two of the dual-material designs were able to exhibit strain-stiffening effects as a metamaterial. The effects of the design parameters on the mechanical behavior of the metamaterials were also demonstrated (Fig. 3) [39]. The results suggested that the fabrication of patient-specific tissue-mimicking medical phantoms with both geometrical and mechanical accuracies is possible with dual-material 3D-printed metamaterials.
《Fig. 2》
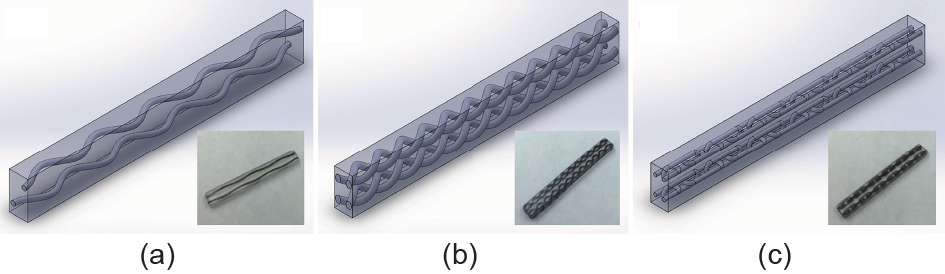
Fig. 2. CAD models and printed samples of three metamaterials: (a) sinusoidal wave design, (b) double helix design, and (c) interlocking chain design [39].
《Fig. 3》

Fig. 3. Stress-strain curves of the four variants of the sinusoidal wave (SW) design [39].
2.3.4. Applications of physiological medical phantoms
In addition to the conventional applications of medical phantoms, physiological medical phantoms are finding new and unique uses in medical fields. In a recent study, Qian et al. [44] demonstrated the efficacy of using physiological patient-specific phantoms to plan the trans-catheter aortic valve replacement (TAVR) procedure. (TAVR is a less invasive treatment option for severe aorta stenosis patients who are at high surgical risk.) In order to achieve optimal clinical outcomes, an individualized assessment of the interactions between the native aortic tissue, the prosthesis, and the blood flow is critical. This study aimed to develop a procedure simulation platform for in vitro TAVR implantation using 3D-printed physiological tissue-mimicking medical phantoms. The researchers also investigated the feasibility of applying this platform to quantitatively predict the occurrence, severity, and location of any post-TAVR paravalvular leaks (PVLs), which are an independent risk factor for increased shortand long-term mortality. In this study, physiological aortic heart valves based on real patient data (i.e., CT images) were fabricated using the integrated metamaterial design and multimaterial 3D printing approach described in Subsection 2.3.3 [39,41]. Fig. 4 [44] shows the CT images of a patient’s aortic root, the 3D computational model, and the 3D-printed physiological phantom. The test and analysis outcomes using the 3D-printed physiological valves indicated the locations of the final PVL in the 12 patients who had a certain degree of PVL after TAVR, as well as the sites of the maximum annular bulge index, a predictor for PVL occurrence (Fig. 5) [44]. The predictions of the location of the dominant PVL from 3D-printed valves matched well with the actual PVL occurrence in the patients, with an accuracy of 75% [44]. In this proof-ofconcept study, the researchers demonstrated the feasibility of using 3D-printed physiological patient-specific phantoms to quantitatively assess post-TAVR aortic root strain in vitro.
《Fig. 4》

Fig. 4. An example of CT images of the aortic root, the 3D computational model, and the 3D-printed physiological phantom. (a), (b), and (c) show the CT cross-sectional views at the ascending aorta and the valves, and the longitudinal view, respectively. (d), (e), and (f) show the 3D computational model viewed from the ascending aorta, the left ventricular outflow tract (LVOT), and the side, respectively. The aortic wall and leaflets are depicted semi-transparently, the calcifications are drawn in red, and the embedded fibers are drawn in green. (g), (h), and (i) show the 3D-printed physiological phantom. The calcifications and the fibers are printed with black materials for better illustration [44].
《Fig. 5》

Fig. 5. Prediction of the PVL locations in 12 patients who had a certain degree of post-TAVR PVL. In the bulge index images, green arrows indicate correct prediction of the dominant PVL sites; red arrows indicate that the maximum bulge index did not predict the dominant PVL site; yellow arrows indicate that a submaximal high bulge index corresponded to the dominant PVL site. In the transesophageal echocardiography (TEE) images, white arrows indicate the dominant PVL sites, and yellow arrows indicate the minor PVL sites [44].
《3. Additive manufacturing of regenerated tissues and organs》
3. Additive manufacturing of regenerated tissues and organs
Owing to the increasing demand for tissue and organ transplantation, and the deficiency of tissue and organ donors, numerous efforts have been made in the field of TE to develop biological substitutes for native human tissues and organs [45–47]. For TE purposes, biodegradable scaffolds with high porosity and interconnectivity can be employed to provide shape, mechanical support, and microarchitecture for cellular growth and reorganization in order to improve and accelerate the healing and repairing process [46,48]. In this regard, the design of TE scaffolds plays a dominant role in the successful rate of treatments. Different strategies for creating 3D scaffolds have been proposed and investigated, such as freeze-drying [49,50], gas foaming [51], phase separation [52], porogen leaching [53], and electrospinning [54,55]. However, precise control of the porosity and internal microstructure of the scaffold manufactured by these routes in order to manipulate oxygen, nutrients, and soluble biomolecules for promoting cell growth and differentiation is still challenging. In addition, directing different types of cell growth in TE scaffolds in order to form functional tissues that are organized at a level of complexity is a major engineering design obstacle [56]. Although a few exciting clinical results have been reported on autologous cell-loaded scaffolds with uncomplicated structural design being capable of guiding the regeneration of multifunctional tissues and organs [45,57,58], advanced strategies for manufacturing acellular or cell-laden bioscaffolds with higher levels of complexity are still in progress [46,59–62]. Advances in AM techniques have been a recent breakthrough in TE and regenerative medicine. A growing amount of interest has focused on manufacturing complex and functional 3D bioscaffolds with specific biomaterials and cells in order to provide a microenvironmental and biological componential similarity to the native tissue for TE application. To date, several types of bioprinting systems that are capable of constructing either acellular or cellladen hydrogel scaffolds have been described in the literature. The three most important and well-established techniques for bioprinting are laser-induced forward transfer (LIFT), inkjet bioprinting, and robotic dispensing.
《3.1. Bioprinting strategies》
3.1. Bioprinting strategies
LIFT is a technique that can deposit cells onto a receiving substrate. In general, a laser pulsed beam is applied on a donor slide or ribbon containing source inks (i.e., hydrogels and cells), followed by the evaporation of the inks; this results in a high-pressure bubble jetting toward the receiving substrate, which is placed underneath the donor slide. By controlling the movement of the donor slide or the substrate, a deposited 2D pattern can be built up to form 3D constructs in a layer-by-layer fusion [49,63–65]. For example, Michael’s group [66] utilized this technique to create a fully cellularized skin substrate that mimicked the microenvironment of the native skin by printing fibroblasts and keratinocytes on top of an acellular dermal substitute (Matriderm®). The results of in vivo experiments demonstrated that the printed cells survived well, and neovascularization could be observed in the skin construct, implying that laser printing may be an adequate strategy for the creation of 3D tissues. Heterogeneous constructs with multiple cell types can also be built up by inkjet printing. Xu et al. [67] fabricated a pie-shaped 3D construct consisting of stem cells, smooth muscle cells, and endothelial cells using a thermal inkjet printer. In contrast to the common method of inkjet bioprinting, which usually ejects inks onto solid substrates to obtain a 3D construct [68–70], the cells were combined with calcium chloride (CaCl2) to form bioinks and the inks were ejected into an alginate-collagen solution. The portion of the polymer solution surface that was impacted by the ink droplets was instantaneously solidified due to the formation of the egg-box structure of the Ca2+-alginate complexes. The results of the in vitro experiments indicated that the printed cells were able to survive, proliferate, and maintain cellular function in the 3D construct. More importantly, the stem cells and endothelial cells were capable of differentiating into bone and blood vessels after respective implantation into mice for six weeks.
In contrast to the LIFT technique, the major disadvantage of inkjet bioprinting is that the viability of the printed cells can be remarkably diminished by the critical shear stress that is generated when the bioinks pass through the nozzle and deposit on the substrates [59,66]. In addition, cell sedimentation occurs due to cell aggregation during printing, and results in nozzle clogging and inhomogeneous cell distribution in the constructs [65,68,71]. Although both techniques possess the ability to precisely create 3D constructs that are composed of multiple cell types on demand, these printing strategies usually produce small-scale constructs, which is an obstacle for practical use in clinical applications [63,71]. Inspired by outstanding performances in the creation of 3D cell constructs using LIFT and inkjet bioprinting, a large number of studies have focused on the development of a robotic dispensing system for bioprinting (i.e., extrusion-based bioprinting, or EBB) owing to its easy-touse nature and good compatibility with different bioinks [72]. EBB allows the manufacture of 3D constructs on a millimeter scale by the pneumatically or mechanically driven dispensation of biopolymers or synthetic biopolymers in a layer-by-layer fashion [72–75]. Mini-tissues (i.e., spheroids and organoids) composed of multiple cellular types can serve as the building blocks for large tissues and organs that are printed using EBB [76,77]. However, several technical gaps need to be addressed in order to improve the structural and componential freedom of these 3D constructs. The primary limitation is that only one bioink can be used for each printing process; this raises the difficulty of the construction of 3D architectures with high levels of complexity. Increasing the number of reservoirs on the printer could be done to achieve printe with multiple bioinks. Of course, the printing speed of the constructs was reduced as a result, since more steps were included in the printing process [72,73,78]. A pneumatic-driven multi-material bioprinter was recently developed by Liu et al. [72]. The printer they developed has the ability to eject seven types of bioink, both individually and simultaneously, by routing different reservoirs into a single print head. It is interesting to note that the novel design of the print head allows different bioinks to mix at a controllable feeding rate before extrusion in order to achieve gradient printing in a single strut.
《3.2. Recent progress and future trends》
3.2. Recent progress and future trends
Recent advances in AM technologies have enabled several new TE pathways. In particular, the following three strategies are gaining momentum now that new AM technologies have become available:① the development of hybrid scaffolding materials to achieve tunable properties of scaffolds; ② the design of special microstructures to achieve convertibility of scaffolds; and ③ the integration of sensors to achieve built-in process-monitoring capability. The details of and future predictions for each strategy are discussed below.
3.2.1. Hybrid scaffolding materials
Biopolymers, such as polycaprolactone (PCL), polylactic acid (PLA), and poly(lactic-co-glycolic acid) (PLGA), are the most commonly used base materials for scaffolding. In most cases, they are not a perfect fit for TE due to their relatively weak mechanical properties, poor cell adhesion, or near-inert bioactivity. By blending additives into the biopolymers, those disadvantages can be mitigated. Many of these additives are bioceramics in powder form. For example, α-tricalcium phosphate (α-TCP) has been added to PCL [53] to improve mechanical properties, cell seeding, and proliferation. β-tricalcium phosphate (β-TCP) has been used as an additive in PCL [79,80], PLA [81], and PLGA [82] to enhance mechanical and hydrophilic properties. In bone TE, it can also improve the biocompatibility and osteoconductivity in the physiological environment due to its bioresorbability and chemical similarity to the mineral phase of bone. It is clinically proven that β-TCP promotes osteogenic and odontogenic differentiation in various types of cells. Hydroxyapatite (HA) is an even more common additive for bone TE because its chemical composition is similar to that of the inorganic part of native bones. It has been used in PCL [83–93], PLA [93–97], poly(D,L-lactic acid)-poly(ethylene glycol)-poly(D,L-lactic acid) (PELA) [98,99], PLGA [82], and poly-D,L-lactide (PDLLA) [100]. Biopolymers with HA additives have exhibited excellent chemical and biological affinity to bone tissues. Other additives used in scaffolding biopolymers include bioactive glass particles [101–103], collagen [104], calcium silicate [91,105], calcium phosphate [106,107], magnesium (Mg) [105,108], and alginates [104,109,110].
More recent studies have explored various nanomaterials as additives for biopolymers. These nanomaterials usually add new functions to the base material. For example, adding magnesia (MgO) [83] to PCL affects the modulation of signal transduction, energy metabolism, and cell proliferation, which promotes new bone formation. Adding magnetic nanoparticles (Fe3O4 or γ-Fe2O3) [89,111] to PCL grants the scaffold the ability of magnetic heating, which significantly stimulates proliferation. Other nanomaterials, such as nanoclay [112], single-wall carbon nanotubes (SWCNTs) [113,114], multiwall carbon nanotubes (MWCNTs) [85,114–119], graphene [117], and graphene oxide (GO) have been added to PCL or PLA to modify the mechanical, electrical, and thermal properties of the base biopolymer.
Current manufacturing methods of scaffolds with hybrid materials all start with premixing the biopolymer and the additives. The prepared mixture is then used to build the scaffold, by either 3D printing or any top-down method. This procedure ensures the homogeneous distribution of additives in the biopolymer matrix. However, the disadvantage is obvious: The composition of the hybrid material is fixed. If the goal is to regenerate a complex, multicell-type organ, different compositions are required at different locations on the scaffold. Using the more recent AM technologies, it is possible to mix the biopolymer and additives in situ during scaffold printing. Fig. 6 shows the conceptual setup for multi-material 3D bioprinting. In this way, future hybrid scaffolds will have tunable properties and extra designed functions, which will benefit the differentiation and growth of multiple cell types to form complicated biological structures.
《Fig. 6》
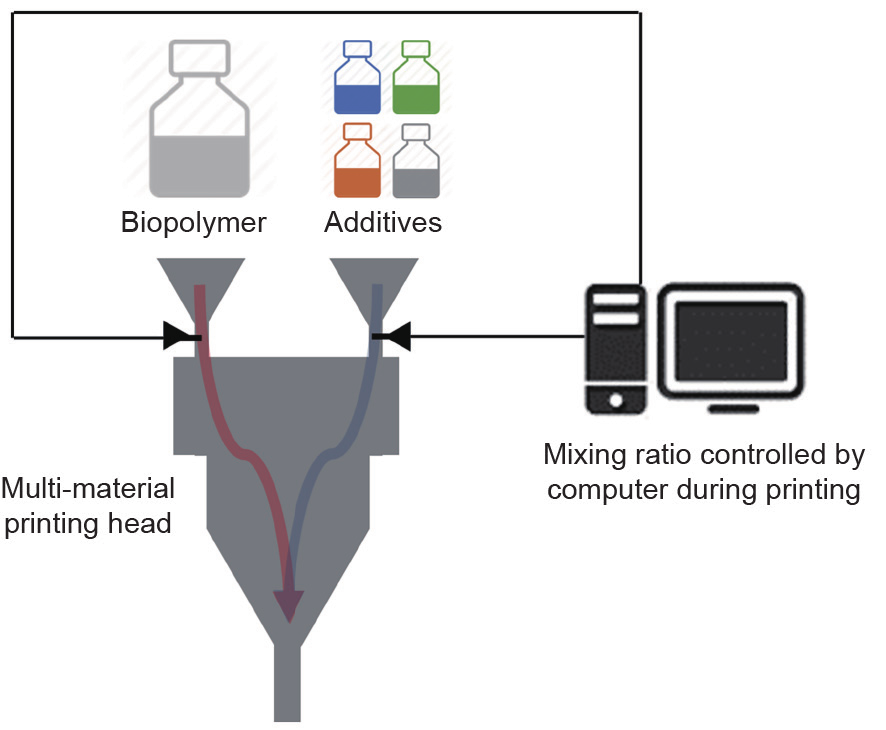
Fig. 6. A conceptual setup for multi-material 3D bioprinting.
3D bioprinting can also be combined with direct-write technologies. Direct-write technologies are conventionally used in the printed electronics industry as an alternative to lithography or screen printing. By integrating direct-write technologies, the 3D bioprinting process can perform selective surface modification during scaffolding. Fig. 7 demonstrates a conceptual setup of 3D bioprinting with in situ growth factor grafting using extrusion and aerosol jet printing technologies.
《Fig. 7》
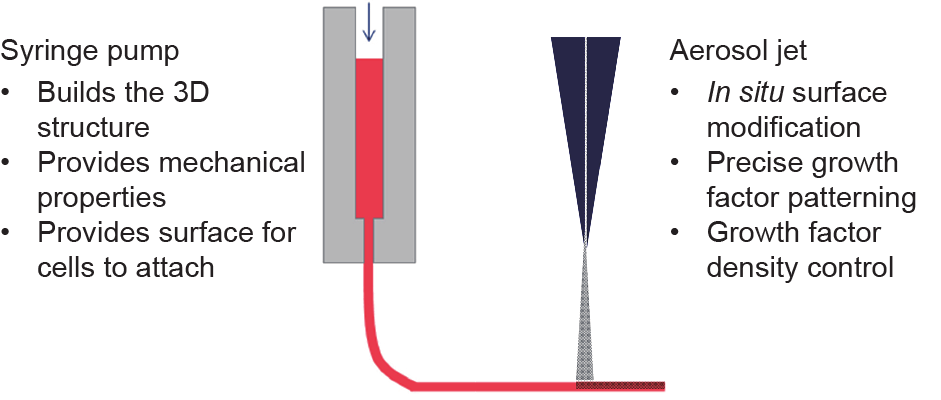
Fig. 7. A conceptual setup for in situ surface modification.
Fig. 8 compares two strategies for fabricating scaffolds with multiple growth factors. Although the pre-mix method requires more material preparation steps, its printing process is relatively straightforward with multi-head 3D printers. The two growth factors are also less likely to cross-contaminate during printing. The in situ grafting method does not require a material preparation step, but requires integration of 3D printing and layer coating for the building of each layer. Depending on the coating technology, there is a risk of cross-contamination between two growth factors. However, the coating step is independent of the scaffold-printing step, which enables a higher degree of design freedom and more detailed coating patterns.
《Fig. 8》
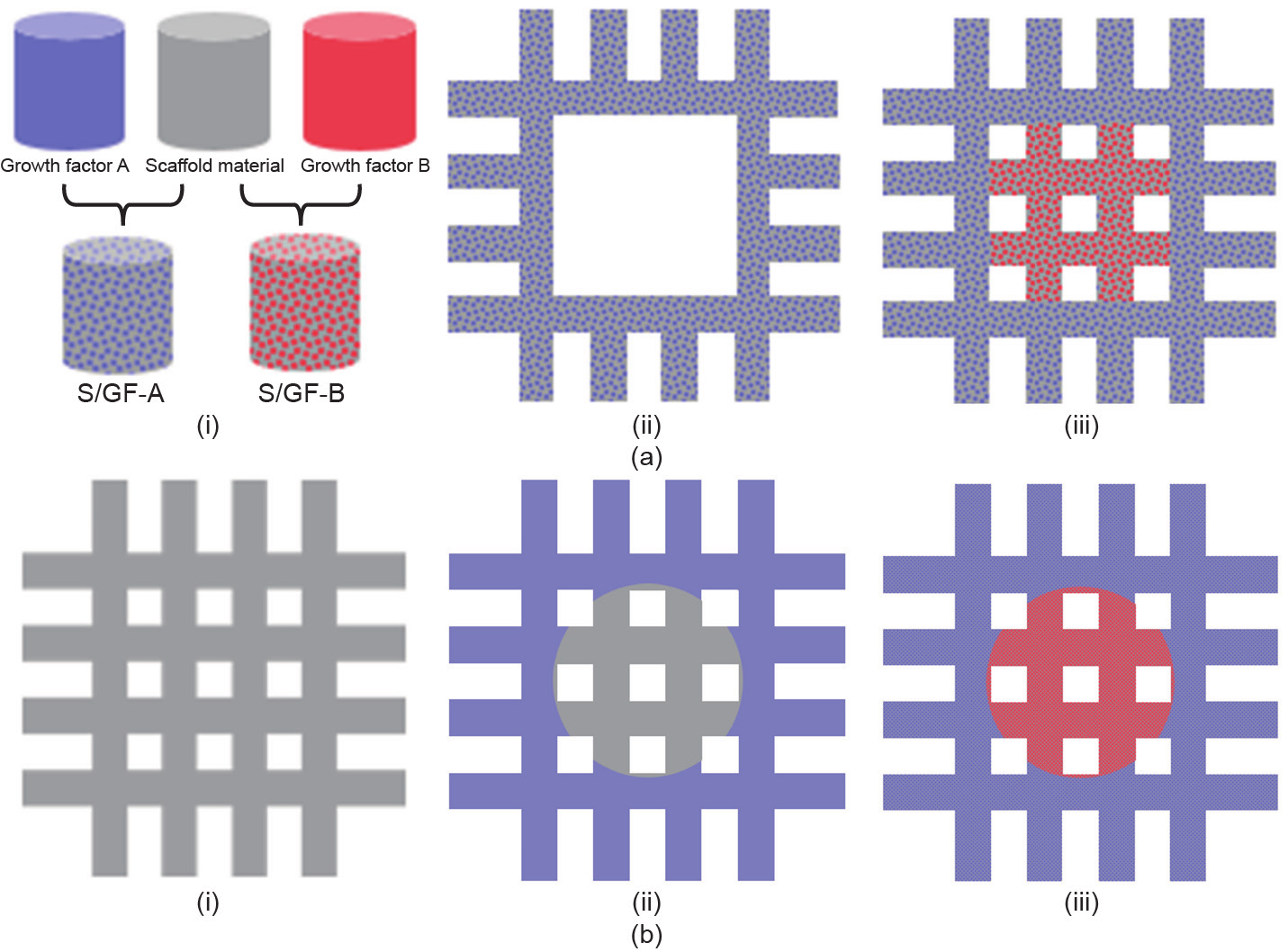
Fig. 8. Comparison of pre-mix method and in situ grafting method for fabricating scaffolds with multiple growth factors. (a) Pre-mix method: (i) mixing scaffold material with growth factors, (ii) printing scaffold material/growth factor A (S/GF-A) as the scaffold at region A, (iii) printing scaffold material/growth factor B (S/GF-B) as the scaffold at region B;(b) in situ grafting method: (i) printing the scaffold with pure scaffold material, (ii) coating region A with growth factor A, (iii) coating region B with growth factor B.
3.2.2. Convertible scaffold
Bioprinting technologies provide precise control over the initial cell distribution in the printed construct. However, once the cells start to grow in the bioreactor and regenerate into the tissue via the self-assembly process, no control method is currently available to ensure an optimal microenvironment throughout the scaffold at all times. In other words, when bioprinting TE scenarios at present, too much of the cell growth process is uncontrolled. Cell growth is a spatiotemporal process with intrinsically high variability in quality, quantity, yield, and other metrics. Although the behavior of each individual cell is not readily predictable, the growth of a cell culture within a large population is largely controllable with environmental factors, including local cell density [75,81] and ion-exchange rate [108,111]. In a cell culture process without a scaffold, certain agitation or perfusion mechanisms are typically used to ensure nearuniform local cell distribution and to promote nutrient, growth factor, and waste exchange. A scaffold will hinder the nutrient, growth factor, and waste exchange of cells. Studies have indicated that cell growth on a scaffold is not optimal in terms of growth rate [102,108] and cell viability [92] once the cell density reaches a certain point.
Using 3D-printed auxetic metamaterials as scaffolds may provide a solution in the near future. Auxetic metamaterials [119,120] are materials with repeated microstructures that exhibit a negative Poisson’s ratio in the macroscale, which allows the volume to change in a unique way for the overall construct. Advances in 3D printing have enabled and accelerated novel designs and applications of auxetic metamaterials [93,104,121]. With auxetic metamaterials as scaffolds, the ability to change volume provides an effective way to control the local cell density. In addition, the change of porosity that comes with the volume change implies that the culture medium flows in and out the scaffold, replenishing nutrients for the internal cells and taking away the waste. Fig. 9 shows a double-arrow type of auxetic metamaterial.
《Fig. 9》
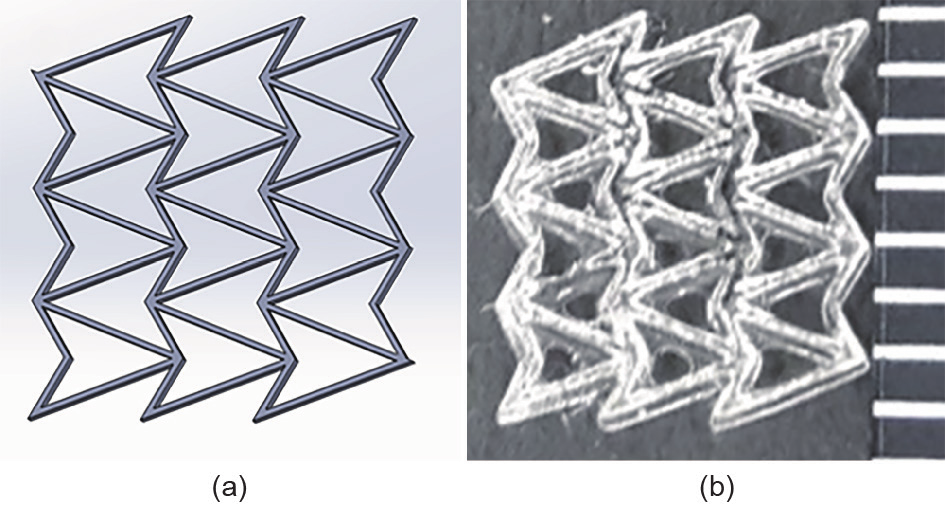
Fig. 9. A double arrowhead auxetic design. (a) Computer-aided design; (b) microscopic picture of a scaffold printed by GeSiM™ bioplotter (distance between marks is 1 mm).
3.2.3. Integrated sensors
Direct-write technologies are a class of AM methods that can fabricate electronic circuits without masking [122]. These relatively new technologies include inkjet printing, aerosol jet printing, syringe dispensing, laser-assisted chemical vapor deposition, laser particle guidance, matrix-assisted pulsed-laser evaporation, and focused ion beam. Direct-write processes are fast and flexible, and have a high tolerance for errors. Some direct-write technologies, such as aerosol jet printing, do not require the substrate to be planar. This provides opportunities to integrate sensors into bio-printed scaffolds.
In most cases, direct-write technologies are used to create conductive patterns. Metallic nanoparticle pastes or dispersions are used as inks in such cases. These include silver, gold, and copper nanoparticles as the three most common materials. Carbon-based inks are also a popular family that is recently adopted in many directwrite technologies and their applications. This includes carbon nanotubes, graphite, graphene, decorated carbon nanotubes, and their mixtures. Some researchers have reported that mixtures of inks with carbon-based nanomaterials and metallic nanoparticles have potential in stretchable electronics printing [122].
With more and more complex functions and designs of printed electronics, there are demands for more types of specialized inks, other than conductive inks. For example, boron nitride nanotubes (BNNTs) can be dispersed into certain solvents to create a piezoelectric ink. There are many applications for a thin layer of patterned dielectric material. Both inorganic and polymeric dielectric inks have been developed, and semiconductor nanoparticle inks and polymer semiconductor inks are on the market. Some recent research is developing biological inks that can be printed by aerosol jet printing. Fig. 10 summarizes the inks that are most commonly used by various direct-write technologies.
《Fig. 10》
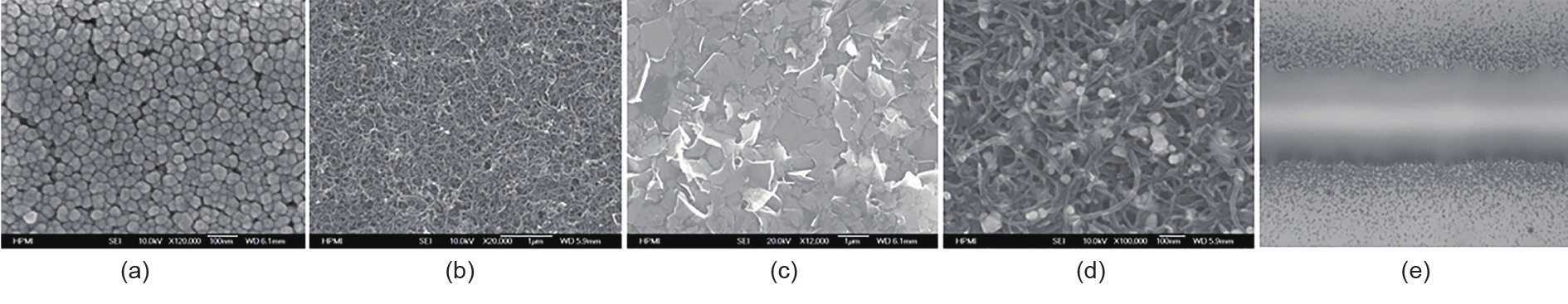
Fig. 10. Typical inks used in various direct-write technologies, including (a) metal nanoparticles, (b) carbon nanotubes, (c) graphite, (d) carbon nanotubes/silver nanoparticles, and (e) polyimide.
On the manufacturing level, the use of direct-write technologies to introduce a sensing capability to smart scaffolds is encountering a set of challenges that include scalability, yield, toxicity, environmental impact, and supply-chain design. Different direct-write technologies are at different manufacturing readiness levels (MRLs). In general, this strategy is still in the proof-of-concept stage. The overall outlook for the smart scaffold field suggests that interest and attention in integrating sensors into TE has increased significantly during the last decade. It is reasonable to believe that more and more smart scaffold designs will include certain kinds of direct-write technologies in the near future.
《4. Conclusions》
4. Conclusions
This paper provides a review of various applications of AM techniques in the construction of functional medical phantoms and in the fabrication of regenerated tissues and organs. Existing work, results, recent progress, and future trends were discussed. In the field of functional medical phantoms, recent work in the 3D printing of tissue-mimicking medical phantoms, radiologically relevant medical phantoms, and physiological medical phantoms has been presented. A detailed discussion was provided on the design and fabrication of physiological medical phantoms. A case for the application of such physiological medical phantoms to surgical planning for the TAVR procedure was presented. In the field of regenerated tissues and organs, existing work and results for 3D bioprinting of these functional bio-structures were reviewed. Recent work in and future trends for the application of emerging AM technologies in this field were presented, including hybrid scaffolding materials, convertible scaffolds, and integrated sensors. From this review of previous and new research work and results, it can be seen that emerging AM technologies have great potential to produce effective functional structures for the advancement of medical care and the realization of personalized medicine.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Kan Wang, Chia-Che Ho, Chuck Zhang, and Ben Wang declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




