《1.Introduction》
1.Introduction
Vegetable oils produced to meet the demands for food, feed, and industrial applications are largely derived from oil palm (Elaeis guineensis) and several major temperate oilseed crops,including soybean (Glycine max), rapeseed (Brassica napus), sunflower (Helianthus annuus), and peanut (Arachis hypogaea); the existing vegetable oil production platforms have been developed from these sources[1,2]. However, due to the booming global population, the consumption of vegetable oils has increased remarkably[3]. In recent years, the shortage of vegetable oils has been further exacerbated by the increasing demand for renewable biodiesel derived from plant oils to serve as an alternative to fossil fuel [4–7]. Research has demonstrated that the chemical structures of vegetable oil and fossil oil are very similar, and that vegetable oil can be processed to reach the applicable criteria of biofuels. The annual global supply of biofuels has been increasing by a factor of 8% since 2000, and reached a staggering 4% of the world’s transport fuels in 2015[8]. This growth is largely due to a dramatic increase in the production of palm oil in the past decade[9]; however, it is unlikely to be sustainable owing to environmental concerns about the excessive plantation of palm trees on virgin rainforest lands and the potentially detrimental ecological impacts[10–13]. Furthermore, reductions in arable land area have already resulted in enormous disturbance to the cultivation of oilseed crops; the major canola-producing areas in Canada and the European Union (EU) are very likely to experience increased prices as a consequence[8]. Oilseed consumption in developing countries will probably continue to outweigh the production level in the coming decades (Fig.1) [3–8], despite the steady increase in production. Therefore, there is a clear need to develop alternative resources or novel platforms for vegetable oil production to make up for this vegetable oil shortfall.
《Fig. 1》

Fig.1.Projection of the oilseeds economy in developing countries. Consumption and production of oilseeds (soybean and other oilseeds) as raw material for vegetable oil in developing countries are predicted to experience an elevating trend in coming years. Black bar represents the oilseed production, open bar represents the oilseed demanding. It is estimated that the oilseeds being produced are less than enough to meet the demand. More vegetable oil sources are required in the less-developed regions of the world[3–8].↑
↑Data result from OECD survey: https://www.oecd-ilibrary.org/agriculture-and-food/data/oecd-agriculture-statistics/oecd-fao-agricultural-outlook-edition-2017_d9e81f72-en? parentId=http %3A%2F%2 Finstance. metastore. ingenta. com%2Fcontent%2F collection %2 Fagr-data-en.
Plant oil, predominantly triacylglycerol (TAG), can be accumulated to high levels in oilseeds in order to support seed germination and early seedling development prior to plant autotrophy by photosynthesis. However, TAG content in non-seed tissues is much lower; for example, it ranges from 0.04% to 0.2% of the dry weight (DW) in leaf tissues of Arabidopsis (Arabidopsis thaliana) [14,15]. In general, TAG in plant vegetative tissues is noncumulative by nature[16], with the exception of a small number of plant species such as the oil palm, olives (Olea europaea), and the yellow nutsedge (Cyperus esculentus). As a consequence,research into increasing vegetable oil content has mainly focused on oilseeds rather than on plant vegetative tissues.
In recent years, plant vegetative tissues, including both photo-synthetic leaf tissue and non-photosynthetic organs such as tuber, have been explored through genetic engineering for their feasibility as TAG accumulation sites. High-biomass plants such as tobacco (Nicotiana tabacum)[17,18], sugarcane (Saccharum officinarum)[19], and potato (Solanum tuberosum) [20,21] have been used to demonstrate the practicality of TAG production in large amounts in non-seed tissues. Owing to the rapid development of multidiscipline-based “-omics”studies and synthetic biology,our understanding of plant lipid metabolism has increased greatly[22]. In particular, transcriptomics, proteomics, and lipidomics have brought more opportunities for a better comprehension of lipid metabolism both in typical oilseeds and in non-typical TAG-accumulating plant tissues[23]. Hence, our knowledge of the bio-chemical pathways and annotation of the key genes contributing to fatty acid biosynthesis , lipid assembly, and turnover has been rapidly expanding, leading to the establishment of multiple meta-bolic models of the lipid dynamics in plants[24–26]. Thus, increasing TAG accumulation in plant vegetative tissues by means of various genetic engineering strategies is now a promising method for generating a new source of vegetable oil.
《2.Efforts to improve lipid production in plants》
2.Efforts to improve lipid production in plants
《2.1.Traditional methods of breeding for high-oil plants》
2.1.Traditional methods of breeding for high-oil plants
Traditional breeding technologies focusing on enhancing the oil production in plants have been mostly concerned with improving the lipid storage capacity of seeds[27]. Earlier studies employed breeding methods such as hybridization and heterosis to improve both the genotypes and phenotypes of oilseed crops in order to expand oil productivity[28]. Genetic mapping and association analyses based on molecular marker technologies including quantitative trait loci (QTL) and single nucleotide polymorphism (SNP) have been extensively used to explore the potential of oil production in plants , especially in Brassica napus, the third-ranked oilseed crop[27,29–32]. QTL studies revealing the loci regulating the oil content in rapeseed have been extensively reported[33–37]. In soybean, through an integrated mapping of major QTLs that correlate with oil biosynthesis, 20 consensus QTLs determining most of the TAG accumulation were similarly identified[38]. Field breeding approaches such as recurrent selection have been used for the genetic improvement of oil content in some non-classic oilseed crops, such as cereal crops. A maize (Zea mays) population containing more than 20% oil in kernels was successfully generated through 103 cycles of selection,far exceeding the original germ plasm, which had merely 4.7% kernel oil[39]. Likewise, a novel oat (Avena sativa) variety with 18% oil in grain was developed by nine recurrent selections between two cultivars that harbor 11% and 3% oil content, respectively[40,41].
Palm oil, which occupies the largest market share (47%) of vegetable oil, has gained a series of improvements by means of germplasm development. Despite controversies regarding its role in causing ecological disequilibrium and soil erosion,the oil palm remains the most popular platform for vegetable oil production[42]. Breeding technologies to increase palm oil productivity have evolved from traditional phenotypic selection to marker-assisted recurrent selection (MARS), which was reported to be highly economical and efficient compared with the 19-year long phenotypic selection cycle[43]. Furthermore, high throughput molecular marker technologies have been used to select germplasms with improved fatty acid composition and to construct oil palm genetic maps[44,45]. For example, an important gene known as SEEDSTICK(STK), which is responsible for the kernel shell loss that directly links to oil productivity in the oil palms grown in sub-Saharan Africa, was recently identified through homozygosity mapping[46].
However, oil yield remains a quantitative trait that correlates with a multitude of factors. Conventional breeding technology largely relies on time-consuming and laborious experiments, in contrast to genetic engineering, which can provide fast and direct means to manipulate lipid metabolism or redirect carbon sources to lipid biosynthesis. This does not mean that traditional breeding will be abandoned; on the contrary,traditional breeding technologies will continue to play an important complementary role to genetic engineering in the future in order to establish highly valid biofactories for vegetable oil production[47,48].
《2.2.Adaption of novel oil crops》
2.2.Adaption of novel oil crops
2.2.1.Jatropha seeds
Jatropha (Jatropha curcas), which contains oil-rich seeds, has been recognized as a potential non-food oil source plant[49,50]. Due to its very good tolerance to droughts, Jatropha can be broadly produced in a wide range of regions with suboptimal growing conditions[51]. Mutation breeding,heterosis breeding, and inter-specific hybridization have all been carried out to improve Jatropha production,in addition to genetic breeding based on molecular marker selection and genetic engineering[52].
2.2.2.Chinese tallow
Despite concerns that it is an invasive plant in some developed countries[53], Chinese tallow (Triadica sebifera) has risen as a potentially novel source of biodiesel[54,55]. A methanol-based transes terification heterogeneous system can transform the Chinese tallow seed oil into biodiesel-standardized product, with an extraordinarily high conversion efficiency of up to 94%[56]. The oil within the mesocarp tissue of Chinese tallow was found to have high oxida tive stability because of its abundance of saturated fatty acids, raising its potential applications as a multifunctional biofuel feedstock[57,58]. Deep transcriptome sequencing specially targeting lipid accumulation in non-seed tissue has also been reported [59,60].
2.2.3.Yellow nutsedge
Yellow nutsedge (Cyperus esculentus), a stolon/tuber plant that is widely viewed as an invasive weed in Australia and the United States, has been reported to contain 26% –30% oil in DW in its tubers[61]. A commercial variety of yellow nutsedge developed in Europe and known as “Chufa”is widely used as a source of cooking/salad oil[62]. It is true that the competition between yellow nutsedge and other crops might bring a number of unpredictable repercussions to the local economy and ecology[63,64]; however, yellow nutsedge may be capable of providing a favorable choice for new vegetable oil platforms. Biochemical studies revealed that the oil biosynthesis of yellow nutsedge tuber initiates at a rather late stage of tuber development,mostly at the expense of sugars[65]. Transcriptomic analysis of the oil metabolism in yellow nutsedge tubers at different developmental stages has been documented;this is particularly interesting not only for further development into the oil tuber crop in its own right,but also as a model system for high-biomass underground tubers, such as potatoes[66].
《3.Genetic manipulation of oil biosynthesis in plant vegetative tissues》
3.Genetic manipulation of oil biosynthesis in plant vegetative tissues
《3.1.Anabolism and catabolism of TAG in plants》
3.1.Anabolism and catabolism of TAG in plants
Fatty acid biosynthesis, neutral lipid assembly, and neutral lipid turnover constitute the basic biochemical cycle of oil in plants. The current understanding of the metabolic networks of plant lipid dynamics is mostly derived from studies of oilseeds; these networks in vegetative tissues remain largely unexplored[67,68]. Nevertheless, the expression of most key genes is detectable in other tissues, suggesting that the biogenesis of plant lipids, to a certain extent,may be similar in reproductive and vegetative tissues[26].
Fatty acid biosynthesis and TAG assembly are highly compartmentalized processes (Fig.2). Pyruvates derived from the phospho-enolpyruvates (PEPs) produced after cytosolic glycolysis play a major role as the direct carbon supply for plastidial de novo fatty acid biosynthesis , from which acetyl-coenzyme A (CoA) is synthesized. In the plastids, the biotin-containing enzyme acetyl-CoA car-boxylase (ACCase) catalyzes the first committed step in fatty acid biosynthesis by activating acetyl-CoA to the 3-carbon intermediate, malonyl-CoA, by adding a carboxyl group. The malonyl group is then transferred from CoA to an acyl carrier protein (ACP) in plastids, which carries the growing fatty acid chain in the fatty acid synthase (FAS) complex. The end products of the de novo fatty acid biosynthesis in plastids are usually acyl-ACPs—mainly 16:0-ACP, 18:0-ACP, and 18:1-ACP. These are subsequently exported into the cytosol by crossing the plastid envelope in the form of free fatty acids (FFAs), facilitated by the acyl-ACP thioesterase family (i.e.,acyl-ACP thioesterase A (FatA) and acyl-ACP thioesterase B(FatB)). FFAs are then reactivated as acyl-CoA to generate a temporary “acyl-CoA pool”in the cytosol as the supplier of acyl groups for the acyl-CoA-dependent Kennedy pathway in the endoplasmic reticulum(ER). During this whole process ,rate-limiting enzymes such as ACCase are highly regulated to ensure regulation of FFA levels because of their cytotoxicities[16].
《Fig. 2》
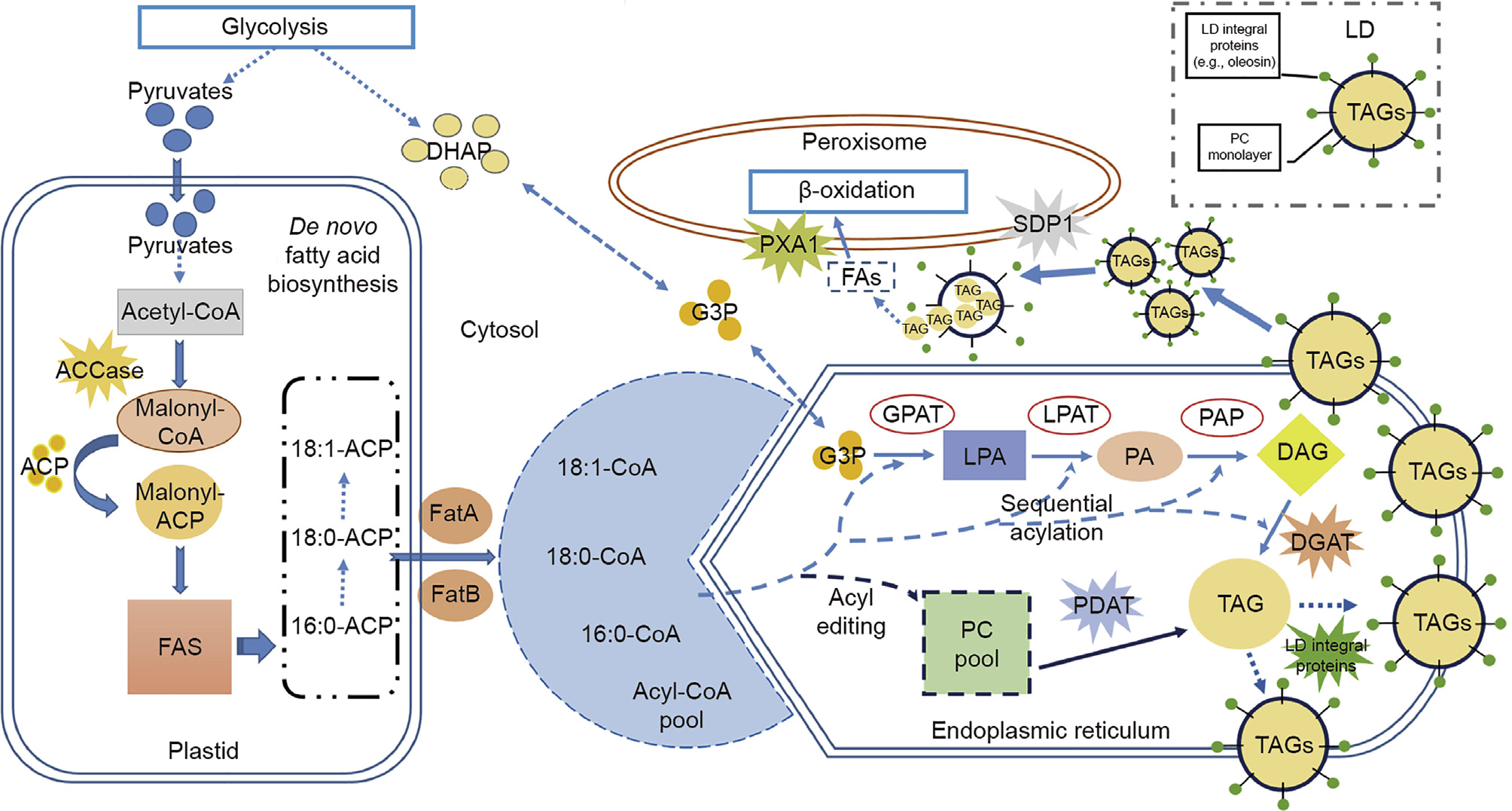
Fig.2.Plant TAG biosynthesis and turnover. Plant cellular plastids (i.e.,chloroplasts) are the dominant place where de novo fatty acid biosynthesis proceeds, within which the production of fatty acids with different chain lengths, such as palmitic acid (16:0-ACP) and steric acid (18:0-ACP), can be initiated by particular FAS systems. The"acyl-CoA pool”generated in cytosol is the major donor of acyls for the subsequent TAG synthesis in ER, where the Kennedy pathway and the acyl-CoA independent pathway cooperate together to support the TAG assembly. The"PC pool”in the ER provides the direct substrate for the acyl-CoA-independent pathway. DHAP: dihydroxyacetone phosphate; CoA: coenzyme A; ACP: acyl carrier protein; FAS: fatty acid synthase; ACCase: acetyl-CoA carboxylase; FatA/FatB: acyl-ACP thioesterase A/B; G3P: sn-glycerol-3-phosphate; GPAT:sn-glycerol-3-phosphate acyltransferase; LPA:lysophosphatidic acid; LPAAT:lysophosphatidic acid acyltransferase; PA:phosphatidic acid; PAP:phosphatidic acid phosphatase; DAG:diacylglycerol; DGAT:diacylglycerol acyltransferase; PC: phosphatidylcholine; PDAT: phospholipid: diacylglycerol acyltransferase;L D: lipid droplet; SDP1: sugar-dependent 1; PXA1:peroxisomal ABC-transporter 1; FAs,fatty acids.
In the classic Kennedy pathway on the ER, sn-glycerol-3-phosphate (G3P) serves as the primary glycerol backbone for glycerolipid biosynthesis. To be specific, sn-glycerol-3-phosphate acyltransferase (GPAT) and lysophosphatidic acid acyltransferase (LPAAT) catalyze the sequential acylation of G3P with acyl groups from the cytosolic “acyl-CoA pool”to synthesize diacylglycerol (DAG), which is converted into TAG by the rate-limiting enzyme diacylglycerol acyltransferase (DGAT)[68]. DGAT is the crucial enzyme that is exclusively responsible for TAG production[68], while other enzymes such as GPAT, which is involved in the initiation of glycerolipid synthesis, play multiple important roles such as the differentiation of “16:3/18:3”plants and the division of eukaryotic and prokaryotic lipid metabolic pathways[69–73]. In addition to the Kennedy pathway, there is an acyl-CoA-independent pathway that usually starts with the transport of plastid-released FFAs directly into the ER for the synthesis of a phosphatidylcholine (PC) pool under the regulation of an acyl editing routine[26]. The PC molecules can thus be transformed into TAG by phospholipid:diacylglycerol acyltransferase (PDAT), which is regarded as another rate-limiting enzyme controlling TAG biosynthesis through the acyl-CoA-independent pathway[74]. Within the acyl-CoA-dependent/ independent pathways, DAG is the fundamental substrate of TAG anabolism. The source of DAG is therefore diverse.In addition to the synthesis from phosphatidic acid (PA) under the catalysis of phosphatidic acid phosphatase (PAP) in the Kennedy pathway, PC acts as an extra donor of DAG and is regulated by the PC:DAG phosphocholine transferase (PDCT), which is a reversible reaction[16]. It is important to note that the PC pool that is formed in the ER, unlike its cytosolic acyl-CoA counterpart, not only donates acyl groups for TAG biosyn-thesis, but also contributes to the maintenance of the intracellular lipid membrane homeostasis of plants[75].
In a typical oilseed, TAG exists in the form of oil body (OB) or lipid droplet(LD) —that is, spherical lipid body surrounded by a phospholipid monolayer onto which a class of LD integral proteins(primarily oleosin and, to a lesser extent, caleosin and steroleosin) are specifically embedded[76]. With this unique structure, the size, mobilization, and fusion of the LDs can be effectively controlled[77]. In addition, it seems that the function of LD is not confined to energy conservation. It has been reported that the leaf LDs in Arabidopsis are able to function as a subcellular factory to synthesize phytoalexins in order to control fungal infection;the associated steroleosin also has the capability to transform estradiol into bioactive ketone-like compounds[78].
In oleaginous tissues other than seeds, such as the avocado (Persea americana) mesocarp, the oleosin type of LD integral proteins were not found in abundance.Rather, a group of small proteins known as LD associated proteins (LDAPs) are the dominant protein in the LD proteome[79]. The LDAPs share a homology with small rubber particle protein (SRPP), which is named for its association with particles storing rubber (cis-1, 4-polyisoprene) in laticifer cells of rubber-producing plants such as the rubber tree (Hevea brasiliensis) and the Russian dandelion (Taraxacum brevicorniculatum)[80]. Unlike oleosin, LDAP may not be integrated into LDs; rather, it may become associated with the particle surface in an isotropic manner[81]. It has been proposed that LDAP proteins may serve two major functions: enhancing the stability of LDs to prevent amalgamation or dispersion, and facilitating the synthesis of new lipids by forming an enzyme complex [80,82–84].
《3.2.Single-gene engineering strategy to increase TAG accumulation》
3.2.Single-gene engineering strategy to increase TAG accumulation
3.2.1.Upstream manipulation
The fatty acids that are reserved to satisfy oil biosynthesis come from a multitude of sources,including starch biodegradation, glycolysis, and direct photosynthetic carbon fixation. However, TAG assembly and LD incorporation, along with the subsequent lipid turnover, are highly regulated biochemical processes, within which several rate-limiting enzymes play dominant roles. Previous strategies aimed at expanding the fatty acid source (push), enhancing TAG synthesis (pull) ,consolidating LD maintenance (packaging), and minimizing lipid degradation (protect) were mostly focused on these steps[18,68]. As the carbon utilization in plants not only is a concerted catabolism, but also has a series of physiological regulations, genes that are individually manipulated may be less effective to realize a global variation on the entire TAG biosynthesis (Table 1) [20,85–100].
《Table 1》
Table 1 Representative studies on the genetic manipulations of major genes within lipid metabolism.

Bn: Brassica napus; Bd:Brachypodium distachyon; St:Solanum tuberosum; At:Arabidopsis thaliana; Rc:Ricinus communis; Si:Sesamum indicum; Jc:Jatropha curcas; VfUSP:Vicia faba unknown seed protein; CaMV:cauliflower mosaic virus; GBSS: granule-bound starch synthase; WRI1:WRINKLED1; LEC2:leafy cotyledon 2; ACCase:acetyl-CoA carboxylase; DGAT:diacylglycerol acyltransferase; PDAT: phospholipid:diacylglycerol acyltransferase; SDP1:sugar-dependent 1; ABHD5:AB-hydrolase domain-containing gene 5; CGI-58:comparative gene identification-58; PXA1:peroxisomal ABC-transporter 1; MGDG:mono-galactosyldiacylglycerol; PG:phosphatidylglycerol.
The single-gene manipulation of the WRINKLED1 (WRI1) transcriptional factor, which was originally discovered in an Arabidopsis mutant with seeds displaying a wrinkled surface and containing higher levels of soluble sugars compared with the wild type[101], can be a representative strategy in the upstream regulation of fatty acid biosynthesis. WRI1 plays a key role in embryo development by specifying the expression of downstream genes toward de novo fatty acid biosynthesis [102]. In vitro experiments have demonstrated that WRI1 binds directly to the promoters of a number of genes involved in fatty acid biosynthesis, including the biotin car-boxyl carrier protein (BCCP) subunit of ACCase, ACP, enoyl-ACP reductase, b-ketoacyl-ACP reductase, plastidial pyruvate kinase, pyruvate dehydrogenase, and FAD2[103–105]. Studies that ectopically overexpressed WRI1 in maize[106] and rapeseed[107] have all found significantly raised oil content in the seeds.WRI1 also induces a 5.8-fold increase in oil production when ectopically expressed in Arabidopsis vegetative tissues[108]. Similarly, a transgenic potato expressing Arabidopsis WRI1 under the transcriptional control of a tuber-specific promoter obtained a significantly increased TAG accumulation, up to 1% of DW[20], demonstrating that WRI1 could function to enhance the lipid biosynthesis not only in seeds, but also in both photosynthetic and non-photosynthetic vegetative tissues. Comparative studies of oil palm and date palm (Phoenix dactylifera) revealed that the WRI1 homologs functioned differently in these disparate palm species by either contributing to the oil storage or facilitating sugar accumulation [109,110].
A highly conserved phosphopeptide-binding protein known as 14-3-3 was identified as interacting with WRI1 by binding with one of its AP2 domains to regulate TAG anabolism[111]. Co-expression of the 14-3-3 and AtWRI1 genes resulted in considerable TAG accumulation in both a transient Nicotiana benthamiana system and in stable transgenic plants, indicating that 14-3-3 is able to enhance the transcriptional activity of WRI1[112]. An earlier study overexpressing the 14-3-3 gene in potato tubers demonstrated that a 69% increase in total lipids was achieved with increased soluble sugar and catecholamine in leaves[113]. As another example,leafy cotyledon 2 (LEC2), the transcriptional fac-tor localized on the upstream of the WRI1 regulatory network[114], has also been examined for its role in carbon allocation control[115]. In Arabidopsis,it was reported that the senescence-inducible expression of the LEC2 gene gave rise to a three-fold increase of TAG in transgenic leaves[88]. However, a drastic reduction of important membrane lipids including mono-/di-galactosyl diacylglycerol (MGDG/DGDG)and phosphatidylglycerol (PG) was observed in the LEC2-expressing plants, reflecting a disruption of membrane lipid homeostasis.
In addition to the transcription factors, overexpression of ACCase led to the enhancement of total lipids in plants[89]. The expression of ACCase in Jatropha was found to be concomitant with oil accumulation during seed development[116]. To divert more carbons to the de novo fatty acid biosynthesis, ADP-glucose pyrophosphorylase (AGPase), which is a rate-limiting enzyme catalyzing a major step of starch biosynthesis, was engineered via RNAi downregulation in Arabidopsis, potato, and maize, albeit with limited success[89,108,117] . In comparison, the acyl-ACP thioesterase family, including FatA1, FatA2, and FatB, which participates in the transportation of fatty acids from de novo plastidial fatty acid biosynthesis into the cytoplasm, was able to further boost TAG biosynthesis when overexpressed in tobacco vegetative tissues[118].
3.2.2.TAG assembly enhancement
In leaf tissues, TAG is normally synthesized as a byproduct of starch production,and its function is beyond merely being an energy donor[16,119]. It has been suggested that TAG can be generated by converting membrane acyl lipids during leaf senescence,and incorporating the residual acyl-CoA into neutral lipids to support the maturity of sink organs[120]. Current genetic engineering strategies in this aspect are mostly focused on the direct enhancement of rate-limiting acyltransferases such as DGAT and PDAT [74,121]. In most plant species, the DGAT gene family consists of three distinct members: DGAT1, DGAT2, and DGAT3[122]. DGAT1 is known to be the predominant gene contributing to the biosynthesis of TAG in Kennedy pathway, whereas DGAT2 is reported to be partly involved in the biosynthesis of unusual fatty acids such as hydroxyl fatty acids[123–125]. More recently, DGAT3 has been hypothesized to function as a scavenger of nomadic acyl-CoAs released from plastidial galactolipids in senescent leaves[126]. Heterologous expression of DGAT1 or DGAT2 was able to enhance TAG accumulation in plants[127,128]. Overexpression of DGAT derived from Jatropha in a yeast mutant showed that DGAT1 could induce a 16.6% increase of TAG and a similar rise (14.3%) of TAG by DGAT2, suggesting their key roles in TAG accumulation[68].
Genetic manipulation of PDAT has been targeted to enhance the acyl-CoA independent pathway[92,129]. It has been proposed that PDAT is a multifunctional gene involved in both TAG biosynthesis and unusual fatty acids metabolism[130]. A recent study on the variations of the liposome in Arabidopsis revealed that under heat stress,the levels of both TAG and polar lipids could be simultaneously altered because of the upregulation of PDAT,indicating that PDAT participates in more sophisticated lipid metabolic networks than DGAT[131]. Moreover , in the acyl editing routine, PDAT is predicted to work cooperatively with an enzyme known as choline phosphotransferase (CPT) that transforms PC to DAG, in order to regulate plant cellular homeostasis[75,132]. The unique features of the PDAT-mediated TAG accumulation pathway warrants further efforts in exploring PDAT functionality in plant lipid dynamics.
3.2.3.TAG packaging
Heterologous expression experiments of plant oleosin and mammalian perilipin in yeast cells have suggested that these LD integral proteins are capable of facilitating the sequestration of TAG and accelerating subsequent LD aggregation [133].Plant oleosin and other oil-body-associated proteins are hence targets for metabolic engineering for enhanced TAG production in oil seeds as well as in vegetative tissues[77,134,135].
At least 17 differentially expressed OLEOSIN genes are known in Arabidopsis,suggesting that these genes are highly regulated[136]. Knockdown or insertion mutants of Arabidopsis oleosins resulted in enlarged and less numerous LDs[137]. Overexpression of soybean oleosin in transgenic rice led to more numerous and smaller LDs, and to an increase in oil content of 37%–46% over the non-transgenic controls,while the overall fatty acid profiles of the TAG remained unchanged[138]. Cysteineoleosin,a modified oleosin, was shown to encapsulate TAG molecules with high efficacy and result in significant increase in the total fatty acid content in diverse vegetative tissues, when it was co-expressed with DGAT1 in Arabidopsis[139].
Furthermore, non-integral proteins, such as LDAPs, apparently play important roles in stabilizing the LD surface to prevent LD amalgamation or dispersion, and in facilitating the synthesis of new lipids through the formation of enzyme complexes[77,140]. Other major non-integral proteins associated with LDs, including oil-body-associated protein 1 (OBAP1) from maize [141,142] and SEIPINs from Arabidopsis[100], have also been studied for their potential role in TAG packaging and accumulation. Three LDAP genes were identified in Arabidopsis, all of which were specifically targeted to the LD surface. LDAP1 and LDAP3 were particularly required for the proper induction of LDs during heat and cold temperature stress[77]. LDAP-overexpressing transgenic Arabidopsis plants exhibited a higher rate of vegetative and reproductive growth as well as a markedly increased tolerance to drought stress[143].
3.2.4.Prevention of LD degradation
Disintegration of LDs, TAG turnover,and fatty acid b-oxidation are the regular cycle of lipolysis.As a result, the maintenance of LDs and the inhibition of lipolysis are targets to minimize oil loss in plant vegetative tissues[144]. Sugar-dependent 1 (SDP1) and peroxisomal ABC-transporter 1 (PXA1) are the currently recognized major enzymes leading to the commencement of oil degradation. SDP1 was initially found in Arabidopsis seeds as the prior enzyme that breaks up the “protective gate”of LDs[94]. The detailed mechanism remained unclear until recent research revealed that SDP1 functions by being delivered in a timely manner through the peroxisomal extension to contact LDs via the peroxisome-LD interaction. Such a process is usually activated at the early stage of plant seedling development and is highly regulated by the number of LD, implying that the expression activity of SDP1 is linked to the TAG content to a certain degree[145,146]. Earlier studies on plant SDP1 were largely focused on mutants and demonstrated that the TAG content could be increased considerably with SDP1 deficiency[147,148]. Further study using transcriptomic analysis indicated that SDP1 expression can be found in nearly all plant tissues , implying that oil degradation is not tissue specific, and thereby improving the feasibility of increasing LD storage in plant vegetative tissues through the inhibition of lipolysis[149]. An 8% increment of the final oil yield was achieved by the RNAi down-regulation of SDP1 expression in rape-seeds, despite deleterious effects on seed vigor[94]. Another lipase PXA1 may be responsible for importing fatty acids to the peroxi some[150]. An Arabidopsis mutant deficient in PXA1 was able to substantially reserve FFAs during lipid hydrolysis[151], albeit mostly a-linolenic acid in the cytosol[98]. It was hypothesized that PXA1 may not work independently, but may interact with a gene known as comparative gene identification-58 (CGI-58) which was originally discovered in mammalian lipophagy and plays a critical role in the plant lipolysis[152,153]. Disruption of CGI-58 in Arabidopsis resulted in an upregulation of lipid biosynthesis, but also demonstrated a positive correlation with the decrease of PXA1 activity[154]. In a subsequent study, it was further indicated that CGI-58 can positively regulate PXA1 activity in most non-seed plant tissues as an expression regulator[155].
Besides the direct manipulation of TAG biosynthesis in plant vegetative tissues, it was also shown that the endogenous replacement of starch branching enzyme (SBE) by an endosperm specific maize homolog was able to boost the total oil content in Arabidopsis by tripling seed production[156]. Although the TAG increase can be largely attributed to the overdeveloped siliques per plant, this finding still yielded a novel insight: that other collateral carbo-hydrate pathways involved in the carbon distribution may possess the potential to enhance oil production.
《3.3.Multigene engineering strategies》
3.3.Multigene engineering strategies
Recent technological progress has significantly expanded our capabilities to manipulate complex metabolic pathways such as lipid biosynthesis through the simultaneous expression of multiple transgenes. TAG biosynthesis in plants is a highly regulated process that interdependently correlates with multiple biochemical path-ways, from the primary carbon allocation to the dynamically equilibrated TAG accumulation[16]. Biochemical pathways involved in plant lipid metabolism are correspondingly regulated by multiple rate-limiting enzymes[157]. As a transient byproduct in plant vegetative tissues, TAG is normally not consecutively synthesized or accumulated in large amounts due to the sophisticated transcriptional regulation of these key genes[68]. Nevertheless, the single-gene manipulations of most of the rate-limiting enzymes have shown considerable effects in increasing oil accumulation, although it would be much more effective to simultaneously engineer different genes together in order to reap a further enhanced oil production. In such integrated strategies, both the expansion of de novo fatty acid biosynthesis as the "source”and the effective formation and protection of TAG in the form of LD "sinks”remain the preferred methodologies[26,158]. Major factors including the upstream transcriptional factors, determinative acyltransferases for TAG assembly , LD integral proteins, and downstream lipases are thus considered to be the primary targets in an integrated or coordinated manner. Nicotiana benthamiana has been adopted for a transient leaf assay, enabling rapid evaluation and iterative improvement of gene combinations in the complex TAG biosynthesis pathways[18,159]. We now have a versatile toolbox for the multigene engineering of lipid production, the coordinated expression of multiple key genes, and the optimization of enzymatic activities, all of which are critical for the successful development of a new oil crop paradigm.
Table 2 [17,19,21,91,108,160–162] summarizes the recent representative research on the enhancement of TAG production in plant vegetative tissues through combinations of multiple transgenes. Simultaneous overexpression of WRI1, DGAT1, and OLEOSIN1 genes was able to increase TAG content in tobacco leaf to 15% of DW, far exceeding the levels achievable by adding up the total effects of expressing these genes separately[18]. Each of these three genes plays a critical role in directing more of the carbon flux to fatty acid biosynthesis, TAG assembly, and LD formation,respectively, and the synergistic functioning of these genes has resulted in significant increases of TAG and, to a lesser extent,of the membrane lipids. TAG content was then raised to an unprecedented level of 30% –33% of DW in transgenic tobacco leaves when SDP1 was further suppressed through RNAi approach or the introduction of Arabidopsis LEC2[17]. Similar results were demonstrated in sugarcane: The expressions of AGPase and PXA1 were suppressed in the WRI1-DGAT1-OLEOSIN1-expressing plants, and up to a 95-fold boost of TAG in aboveground vegetative tissues was reached,while single-gene engineering only resulted in 1.5–9.5-fold increase in TAG in each of the mono-transgenic lines[19]. The synergistic effect of multiple transgenes on TAG accumulation was also explored in potato tubers[17]. Potato tubers are an important starch source, harboring 16% –20% starch, 2%–2.5% patatin, and 72% –75% water of the fresh weight, whereas TAG merely account for approximately 0.01%–0.03%[163]. When genetically modified by cooverexpressing the WRI1,DGAT1, and OLEOSIN1 genes, the highest oil transgenic potato line had about 3% TAG of DW in tuber, a nearly 100-fold increase compared with the wild type[37]. This is in sharp contrast to the transgenic potato that individually expresses the Arabidopsis WRI1 transcriptional factor,which obtained a 1% TAG accumulation of DW in tubers[20]. Even though detailed information on the underground oil synthesis in planta still remains poorly known, it has become clear that multigene manipulation strategies targeting the global lipid metabolic network are powerful in redistributing carbon to the synthesis of the most stable and energy-rich chemical forms in plant vegetative tissues.
《Table 2》
Table 2 Representative studies on the multigene engineering of lipids in plants.
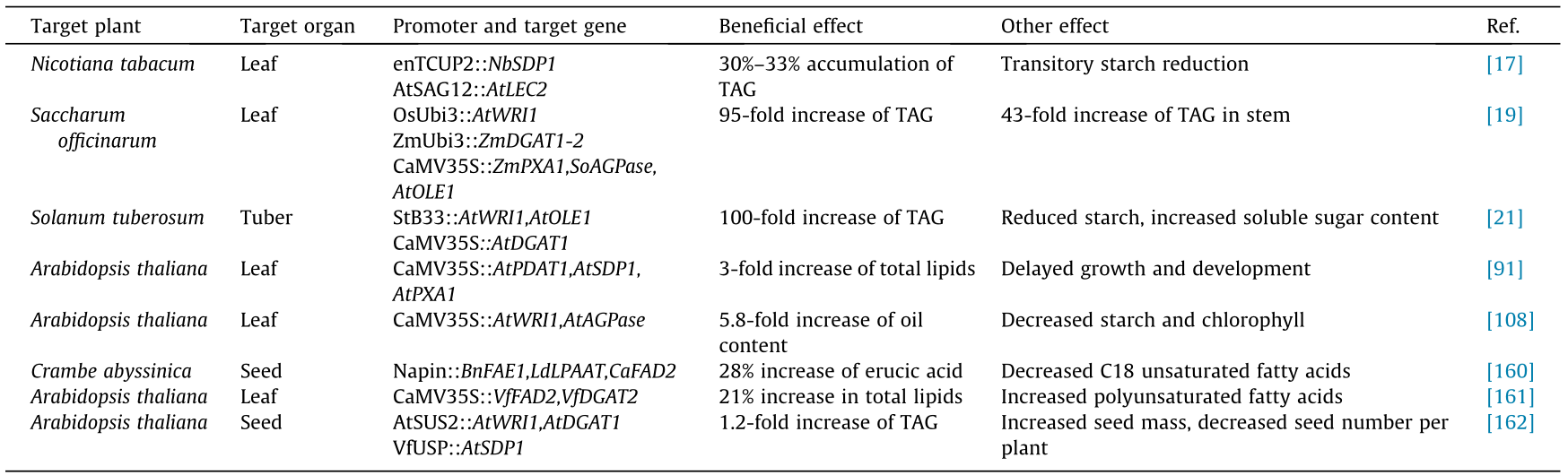
Nb:Nicotiana benthamiana; Zm:Zea mays; Ld:Limnanthes douglasii; Vf:Vernicia fordii; enTCUP:tobacco constitutive expression cryptic promoter; OsUbi3:Oryza sativa ubiquitin promoter; B33:potato tuber-specific patatin promoter B33; SUS: sucrose synthase; VfUSP:Vicia faba unknown seed protein; SAG:senescence associated gene; Napin:Brassica napus seed specific promoter; AGPase:ADP-glucose pyrophosphorylase; OLE:oleosin; FAE1:fatty acid elongase1; LPAAT:lysophosphatidic acid acyltrans-ferase; FAD2:microsomal oleate desaturase.
《4.Transcriptional control of transgenes for TAG enhancement》
4.Transcriptional control of transgenes for TAG enhancement
Unlike the cytotoxic FFA,abundant accumulation of TAG in the plant cytoplasm is largely innocuous because it is a generally phys iochemically stabilized substance with good hydrophilicity and non cytotoxicity[164]. However, there is often a tradeoff between biomass production and TAG production in high-oil transgenic lines[37,91]. Channeling carbon away from common metabolic process and toward an added TAG sink may present a metabolic conflict for vegetative tissues that normally do not accumulate TAGs. Such a metabolic burden is commonly reflected as slow plant growth and reduced biomass production;these are undesirable because high biomass accumulation is required to make a crop economically viable. The CaMV35S promoter,which is widely used to drive constitutive overexpression of target genes to enhance plant TAG biosynthesis, is generally believed to cause disturbance in the biochemical network equilibrium of transgenic plants[165–167]. Therefore, constitutive production of TAG in plant vegetative tissues may not be an appropriate production mode for universal applications[68]. It is imperative to precisely regulate the target gene expressions at both temporal and spatial levels[168,169].
To avoid these undesirable effects, engineered pathways aiming to enhance TAG production are usually regulated through promoter-mediated inducing systems, as illustrated in Tables1 and2. A chloroplast-specific RuBisCO small subunit (SSU) promoter was used in the expression regulation of WRI1 and OLEOSIN1 in transgenic tobacco leaf, in order to control the transcription activity in green tissues[18]. This attempt successfully increased the leaf oil content to the industrial standard without causing major undesirable repercussions upon other important physiological and bio-chemical traits. The senescence stage of a plant has been suggested as a possible target for oil production at high levels, following the earlier establishment of the entire plant biomass[170]. Some significant successes have been reported with a senescence-inducible promoter driving further TAG increase in plant vegetative tissues[170], despite concerns that carbon fixation will be weakened during the natural degradation of plant cellular organelles, such as the chloroplast, during senescence[171–174].
《5.Other key issues in the enhancement of TAG accumulation in non-seed plant tissues》
5.Other key issues in the enhancement of TAG accumulation in non-seed plant tissues
Although significant progress has been made in gene discovery, the roles of many genes in fatty acid biosynthesis and lipid metabolism remain elusive. Even in model plants such as Arabidopsis with known genome sequences, genome-wide association studies have recently revealed large numbers of previously unknown genes that are highly relevant to lipid metabolism[175]. For many oleaginous plants without a sequenced genome, comparative transcriptomics is useful in generating large datasets that can be mined to identify candidate enzymes, as exemplified by the comparative studies between the oil palm and the date palm[109,176].
Prior to embarking on lipid metabolic engineering, it is necessary to determine whether a given target plant species is theoretically capable of commercial viability. This is especially true for plant-oil-based biodiesel feedstock, a high-volume yet low-value product. For example,a high-yield and high-biomass crop such as potato tuber has been considered as a potential target oil crop. The recently achieved moderately enhanced oil accumulation in potato tubers warrants further studies on TAG metabolism and on the intricate relationship between oil and starch in a classic starch-accumulating storage organ[20,177]. Because the TAG content reaches approximately 30% of DW in the anatomically similar yellow nutsedge tuber, it might be possible,at least theoretically, to further raise the TAG level in transgenic potato tubers without serious impacts on plant growth and tuber yield[65].
The selection criteria of gene donors for a given target oil crop, including the evolutionary distance between the host and donor species, are of great importance. Despite sporadic reports regarding the incorporation of genes into hosts from an evolutionarily distant donor, the success rate is rather limited[178]. For example, the ectopic expression of specific transcription factors, such as WRI1, needs to be considered in terms of the compatibility of these factors with their cognate binding sites. On the other hand, high expression of WRI1 was recently found to be fatal, especially when the homology is very high between the gene donor and the host plants[86]. Such observations necessitate detailed studies on the metabolic networks of target crops and gene donors at the molecular and biochemical levels.
The traditional approach of introducing more than one trans-gene involves the stacking of expression cassettes in the transfor-mation vector. The physical arrangement of the multiple cassettes within a linear vector is critical in minimizing the read-through transcription effects that potentially give rise to aberrant RNA and that may trigger transgene silencing[179]. The coordination of gene expression by including alternative promoters requires further consideration, as the repetitive use of a same promoter may result in homology-related instability.
This transgene-stacking approach is only suitable for a small number of genes because of the increased recombination frequency associated with large insert sizes. Efforts to introduce multiple transgenes encoding the lipid biosynthesis pathway on a single vector, therefore, are often hindered by the technical difficulty of gene cloning into large vectors. This limitation could be overcome by co-transformation with two or more transformation vectors, or by sequential transformation steps and subsequent combination by sexual crosses. Furthermore, with the rapidly falling costs of gene synthesis and steady progress in synthesizing and assembling large DNA inserts , it is envisaged that a lipid biosynthesis pathway with a higher degree of complexity could be successfully engineered into the target crop genome in the near future.
As some studies have noted,the synthesis of important membrane lipids such as phospholipids and galactolipids can be affected by activating the available FFAs to participate in the TAG biosynthetic pathway[180,181]. The plasma membranes of plant cells provide an osmotic adjustment associated with drought and extreme temperatures,as well as acting the primary barrier to bacterial, fungal, and insect pathogens. In addition, membranes provide a repository of lipids that can generate signal molecules for localization or distal responses to environmental perturbations.For example,an alteration in the levels of intermediate chemicals such as PA,which plays a critical role in the endogenic signal transduction and stress-responding systems of the plant,may affect plant growth and physiology[182,183]. Furthermore, the genes involved in lipid signaling have only begun to be revealed,and it is likely that many of the large number of unstudied putatively lipid-related genes may play signaling or membrane-remodeling roles (e.g.,PDAT). Therefore, a systemic evaluation of genetically modified oil crops with introduced TAG biosynthesis pathways in vegetative tissues on the omics level is clearly required[184–187]. Multisite field tests of the new oil-rich plants to assess their environmental adaptability, actual productivity, and market feasibility are also of high importance[20]. Owing to the deteriorating global ecology, the survival of crops under adverse conditions should also be taken into account.It has recently been revealed that plants deficient in TAG hydrolysis are able to withstand extended darkness and oxidative damage, demonstrating that TAG abundance in plant tissues may grant the potential to resist abiotic stresses more effectively[188].
《6.Summary and perspectives》
6.Summary and perspectives
Vegetable oils are of great importance in terms of land usage,food security, biofuels production,and many non-food applications. Conventional plant breeding is critical for improvements in oil yield, but is limited due to the lack of genetic diversity that can be introduced to obtain complex oil-accumulation pathways. Excellent progress has been made by addressing the rate-limiting steps in lipid biosynthesis through enzyme overexpression or the introduction of enzyme variants that are insensitive to negative feedback regulation, especially with the concerted expression of multiple transgenes.
The appropriate selection and collocation of transgene sources, inducible promoters, and the target plant species are of great importance in establishing an industrially viable plant biomass oil production platform, without severely compromising the normal development of transgenic plants. Further improvements in oil production require system biology approaches in order to obtain a greater understanding of not only lipid metabolic networks, but also the complex multilevel regulation of biosynthetic pathways, including carbon partitioning and the transcription factors that orchestrate lipid metabolism and turnover. Achieving this goal will require significant strides forward, especially in systems biology and metabolic network reconstruction.Ultimately, a combination of omics technologies and advanced genome-editing capabilities will greatly expand our ability to enhance lipid accumulation in an appropriate chassis.
The application of synthetic biology to lipid metabolic engineering offers the possibility of rapid assembly of multiple genes for the introduction of complex pathways, developing interchangeable, modular assemblies of transgene cassettes. It is also envisioned that the future high-oil trait could be coupled with other output traits with health benefits or industrial values, such as omega-3 long-chain polyunsaturated fatty acids. Such"dual-purpose”crop strategies may have potential for direct use as a niche health food, animal feed,or oleochemical feedstock. Currently popular genome-editing tools, such as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9, could be used to remove or minimize metabolic competition while directing metabolic flux toward the TAG biosynthesis route,or to modify specific amino acids in lipid biosynthesis enzymes in order to improve enzyme activity or generate altered substrate specificities.
《Acknowledgements》
Acknowledgements
Xiao-Yu Xu wishes to thank the China Scholarship Council (CSC) for financial support.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Xiao-Yu Xu, Hong-Kun Yang, Surinder P.Singh, Peter J. Sharp, and Qing Liu declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




