《1. Introduction》
1. Introduction
Ramsden [1] and Pickering [2] were the first to identify and describe Pickering emulsions at the beginning of the 20th century. In these colloidal suspensions, stabilization is achieved by using only solid particles (hence the alternative name of solidstabilized emulsions) in the place of organic surfactants and polymers. In classical emulsions, the system stability is ensured by the adsorption of amphiphilic compounds that modify the interfacial properties of the two phases. In Pickering emulsions, the absorption of solid particles at the interface between liquids should form an obstacle to limit the merging (coalescence) between droplets.
Solid particles can be adsorbed at the interface between oil and water only upon partial wetting by both phases (i.e., dual wettability). Their adsorption leads to a decrease of the surface of the high-energy oil–water interface. This is one of the driving forces contributing to particle transfer at the interface. Different factors must be taken into account in the energy expression, including the interface between the particle/oil and particle/water phases. On the basis of the relative particle wettability, Pickering emulsions can be categorized into two types: ① oil-in-water (O/W) emulsions that are stabilized by hydrophilic particles; and ② water-in-oil (W/O) emulsions that are stabilized by hydrophobic particles.
Despite the advantages (e.g., higher stability and lower toxicity) of solid particles relative to surfactant-based emulsions, the number of studies on Pickering emulsions has increased significantly in recent years because of the potential value of these emulsions for different and novel applications (Fig. 1), particularly in industry (i.e., food technology [3], cosmetic products [4], oil recovery [5] and, more recently, drug delivery [6]). Many types of organic [7–9] and inorganic solid particles can be used as Pickering emulsifiers. The main features of a stabilizing particle are its dual wettability, morphology (dimension and shape), and concentration [10]. This review will focus particularly on inorganic particles.
《Fig.1》
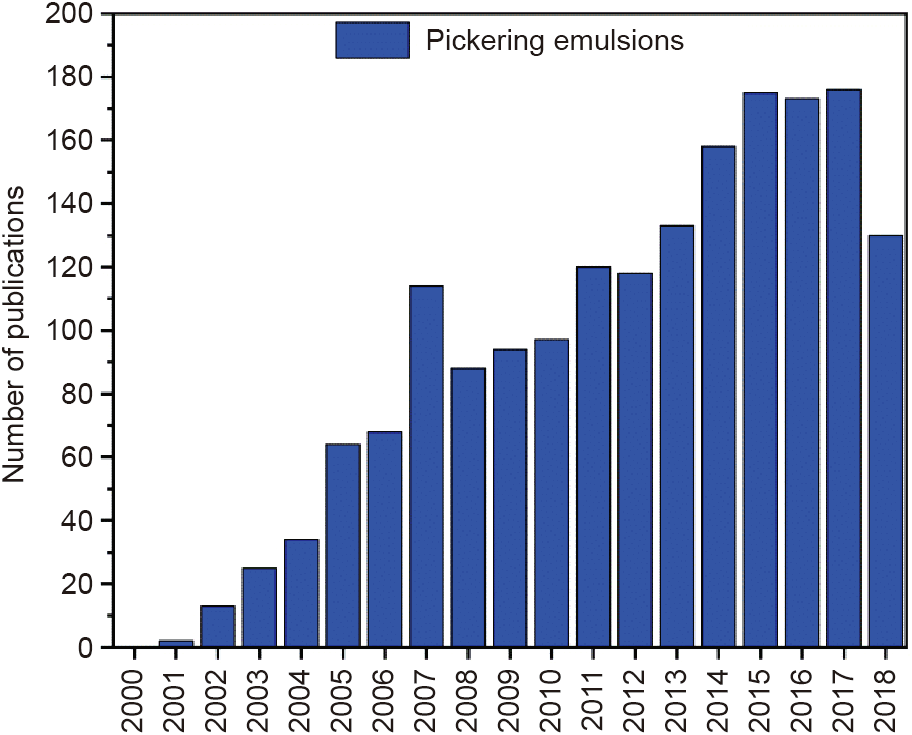
Fig. 1. Number of publications with the key word "Pickering emulsions” per year from the first description of Pickering emulsions (2000–2018).
Previous review articles on Pickering emulsions have described their preparation and properties, how their stability can be influenced, and what materials can be fabricated using these mixtures. For example, Aveyard et al. [11] focused on Pickering emulsions that are stabilized only by solid particles adsorbed at the oil–water interface, particularly silica nanoparticles that show wellcontrolled surface properties. They also extended the analysis by Levine et al. [12] based on the free energy required for the emulsification of drops surrounded by tightly packed monolayers of monodispersed spherical particles. The researchers mainly considered the potential effects of the free energy of the line tension in the three-phase contact angle around particles adsorbed at the droplet interface during emulsification. A positive line tension (even a small one) might lead to positive free energies of particle adsorption, especially for contact angles lesser or greater than 9°. Conversely, a negative line tension might lead to negative free energies, mostly for angles close to 9°. The effect is a function of the particle radius, and is higher for smaller particles [12]. Binks and Horazov [13] published a book on the behavior of particles at planar interfaces. They described simulations and theoretical approaches to the structure and dynamics of particle monolayers, and attempted to explain how particles can contribute to wettability in oil–water interfaces.
Hunter et al. [14] evaluated the major factors influencing the stability of particle-stabilized foams and emulsions to determine the similarities and differences between foam systems and emulsions. Toor et al. [15] discussed the self-assembly of different materials (e.g., nanoparticles, nanorods, and nanosheets) at fluid interfaces. They found that at fluid interfaces, self-assembly is influenced by the nanomaterial shape, and that the nanoparticle interfacial assembly is mainly driven by a reduction of the interfacial energy. They obtained anisotropic nanoparticles with different orientations by controlling the bulk particles concentration. The segregation of single-walled carbon nanotubes (SWCNTs) between immiscible liquid phases could allow the fabrication of flexible electronic thin films, producing porous SWCNT/polymer composite foams. Moreover, the assembly of atomically thin graphene oxide (GO) sheets at fluid interfaces could represent a new way to fabricate graphene films for electronic applications [15].
Several studies have also reported advances in nanoparticle self-assembly at liquid–liquid interfaces [16,17]. Indeed, nanoparticles can be manipulated by modulating the interface nature, their surface features, and their size. A reduction of the interfacial energy promotes nanoparticle segregation at liquid–liquid interfaces that are suitable templates for the fabrication of nanoparticle-based structures. Nanoparticle self-assembly at liquid–liquid interfaces provides new insights into the design and fabrication of novel soft materials, such as structured liquids [18].
Chevalier and Bolzinger [19] reviewed the fundamental physical–chemical features of Pickering emulsions (i.e., droplets size, emulsion stability, and rheology). They also investigated the methods used to control the key parameters in the preparation of solidstabilized emulsions for specific applications, such as drug delivery or the manufacturing of porous materials. Schrade et al. [20] summarized the progress in Pickering-type nanoparticles production using heterophase polymerization methods, and the applications of such methods for preparing emulsions, micro-emulsions, dispersions, and suspensions. Tang et al. [21] provided a comprehensive study of Pickering emulsion systems that can respond to different external triggers, and their potential applications. Wu and Ma [22] described how to prepare Pickering emulsions, especially the techniques that allow the production of uniformly sized emulsion droplets. They also discussed how the microparticles and nanoparticle characteristics can affect the production and features of Pickering emulsions, and the biomedical applications of these emulsions. Yang et al. [23] focused on solid particles that are commonly used as emulsifiers, such as clay, chitosan, and carbon nanotubes (CNTs). They also classified the three different types of materials fabricated from emulsions: microspheres, microparticles, and Janus particles. Finally, Yang et al. described many biomedical, chemical, and physical applications of materials produced using Pickering emulsion systems.
However, neither the particle morphology effects on Pickering emulsions production nor the current applications of Pickering emulsions have been reviewed so far. Here, we describe the different morphologies of particles used as stabilizers, with a particular focus on two-dimensional (2D) materials. This special class of materials offers some advantages due to their direct participation in emulsion stabilization and their role as molecular barriers. First, we will describe different factors that affect emulsion stability and the destabilization mechanisms. Next, we will list the morphology of different particles used to stabilize Pickering emulsions. Finally, we will summarize different applications of Pickering emulsions as active systems.
《2. Emulsion stability and the destabilization mechanisms 》
2. Emulsion stability and the destabilization mechanisms
《2.1. Emulsion types》
2.1. Emulsion types
In particle-stabilized emulsions, the choice of emulsion type is driven by its wettability, which is quantified using the contact angle. According to the Bancroft rule, hydrophilic particles (i.e., with a contact angle < 90° measured through the water phase) are better for stabilizing O/W emulsions. Conversely, hydrophobic particles (i.e., with a contact angle > 90°) are more suitable for stabilizing W/O emulsions (Fig. 2). Particles that are fully wetted by water or oil remain dispersed in that phase, and cannot form an emulsion [24]. The term dispersed phase refers to droplet-forming liquid, whereas the term continuous phase refers to a liquid in which droplets are separated, one from the other.
《Fig.2》

Fig. 2. Description of the relationship between the particle contact angle (θ) and the emulsion type.
According to the Bancroft rule, emulsions with high potential for long-term stability will be preferentially formed when mixing the same volume of oil and water. It is possible to modify the type of emulsions formed by modulating the ratio between the volumes of the dispersed and continuous phases. When this ratio is extremely high, the emulsion will undergo "catastrophic phase inversion” [25,26], and will be unstable against coalescence because it is a non-preferred emulsion [12]. Eq. (1) is used to quantify the energy needed to displace a particle from the oil/water interface:

where r is the particle radius, cO/W represents the oil/water interfacial tension, and θ is the contact angle of the three phases (normally defined by the aqueous phase) [27,28].
The adsorption of perfectly soft and rigid particles at the interface is determined by the surface tensions of the oil–water, oil– particle, and water–particle interfaces (γO/W, γO/P, and γW/P, respectively). Soft particles are better emulsifiers than hard particles because they stretch at fluid interfaces. This deformation increases the adsorption energies compared with rigid particles. In addition, soft particles can spread to cover a larger oil–water interface area than hard particles [29].
《2.2. Emulsion stability》
2.2. Emulsion stability
The effectiveness of solid particles as emulsifiers mainly depends on their wettability and morphology. Additional critical parameters are the oil nature, particles concentration, phase volume fraction, and order of addition during manufacturing.
2.2.1. Wettability
The adsorption of a particle at the oil–water interface is strongly influenced by its hydrophobicity, which depends on the oil–water interface contact angle. The wettability of solid particles at the oil– water interface will determine the Pickering emulsion type (O/W or W/O). Indeed, the liquid by which particles are predominantly wetted will be the continuous phase, whereas the other liquid will be the dispersed phase. In general, particles with a contact angle in the range of 15° < h < 90° should stabilize O/W emulsions, whereas particles with a contact angle in the range of 90° < h < 165° should stabilize W/O emulsions [30]. The particle wettability can be tailored by surface functionalization.
The influence of wettability on the emulsion stability has been studied using silica particles. For example, Binks and Lumsdon [31] used spherical silica particles and emulsions formulated with water and toluene. They found that catastrophic phase inversion (from W/O to O/W emulsion) occurs when the water volume fraction (Φw) is increased. Moreover, with hydrophobic particles, higher Φw values are needed to observe this type of inversion. Björkegren et al. [32] reported that emulsions with smaller drops are obtained when colloidal silica particles modified with hydrophobic groups are used, in comparison with the use of nonfunctionalized silica (Fig. 3) [33]. Silica nanoparticles with different wettability (i.e., hydrophobic, hydrophilic, and intermediate wettability) were used to investigate the effect of wettability on emulsion formation and separation kinetics. A comparison of the separation kinetics of the emulsions prepared with these different nanoparticles revealed that the emulsions stabilized by hydrophobic nanoparticles were the most stable [34].
《Fig.3》

Fig. 3. Interfacial assembly of spherical particles at the oil–water interface. Reproduced from Ref. [33] with permission of The Royal Society of Chemistry, © 2017.
Briggs et al. [35] found that the inherent hydrophobicity of functional groups at the surface of multi-walled carbon nanotubes (MWCNTs) could be modified into hydrophilic behavior by oxidation with nitric acid. The emulsion droplet size displayed parabolic behavior, whereby the smallest emulsion droplets were stabilized by amphiphilic MWCNTs and larger droplets by more hydrophobic or hydrophilic MWCNTs. Moreover, hydrophilic MWCNTs stabilized O/W emulsions, whereas non-functionalized MWCNTs stabilized W/O emulsions. This study highlights that the emulsion type can be influenced by the surface modifications of the stabilizing nanoparticles.
Xiao et al. [36] discussed advances in tailoring the wettability of colloidal particles for Pickering emulsions, along with the related applications. They focused on switchable Pickering emulsions with their environmental-responsive properties, and the effect of surface roughness. They also thoroughly described the methods to finely tune the particles' wettability by modifying their surface functional groups (physical adsorption or chemical anchoring) or topology. Xiao et al. stated that tuning the wettability of small molecules or polymers offers ample opportunities for industryrelated applications.
2.2.2. Particle concentration
The emulsion stability and average droplets size are strongly influenced by the particle concentration [12]. As solid particles must be adsorbed at the oil–water interface in order to act as emulsifiers, the emulsion stability tends to proportionally increase with the particles concentration. This trend has been confirmed by Gelot et al. [37], who observed that when the particles concentration increases, the emulsion is stable against coalescence for a longer time because more particles can go to the interface and improve the emulsion stability. Moreover, coalescence can be prevented by covering droplets with a tightly packed layer of particles [38].
Besides the classical arrangement of two densely packed monolayers, other particle-based structures can prevent droplets coalescence, such as a dense layer of bridging particles and a layer of aggregated particles organized in a low-density network. These structures typically involve some particles aggregations or droplet flocculations. For example, in a single dense layer of bridging particles, each particle is partially wetted by the two dispersed phases, although it is still in the aqueous continuous phase. In a third type of inter-droplet arrangement, droplets are stabilized through the adsorption of aggregated colloidal particles. This structure consists of a rigid disordered layer/network of particles that are adsorbed to the oil–water interface(s), and are held together by attractive interparticles forces [39].
Binks et al. [40] showed that in Pickering emulsion mixtures, droplets coalescence is triggered by compositional ripening. Conversely, coalescence is inhibited by the addition of an excess of particles because they attach to and stabilize the liquid–liquid interface. Interestingly, particles protruding from a droplet can simultaneously adsorb to another interface, thus bridging two droplets through a shared particles monolayer. This configuration satisfies the equilibrium contact angle on both sides of the bridging particles, thus preventing coalescence [41,42]. However, this is not a general rule for emulsion stability, because in some cases, an increase in the number of particles leads only to a particle excess in one liquid [43,44].
A study by Frelichowska et al. [45] on the influence of particles concentration on emulsion stability showed that O/W emulsions are not stabilized by low silica nanoparticles concentrations, and that their stability is improved by increasing the nanoparticle content. Moreover, particles concentration variations modulate droplets size. All the researchers who assessed the effect of solid particle content on droplet size found an inverse correlation between droplets size and particles concentration [46–48]. Indeed, the number of particles adsorbed at the droplet surface increases with their concentration to finally form a packed monolayer. When this occurs, the total oil–water interfacial area is defined by the number of solid particles.
Gavrielatos et al. [34] revealed that the presence of nanoparticles in an emulsion, even at very low concentrations (0.005% or 0.01%), significantly increases the O/W emulsion separation time from a few minutes (in the absence of nanoparticles) to several hours or even days. The emulsion stability typically increases proportionally with the nanoparticles concentration. It was demonstrated that the emulsion stability could be influenced by the shearing time, with longer shearing times resulting in slow separation rates due to the dispersed droplets having a smaller size. However, this effect decreases when droplets reach the equilibrium size, and the separation kinetics will not be further retarded.
Packing density is also crucial for droplets stability. The most commonly reported configuration of spherical particles is dense, hexagonally close-packed particles. This packing model predicts the formation of larger drops when the droplet volume fractions increase. If the droplets can adsorb more colloidal particles, the surface tension values decrease and smaller droplets will form [49]. The volume fraction of the droplets is an important factor in determining the packing. In a monodispersed emulsion, 74% is the maximum volume fraction (Φm) in which droplets are hexagonally close-packed without being distorted. If the volume fraction increases to a certain critical value (Φc), droplets will coalesce together [50]. Under such conditions, simple geometrical considerations (Eq. (2)) describe the link between the droplets diameter and the dispersed phase and solid particles mass ratio (Moil/Msolid):

where ρoil is the oil density and αoil is the interface area covered by the solid particles mass. This equation has been applied in several studies [51].
Therefore, very large droplets can be obtained by using a limited number of solid particles, leading to the stabilization of a small interface surface. A mild emulsification method (termed "handshaking”) can be used to prepare stable coarse emulsions [46,52]. A highenergy emulsification process generates smaller droplets that will rapidly undergo coalescence until the interface surface is fully covered by particles (Fig. 4) [53]. On the other hand, with a higher solid particle mass, very fine emulsions might be obtained; however, this requires an adapted emulsification method.
《Fig.4》

Fig. 4. Schematic representation of droplet coverage by nanoparticles and nanosheets.
2.2.3. Oil type and volume fraction
The oil type used to prepare the emulsion and the ratio between the dispersed and continuous phases are two other important factors that affect the stability, and sometimes also affect the emulsion type. The type of oil phase is crucial because it determines the interfacial tension of the oil–water interface, and can affect the interactions with the particles. He et al. [48] used several aromatic and non-aromatic solvents as the oil phase to prepare GOstabilized Pickering emulsions, and found that only O/W emulsions were obtained. Moreover, GO-mediated stabilization was much higher with aromatic solvents than with non-aromatic solvents. Thickett and Zetterlund [54] theoretically described O/W emulsions stabilized by GO sheets in which the polarity of the oil phase was thoroughly analyzed. They found that GO-stabilized emulsions could be prepared only when using hydrophobic and aromatic solvents (e.g., styrene), and not with polar solvents as the oil phase.
The emulsion stability and type are greatly influenced by the dispersed-phase volume. Variations in the oil/water ratio at constant particle wettability or with progressive changes in the particle wettability might lead to catastrophic phase inversion. Binks and Lumsdon [25] found that hydrophilic silica-stabilized emulsions underwent catastrophic phase inversion from O/W to W/O when the dispersed-phase volume fraction was around 0.7. He et al. [48] observed that GO-stabilized emulsions display huge stability variations when different oil volume fractions are used, because the oil/water (benzyl chloride/water in this study) ratio changes. To be specific, the stable emulsion fraction increased progressively starting from oil/water ratios greater than 0.5, and decreased with oil/water ratios lower than 0.5.
2.2.4. pH and ionic strength
As the particle surface wettability can influence the Pickering emulsion stability, nanoparticles with switchable partial surface wettability represent an interesting option for producing O/W and W/O emulsions. Moreover, pH variations can change the hydrophobicity and consequently the wettability of particles harboring surface groups that can be ionized. Therefore, changes in the solution pH could be used to modulate the particle adsorption at the interface, and possibly affect the emulsion type [48,55]. Hao et al. [56] described a pH-responsive emulsion system that is stabilized by interfacial active titanium dioxide (TiO2) nanoparticles. At pH values of 3–4 (Fig. 5) [56], the emulsion droplets were completely destroyed and the nanoparticles were distributed in the aqueous phase. Increasing the pH value to 7–8 with sodium hydroxide (NaOH) allowed the emulsion to re-form.
《Fig.5》

Fig. 5. Schematic illustration of the pH-switched Pickering emulsion strategy. Reproduced from Ref. [56] with permission of Elsevier B.V., ©2018.
It is crucial to understand how pH changes affect the stability of particle-stabilized emulsions. The pH influences the surface charges of particles and molecules, and consequently also their interactions with the different phases. For example, a study on the stability, microstructure, and macroscopic behavior of pHcontrolled O/W emulsions containing chitosan-modified silica nanoparticles as stabilizers showed that the behavior of these particles is pH-dependent, resulting in emulsions with different length structures. Moreover, chitosan adsorption on silica is reversible at pH 5.5. That study concluded that the microstructure and properties of the network-stabilized emulsion could be switched by simply changing the pH [57].
In other words, silica nanoparticles can be used to analyze the emulsion properties when they undergo pH changes. For example, Ren et al. [58] prepared pH-switchable emulsions using dynamic covalent silica nanoparticles. They found that the particle hydrophilicity could be modulated by adjusting the pH between 7.8 and 3.5. At pH 7.8, the particles were partially hydrophobic and stabilized O/W Pickering emulsions. Conversely, when the pH was decreased to 3.5, the particles became highly hydrophilic, leading to phase separation.
Mwangi et al. [44] investigated how the stabilizing activity of self-aggregated chitosan particles is influenced by chitosan concentration and environmental factors (i.e., ionic strength, temperature, and pH). They found that droplet coalescence and creaming were promoted by progressively decreasing the pH, with demulsification occurring at low pH. Furthermore, emulsion stability was improved by particle aggregation at the oil/water interface, and by the formation of chitosan networks in the continuous phase, which limits droplets interactions.
In conclusion, the emulsion stability and type can be modulated just by controlling/adjusting the pH and ionic strength. Variations in pH can dramatically modify the emulsion's microstructure and properties, as well as the particles hydrophobicity, and ultimately affect its stability by destroying the droplets. In the next section, we will briefly describe the different phenomena that can lead to emulsion destabilization.
《2.3. Destabilization mechanisms》
2.3. Destabilization mechanisms
Emulsion properties can remain unchanged for some time, which is a characteristic known as "emulsion stability.” However, as emulsions are thermodynamically unstable, their properties will change over time. The speed at which properties of an emulsion change defines the emulsion stability. A large interfacial water–oil area strongly reduces the thermodynamic stability upon emulsification, while the interfacial Gibbs free energy increases (Eq. (3)):

where ΔA (m2 ) is the total interfacial area in the system.
The positive Gibbs free energy associated with interface formation compensates for the negative energy due to particles adsorption. Although attached particles are not in equilibrium, they are irreversibly attached, thus leading to the emulsion’s kinetic stability. The destabilization of Pickering emulsions and consequent separation of the macroscopic phases can be caused by different mechanisms that occur on their own or concomitantly. A brief summary of these breakdown processes is provided in the following section.
Droplets with higher density than the continuous phase tend to descend and form a layer at the bottom of the emulsion (i.e., sedimentation). Conversely, droplets with lower density than the continuous phase tend to rise up and form a layer of droplets on top of the emulsion (i.e., creaming) [59]. Flocculation occurs when two or more particles or droplets associate with each other to form a larger aggregate, while maintaining their initial size [60]. Ostwald ripening is another destabilization process whereby smaller droplets gradually grow into larger ones due to the massive diffusion of molecules of the dispersed phase through the continuous phase [61]. Finally, coalescence describes the fusion of two or more droplets into larger droplets upon the thinning and disruption of the liquid film between droplets [62].
In the next section, we will discuss how solid particle morphology can affect the type and stability of Pickering emulsions.
《3. Morphology of solid particles 》
3. Morphology of solid particles
Solid particles type and morphology can modulate the properties of Pickering emulsions (Table 1) [29,42,49,57,63–92]. To be specific, the particles' shape governs their behavior at the interface and, consequently, their ability to stabilize the emulsion. In this section, we will describe the type of emulsions obtained using different particle morphologies and formulations.
《Table 1 》
Table 1 Particle morphology and emulsion types.

《3.1. Spherical shape 》
3.1. Spherical shape
In the last ten years, many studies on spherical particles emulsifiers have been published. Binks and Lumsdon [93] demonstrated that silica nanoparticles can form O/W or W/O emulsions as a function of the particles mixture wettability. Silica particles were used to investigate various features of oil–water emulsions, such as phase inversion [25,26], solid wettability [31,94], and emulsions’ thermal and pH responses [56,95]. Arditty et al. investigated the coalescence aspects and rheological properties [46] of emulsions stabilized by silica particles [51] in order to better understand the mechanisms underlying emulsion destabilization. Björkegren et al. [32] explored the covalent modification of silica nanoparticles harboring hydrophilic and hydrophobic groups that mimic the properties of surfactants. They showed that such nanoparticles can be used to produce emulsions with smaller droplets compared with unmodified silica, and that such emulsions are stable for up to 1.5 years. Stimulated by the emulsion stability improvement that was obtained using modified silica nanoparticles, other researchers investigated other modifications. Alison et al. [57] found that emulsions can also be stabilized by modifying silica nanoparticles using non-covalently bound chitosan oligomers (Fig. 6). Thanks to their interfacial adsorption, these particles make it possible to produce O/W emulsions with small droplets sizes (i.e., a few micrometers) by means of high-pressure homogenization. Otero et al. [63] studied how two adjacent water drops (one enriched in ethanol and the other pure water) could be stabilized in a bath of toluene, ethanol, and excess colloidal silica. They found that the presence of a bath composition gradient between the drops could induce a self-assembly process whereby new droplets were formed along the path to the ethanol-enriched drop until they made a complete bridge. They also observed self-assembled bridges between drops with different compositions.
《Fig.6》

Fig. 6. Pickering emulsion prepared with chitosan-modified silica nanoparticles. Reproduced from Ref. [57] with permission of American Chemical Society, © 2016.
TiO2 nanoparticles have also been investigated as emulsion stabilizers. Chen et al. [64] prepared organic–inorganic hollow spheres using a Pickering emulsion polymerization technique and TiO2 nanoparticles self-organize at the oil–water interface. Zhao et al. [65] prepared microspheres by O/W Pickering emulsion polymerization with modified TiO2 nanoparticles. They showed that different microsphere types were obtained as a function of the surface modification. Hollow spheres were formed when using TiO2 modified with oleic acid or sodium oleate, and solid spheres were formed with non-modified TiO2.
Other researchers have studied the behavior of emulsions stabilized by Fe2O3 particles for the preparation of novel composites. For example, Kim et al. [66] stabilized an O/W emulsion with core–shell-structured magnetic polystyrene (PS)/Fe2O3 particles that were placed at the interface of styrene droplets. In addition to PS, other polymers have been used for the same purpose. Wei et al. [67] prepared biocompatible poly(lactic-co-glycolic acid) (PLGA) microcapsules using Fe2O3 nanoparticles as O/W emulsion stabilizers. Ahn et al. [68] prepared poly(methyl methacrylate) (PMMA)/Fe2O3 magnetic composite particles synthesized by Pickering emulsion polymerization (Fig. 7) by using Fe2O3 nanoparticles (< 50 nm) as a solid stabilizer of the methyl methacrylate (MMA) monomer droplets in the aqueous phase of an O/ W emulsion.
《Fig.7》

Fig. 7. Description of the protocol to produce PMMA/Fe2O3 particles. AIBN: azobisisobutyronitrile; AIBA: 2,2'-azobis(2-methyl propionamidine) dihydrochloride; PMMA: poly (methyl methacrylate). Reproduced from Ref. [68] with the permission of American Chemical Society, © 2014.
Spherical PS latex particles with different wettabilities can be used to prepare emulsions with different oil types. To be specific, Binks and Lumsdon [69] and Nallamilli et al. [70] studied different variables (i.e., oil to water ratio, mixed particle composition, and pH), and demonstrated that the emulsion type and stability were related to the behavior of the particles dispersed in the aqueous phase before emulsification. They also found that the kind of emulsion formed (i.e., droplet diameter, stability) depended on the particle wettability.
《3.2. 2D structures》
3.2. 2D structures
Pickering emulsions can also be stabilized with 2D components, such as GO [49,96–100] and hexagonal boron nitride (h-BN) [42,101]. However, the interfacial behavior and emulsifying performance of these 2D materials are largely unexplored. Creighton et al. [71] developed a thermodynamic model to explain the Pickering emulsion stabilizing activity of ultrathin plate-like solids. They hypothesized that atomically thin plate-like solids exhibit the following unique features and behaviors (Fig. 8):
《Fig.8》
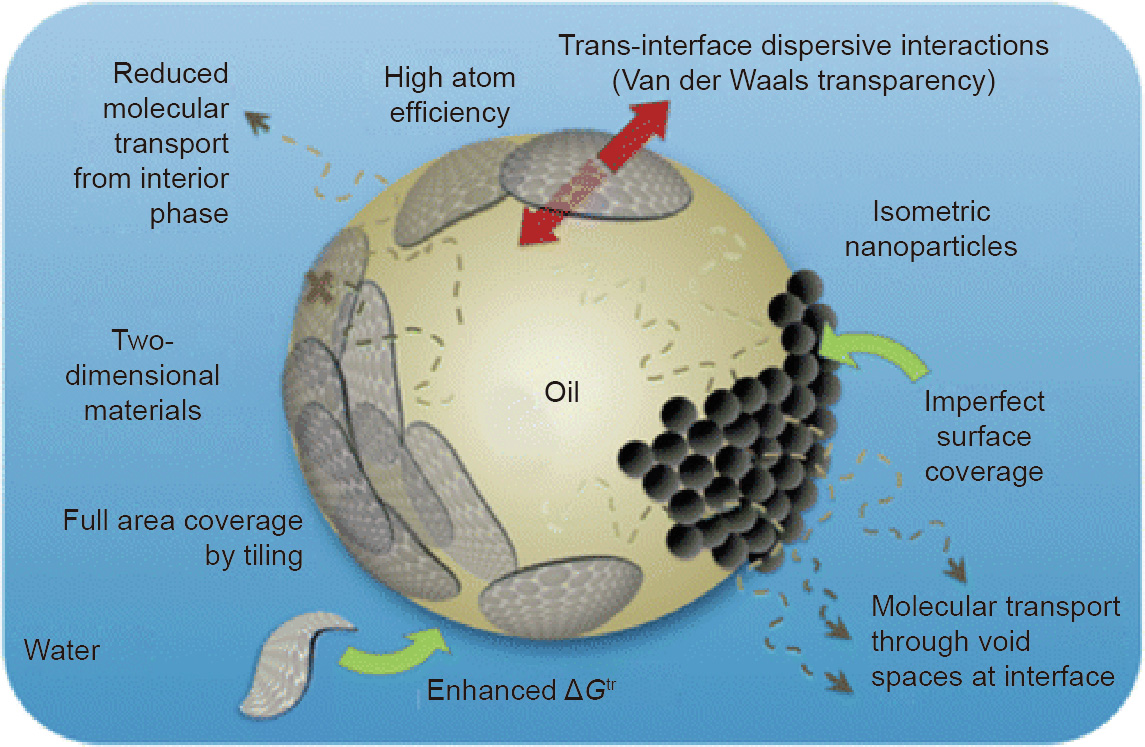
Fig. 8. Schematic overview of the features of 2D stabilizing agents. ΔGtr: transfer free energy. Reproduced from Ref. [71] with the permission of American Chemical Society, © 2014.
(1) High atom efficiency (all atoms are at the liquid–liquid interface and directly contribute to stabilization);
(2) Multilayer tiling that increases interfacial coverage and passivation;
(3) Barrier properties;
(4) Van der Waals transparency, linked to GO wetting transparency on solid substrates;
(5) Elasticity and conformation to curved interfaces;
(6) Templating for fabricating novel materials.
Creighton et al. used this model to find the most suitable material surface chemistry and geometry. They also analyzed some of the features that might be specific to 2D material-stabilized emulsions using graphene-based particles to evaluate the model predictions. They hypothesized that 2D materials can be deposited either by ① ordered layer-by-layer deposition (i.e., sequential close packing), or by ② random ballistic deposition, where each disk center point has an equal probability of residing on any surface patch.
Kim et al. [102] were the first to describe GO-stabilized emulsions. GO has an amphiphilic behavior and can adsorb onto the oil–water interface, thus lowering the surface and interfacial tension. He et al. [48] prepared Pickering emulsions stabilized exclusively with GO and assessed how their stability, type, and morphology can be modulated. They showed that the droplet size decreases when sonication time or GO concentration increases. Moreover, emulsions tended to be more stable with intermediate oil/water ratios and low pH values. Interestingly, although they produced only O/W emulsions despite using different solvents, these scholars observed some water-in-oil-in-water (W/O/W) emulsion droplets.
Other 2D materials are also good stabilizers of Pickering emulsions. For example, Gonzalez Ortiz et al. [42] found that when h-BN nanosheets (h-BNNSs) are used as stabilizers, only W/O emulsions are generated due to h-BNNS hydrophobic behavior. Moreover, the droplets size is inversely correlated with h-BNNS concentration and is not dependent on the sonication power.
Among all the known 2D materials described in the literature, only emulsions stabilized with GO and h-BN have been compared (i.e., type of emulsions, droplet size, and rheological behavior). The main difference is that GO can establish interactions through hydroxyl or carbonyl groups. Consequently, two emulsion types (direct O/W and indirect W/O) may be produced due to the different hydrophilic properties of such 2D materials. Conversely, h-BN displays an inert character [103]. Although this does not hinder its use for the production of stable emulsions, it must be functionalized to allow interactions with other groups.
Xu and Antonietti [72] exfoliated graphitic carbon nitride (g-C3N4) into few-layers nanomaterials, and found that the obtained nanosheets were able to stabilize either a W/O or O/W emulsion depending on the selected oil phase. The volume of the emulsion phase increased with increasing g-C3N4 concentration. The sizes of the hexane droplets also varied with the g-C3N4 concentration. The researchers assumed that the surface activity of the g-C3N4 depended on the pH. They noticed that the optimal emulsification was achieved in rather pure water (pH 6.4); any major pH variation led to suppression or even no formation of an emulsion. In addition, the emulsion could be reversibly broken and regenerated by sonication and shaking, respectively.
Further investigations of other 2D structures have been published. Inam et al. [73] investigated the design of water-in-water (W/W) Pickering emulsions using uniform diamond-shaped nanoplatelets with a range of different sizes (up to ~9.5 μm in length) and poly(lactide) block copolymers. Emulsion stabilization was achieved using a crystallization-driven self-assembly methodology. In addition, they managed to obtain significant control over the surface area while maintaining a single-crystal thickness. Yan et al. [74] carried out other studies based on 2D structures stabilizing Pickering emulsions. They explored the ability of Janus mesosilica (mSiO2) nanosheets with perpendicular mesochannels to be used as an interfacial catalyst for biphasic reactions. The interfacial activity of the Janus mSiO2 nanosheet was examined by testing its capacity to emulsify a toluene/water system, resulting in a well-defined W/O emulsion. The unique 2D structure showed enhanced catalytic activity in aqueous nitroarene hydrogenation reactions, which was 13 times higher than that of a conventional silica-based interfacial catalyst.
《3.3. Anisotropic particles》
3.3. Anisotropic particles
Due to the abundance of anisotropic particles, many studies have focused on the behavior of these particles at liquid–liquid interfaces, and their stabilizing effect. Demulsification behavior may be dependent on particle morphology. It is known that depending on the particle morphology, the adsorption energy at the liquid–liquid interface is different. De Folter et al. [75] used hematite particles with different morphologies (cubic or peanutshaped) to study how shape affects Pickering emulsions. These particles formed O/W emulsions that did not undergo coalescence for about one year post-preparation. At the oil–water interface, the cubes formed monolayers with an intermediate packing between hexagonal and cubic, and with an orientation parallel to one of their flat faces. Conversely, the peanut-shaped microparticles formed interdigitating stacks, and their long axes were parallel to the interface (Fig. 9).
《Fig.9》

Fig. 9. Cubic and peanut-shaped particles assembled at the oil–water interface. Reproduced from Ref. [75] with the permission of American Chemical Society, ©2014.
Hou et al. [104] also investigated cubic morphologies—in this case, in silica nanoparticles. They chose to study polyhedral oligomeric silsesquioxane (POSS), which is the smallest nanoparticle, and is a monodispersed caged-shaped silica cubic nanoparticle. When these nanoparticles are amine-functionalized, they were shown to be exceptionally interfacially active, and to form assemblies that jammed at the oil/water interface. This type of nanoparticle allows the formation of W/O Pickering emulsions. The packing density of the amine-functionalized POSS assemblies at the water/ oil interface could be tuned by changing the concentration, pH, and degree of functionalization of the POSS. Functionalized POSS showed a higher interface coverage and, hence, a lower interfacial tension, in comparison with nanoparticles surfactants formed by interactions between functionalized nanoparticles and polymeric ligands.
Yang et al. [76] investigated another kind of morphology of anisotropic particles: dumbbell-shaped bi-component mesoporous carbon–organosilica Janus particles. This morphology was obtained through the one-step compartmentalized growth of a mesoporous organosilica sphere attached to a mesoporous resorcinol–formaldehyde (RF) sphere. The researchers demonstrated that the synthetized Janus catalysts could assemble at the oil–water interface, stabilizing a W/O Pickering emulsion. The morphologies and droplets sizes were well retained after a few weeks, indicating the high stability of these Pickering emulsions.
《3.4. Other particle morphologies》
3.4. Other particle morphologies
Other particle morphologies have been tested as candidates to stabilize Pickering emulsions, including nanotubes, rods, ellipsoids, fibrous-like particles, disc-like particles, and layered double hydroxides (LDH). The different emulsions formulated with these particles are described below.
3.4.1. Carbon nanotubes
CNTs are interesting materials due to their specific features, such as electrical conductivity, elevated mechanical strength, good thermal conductivity, and chemical compatibility with biomolecules [77].
Menner et al. [78] described the preparation of porous polymer foams through the stabilization of a medium internal-phase emulsion (MIPE) using CNTs. CNT hydrophobic behavior results in a W/O emulsion. Their addition yields processing advantages and improves the mechanical and electrical features of the fabricated materials. Chen et al. [79] prepared CNT microcapsules using a Pickering emulsion method that allows the self-assembly of oxygen plasma-treated CNTs at the interface of oil droplets dispersed in water. The resulting O/W emulsions had good long-term stability. The microcapsule morphology depends on the plasma-treated CNT content.
3.4.2. Rod shape
Fujii et al. [80] synthesized hydroxyapatite (HAp) nanoparticles with a rod-shaped morphology using a wet chemical method in aqueous media. They studied the emulsion type, droplet diameter, and morphology, and found that in general, O/W emulsions are stabilized by HAp nanoparticles, and that this effect depends on the oil characteristics and the pH of the system. Capron and Cathala [81] showed that high internal-phase O/W emulsions can be stabilized for more than one year by rod-shaped cellulose nanocrystals that irreversibly adsorb at the oil–water interface. They also found that the quantity of particles added to the emulsion and irreversibly adsorbed at the interface regulate the interface area and consequently the droplet size.
3.4.3. Ellipsoid shape
Madivala et al. [82] used W/O emulsions stabilized by hydrophobic prolate ellipsoids (obtained by stretching PS latex particles) and O/W emulsions stabilized by hydrophilic spindletype hematite particles to highlight that the particles aspect ratio significantly influences emulsion stability. Li et al. [83] compared two ellipsoid-shaped cellulose nanocrystals (CNCs I and II) with different crystalline allomorphs as stabilizers for O/W Pickering emulsions. The emulsion ratio was higher, the droplet dimension was two times smaller, and the stability was better in the emulsions obtained with CNCs I compared with those prepared with CNCs II. The researchers concluded that the crystallinity of CNC allomorphs is strongly implicated in their emulsion-stabilizing activity.
3.4.4. Fibrous-like shape
Zein colloidal particles also show emulsion-stabilizing properties. De Folter et al. [84] showed that O/W emulsions are efficiently stabilized by the addition of zein particles prepared with an antisolvent precipitation method. Moreover, the particle size, charge, wetting properties, and emulsion stability were strongly influenced by the particle concentration, pH, and ionic strength. Feng and Lee [85] prepared O/W Pickering emulsions stabilized with zein colloidal particles and zein/sodium caseinate (NaCas) nanocomplexes. The addition of NaCas strongly increased the wettability in the oil phase of zein colloidal particles that are normally wetted by the aqueous phase (Fig. 10). NaCas addition (with a ratio of 10:3 or 10:4 in zein/NaCas nanocomplexes) increased Pickering emulsion stability.
《Fig.10》

Fig. 10. Simplified drawing of the interfacial arrangements in the presence of different zein/NaCas ratios. Reproduced from Ref. [85] with the permission of Elsevier Ltd., © 2015.
3.4.5. Disc-like shape
Ashby and Binks [86] found that Pickering emulsions can be stabilized by disc-like Laponite RD clay particles. By setting the phase diagram of aqueous dispersions as a function of the clay and salt (NaCl) concentrations, they demonstrated that Laponite RD particles are good stabilizers of toluene–water (O/W) emulsions, and that Ostwald ripening causes changes in the droplet size distribution over time. Bon and Colver [87] investigated the preparation of mini-emulsions stabilized with Laponite clay disks. In pure water, these disks were present as individual colloids with an overall negative charge. Upon NaCl addition, they observed a modest colloidal instability that induced flocculation of the clay disks, thus increasing their stabilizing effect on the O/W emulsions. Dinkgreve et al. [88] evaluated high internal-phase emulsions (HIPEs) stabilized by Laponite, and found that such O/W emulsions were stable to shear, after salt addition to the system. Teixeira et al. [89] studied the different mechanistic aspects of Pickering emulsions stabilized by Laponite clay with different monomer mixtures. They demonstrated that Laponite clay discs stabilize W/O emulsions and are strongly implicated in the particle formation (nucleation) stage during Pickering emulsion polymerization. The particle size progressively decreased and the nucleation time increased when higher stabilizer concentrations were used, thus expanding the particle size range. Luo et al. [90] carried out experimental and modeling studies to establish a correlation among the electrostatic energy, interface geometry, and interfacial jamming states. They tested the ability of Janus kaolinite nanoplatelets to form O/W Pickering emulsions under different conditions (temperature, ionic force, and pH). They found that the emulsions with a nonequilibrium shape were stable against changes in temperature and ionic force, while morphological transitions could be achieved effectively and repeatedly by adjusting the pH values. These researchers also proposed an electrostatic model to understand the dynamic jamming of smart jamming platelets and the reversible shape transition of droplets, which was further verified by Monte Carlo calculations.
3.4.6. Layered double hydroxides
Yang et al. [91] investigated how LDH particle concentration, salt concentration, salt concentration, and oil phase volume fraction (/o) influence several emulsion parameters (particularly type and stability). They found that LDH exhibit hydrophobic behavior and therefore are effective stabilizers of W/O emulsions, and that salts are needed for emulsion formation and stabilization. The emulsion droplet size becomes progressively bigger with higher /o values, and becomes smaller with increasing salt and particle concentrations. The emulsion is more stable against creaming when /o, salt, and particles concentrations are increased. Zhang et al. [92] analyzed how the emulsification method affects the macroscopic properties and microscopic morphology of Pickering emulsions stabilized with LDH particles. To achieve this aim, they used ultrasonication or vortexing to prepare a series of W/O emulsions with the same composition, stabilized with plate-like LDH particles that were modified with sodium dodecyl sulfate to increase their hydrophobicity. The researchers found that the emulsions obtained by ultrasonication displayed longer stability and gel-like characteristics at dispersed-phase volume fractions much lower than the random close-packing limit. Conversely, the emulsions obtained by vortexing showed some sedimentation and liquid-like behaviors.
Other types of morphology have also been briefly investigated. For example, Zhao et al. [105] prepared asymmetric nanoparticles, such as dual-mesoporous Fe3O4@mC&mSiO2. The mesoporous nanoparticles were made up of one-dimensional (1D) mesoporous SiO2 nanorods and a closely connected core–shell-structured Fe3O4@mC nanosphere. This distribution offered spatial isolation for the carbon and silica, meaning a total separation of the hydrophobic and hydrophilic domains in every single nanoparticle. An advantage of this system is that due to the spatial isolation of the carbon and silica domains, the hydrophilic/hydrophobic ratio of the obtained asymmetric dual-mesoporous Fe3O4@mC&mSiO2 Janus nanocomposites can be easily adjusted by changing the volume ratio between the two domains.
In this case, both W/O and O/ W Pickering emulsions can be prepared by adjusting the ratio between the two domains. In conclusion, in addition to wettability, particle morphology plays a role in determining the type (O/W or W/O), stability, and behavior of Pickering emulsions. Experimental studies have shown that the particle shape influences particles self-assembled structure. This finding implies that the formation of self-assembled structures of surface-active particles is regulated by particles wettability and shape [106]. Other studies have demonstrated that particle shape influences the packing and therefore the stability of emulsions, and can induce capillary interactions [82].
《4. Applications of Pickering emulsions》
4. Applications of Pickering emulsions
In the last decades, Pickering emulsions have become ideal candidates for various applications due to their many attractive features, such as stability, easy formation, droplet-size tailoring, special surface chemistry, and uniform droplet size. Table 2 lists different applications involving the use of Pickering emulsions [100,101,107–124]. We will then discuss the different particle morphologies used to formulate the active emulsions.
《Table 2》
Table 2 Pickering emulsion applications and particle morphology.
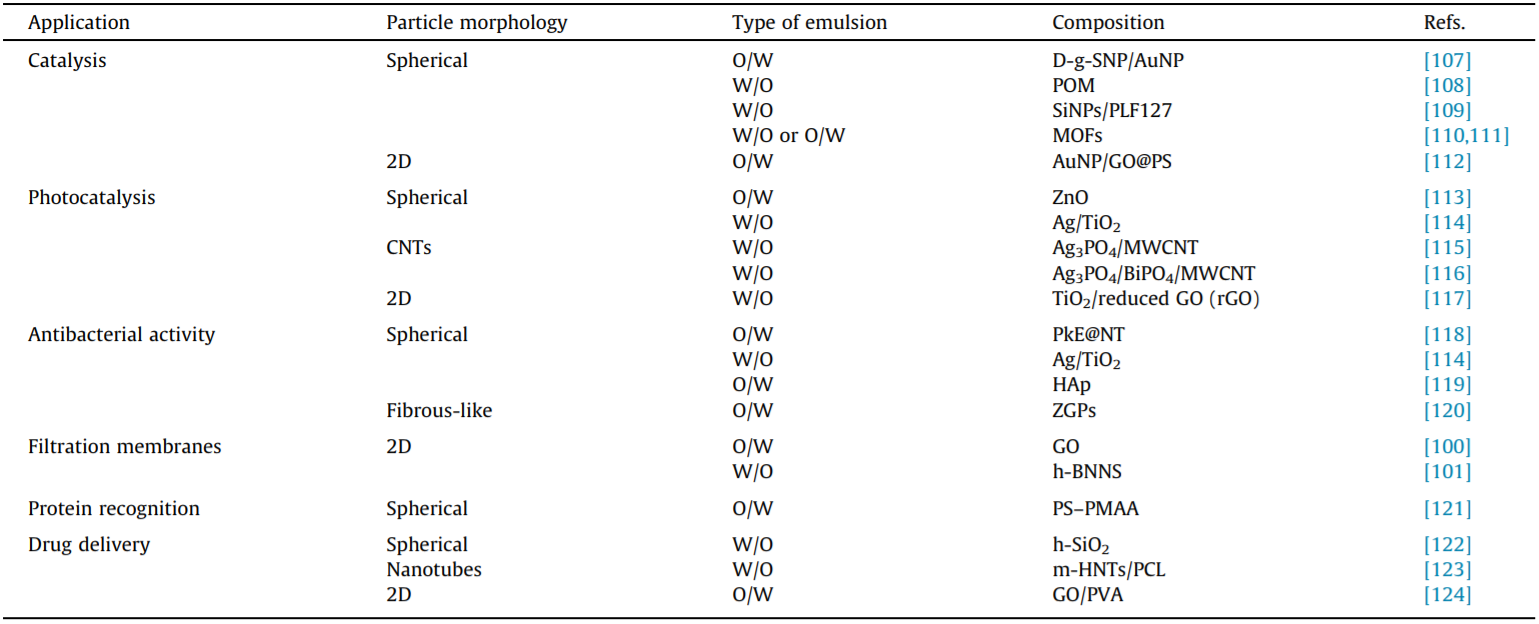
D-g-SNPs/AuNPs: starch-based nanoparticles with growth gold (Au) nanoparticles; POM: polyoxometalate nanoparticles; SiNPs/PLF127: silica nanoparticles with Pluronic F127; MOFs: metal–organic frameworks; AuNPs/GO@PS: gold nanoparticles/graphene oxide-coated polystyrene microspheres; PkE@NT: epoxy-acrylate copolymer@nanoTiO2; ZGPs: zein/gum Arabica nanoparticles; PS–PMAA: polystyrene–polymethacrylic acid; h-SiO2: hydrophobically modified silica nanoparticles; m-HNTs/PCL: poly(L-lactic acid)-modified halloysite nanotubes/poly(ε-caprolactone); GO/PVA: graphene oxide/polyvinyl alcohol.
《4.1. Pickering emulsions for catalysis》
4.1. Pickering emulsions for catalysis
Pickering emulsions are able to largely improve the catalytic efficiency of classical systems due to their larger interfacial areas. Moreover, they can be easily prepared and recycled. Qi et al. [107] employed starch-based nanoparticles/gold (Au) nanoparticles (D-g-SNPs/AuNPs) that localize at the interface to produce pH-responsive Pickering emulsions, which were then used as catalytic micro-reactors for p-nitroanisole hydrogenation at the oil– water interface. The reaction exhibited elevated catalytic activity and good recyclability. Leclercq et al. [108] prepared Pickering emulsions in the presence of water (75%), an organic solvent (25%), and solid amphiphilic catalytic polyoxometalate nanoparticles with competitive reaction rates and good yields. These reagents show good selectivity and are particularly efficient for the epoxidation of olefins. Such catalytic emulsions allow the easy separation of products and catalysts in two phases, thus combining the advantages of biphasic catalysis and heterogeneous catalysis without their limitations. In comparison with other techniques, such as silica immobilization, the preparation of this catalyst is much simpler, and the catalyst can be readily recycled after its recovery. The fabrication of micro-sized catalytic reactors using Pickering emulsions as scaffolds has been recently reported as well. Liu et al. [109] developed a method to synthesize interiorstructured mesoporous carbon microspheres (MCMs). The method consists of surfactant-assembly inside Pickering droplets. The researchers used silica particles as the emulsifier and an amphilphilic triblock polymer (Pluronic F127), which after polymerization and carbonization resulted in MCMs. Huo et al. [110] were the first to prepare O/W Pickering emulsions stabilized with metal–organic frameworks (MOFs). When a monomer is added to the system, MOFs nanoparticles act as polymerization reactors forming microcapsules composites, which can be further used as encapsulation systems. Furthermore, Xu et al. [111] used the same strategy to prepare a hollow structure that served to encapsulate species offering catalytic activity using MOFs nanoparticles-stabilized W/O Pickering emulsions. Tang et al. [112] used gold nanoparticles and GO (AuNPs/GO) in benzyl chloride to stabilize Pickering emulsions, and studied the factors affecting emulsion stability, such as the oil/water ratio, AuNPs/GO concentration, pH value, and electrolyte type and concentration. They then polymerized the O/W Pickering emulsion to produce AuNPs/GO-supported PS microspheres (AuNPs/GO@PS) that displayed acceptable catalytic activity for 4-nitrophenol reduction.
In the case of catalytic reaction in continuous flow, Zhang et al. [125] developed a conceptually new method termed flow Pickering emulsions (FPEs) to process biphasic reactions in continuous flow. This method involves the compartmentalization of bulk water into micrometer-sized droplets based on a W/O Pickering emulsion; the droplets are then packed into a column reactor. This method allows the oil phase containing the reactant molecules to continuously pass through the column in the manner of plug-type flow, while retaining the compartmentalized water-droplet integrity and the water-soluble reagent in the column reactor. The advantage of this method is that the interfacial adsorption force acting on the particles of the solid emulsifier is significantly larger than the drag force caused by the oil flow. Furthermore, the FPE method has been successfully applied in three different types of reactions, including H2SO4-catalyzed addition reactions [125]. In comparison with its conventional batch counterparts, the FPE reaction features a "greener” footprint because it avoids continuous agitation and intermittent separation of the product.
Yang et al. [91] developed a new method to prepare solid–liquid hybrid catalysts via the interfacial growth of a porous silica crust around a Pickering emulsion. This method makes it possible to obtain a high-quality solid–liquid hybrid catalyst that is suitable for continuous-flow chemical and enzymatic reactions. The researchers proposed a catalyst particle consisting of a molecular catalyst (or enzyme)-containing ionic liquids pool and a porous solid outer crust. This type of catalyst particle can be packed in industrially preferred fixed-bed reactors for continuous-flow reactions. Furthermore, the catalytic efficiency can be modulated by rationally engineering liquid–solid hybrid catalysts, which is not possible for typical homogeneous or heterogeneous catalysts.
《4.2. Pickering emulsions for photocatalysis 》
4.2. Pickering emulsions for photocatalysis
Pickering emulsions have been used as photocatalytic systems for the degradation of organic contaminants, with good results. The nanoparticles used for stabilizing O/W or W/O emulsions also typically act as active sites for the degradation of pollutants. Alternatively, they can be subjected to surface modification or just decorated with photoactive compounds.
Wu et al. [113] showed that organic contaminants in wastewater can be degraded by photocatalysis using Pickering emulsions prepared with modified zinc oxide (ZnO) nanoparticles. Wang et al. [114] prepared macro-porous silver (Ag)/TiO2 composite foams via Pickering emulsions stabilized by hydrophobic TiO2 decorated with Ag nanoparticles that display antibacterial activity and photocatalytic properties. As the obtained emulsion foams had interconnected pore channels, the Ag ions could diffuse from the foam interior to provide bactericidal activity in the water medium. Zhai et al. [115] developed a photocatalytic approach for producing a W/O Pickering emulsion in which silver phosphate (Ag3PO4) is the photocatalytic active semiconductor and MWCNTs are the hydrophobic conducting nanostructure. An analysis of the photocatalytic activity (i.e., dye degradation and oxygen evolution) of the Pickering emulsion stabilized by Ag3PO4/MWCNT showed that the system was more efficient than the traditional solutiondispersed photocatalytic systems. Mohaghegh et al. [116] demonstrated the good photocatalytic activity (i.e., Acid Blue 92 dye degradation under visible and ultraviolet (UV) light irradiation) of a system in which a p–n heterojunction Ag3PO4/BiPO4 (AB) was used as the photocatalytic active component, while MWCNTs and graphene were used as the hydrophobic conducting nanostructures and as W/O Pickering emulsion stabilizers.
Zhang et al. [117] prepared a TiO2/rGO composite using an approach in which highly exfoliated GO sheets were extended at the W/O interface; TiO2 nanoparticles were then grown on the GO sheets to ensure smooth connection between materials (Fig. 11). The close aggregation of the TiO2 nanoparticles produced mesoporous packing voids that enhanced the system’s photocatalytic degradation performance.
《Fig.11》

Fig. 11. Production of a TiO2/rGO composite using the interfacial growth method induced by the GO-stabilized Pickering emulsion. TBT: tetrabutyl titanate; TCH: tetracycline hydrochloride; rGO: reduced GO; CB: conduction band; VB: valence band. Reproduced from Ref. [117] with the permission of American Chemical Society, ©2017.
《4.3. Pickering emulsions for antibacterial activity》
4.3. Pickering emulsions for antibacterial activity
Pickering emulsions are good templates for fabricating different types of materials, such as microcapsules or porous scaffolds, with antibacterial activity.
Zhai et al. [118] developed a waterborne epoxy-acrylate copolymer@nano-TiO2 (PkE@NT) composite with antibacterial activity via Pickering emulsion polymerization. The researchers used butyl acrylate styrene and glycidyl methacrylate as the monomers and nano-TiO2 as the stabilizer adsorbed on the surface of the copolymer particles. In another study, core–shell microcapsule composites composed of a core of citronella oil (CTO) and a shell of HAp/quaternary ammonium salt of chitosan/sodium alginate were fabricated by templating CTO-in-water Pickering emulsions. The CTO-loaded microcapsules displayed bactericidal properties against Staphylococcus aureus and Escherichia coli [119].
Li et al. [120] showed that O/W Pickering emulsions fabricated using zein/gum Arabica nanoparticles (ZGPs) as stabilizers contained an oil fraction of 0.3, with 6.25% (w/v) of ZGPs. The emulsion droplets size and creaming index were significantly influenced by the ZGP concentrations and oil fractions. When thymol was loaded into the ZGP-stabilized Pickering emulsions, the growth of Escherichia coli was inhibited, and the emulsions displayed the controlled release of thymol for antibacterial activity.
《4.4. Pickering emulsions for filtration membranes》
4.4. Pickering emulsions for filtration membranes
Nagarajan et al. [100] prepared porous gelatin membranes for water filtration using an ethyl benzoate-in-gelatin (EthB-gelatin) emulsion stabilized with 3 g·L-1 GO. The membranes remained stable in water for more than 13 days with water permeability values reaching (5.8 ± 1.3) L·h-1·m-2·bar-1 (1 bar = 100 kPa). Gonzalez-Ortiz et al. [101] used emulsion templating to prepare poly(vinyl alcohol)-based porous membranes for water filtration that were stabilized with h-BNNS. Membranes with a pore size of around 1 mm exhibited water permeability values greater than 2000 L·h-1·m-2·bar-1 and a rejection efficiency of about 100%.
《4.5. Pickering emulsions for protein recognition 》
4.5. Pickering emulsions for protein recognition
Pickering emulsions have also been used for protein recognition. Xu et al. [121] fabricated a Pickering HIPE with an amphiphilic PS–PMMA block copolymer as the stabilizer. The porous materials obtained after polymerization with dopamine displayed specific imprinted surface sites with protein-recognition capacity.
《4.6. Pickering emulsions for tissue engineering and drug delivery》
4.6. Pickering emulsions for tissue engineering and drug delivery
Porous scaffolds are interesting for tissue engineering [126] because they can provide a suitable setting for cell proliferation and differentiation, and a physical support for tissue engineering. Different approaches are available for producing porous scaffold materials, such as electrospinning [127] and thermally induced phase separation (TIPS) [128]. Nevertheless, using Pickering emulsions as templates is the approach with the best potential due to their stability, easy processing, and eco-friendly properties.
Hierarchical macro-porous biocompatible (HmPB) frames were made using poly(L-lactic acid) and poly(ε-caprolactone) (PCL), the solvent evaporation of three-dimensional (3D) printed Pickering HIPEs, and hydrophobic silica nanoparticles as the stabilizer. In vivo studies assessing the release of enrofloxacin (ENR) suggested that the frames are a suitable substrate for drug delivery [122].
Porous scaffolds of poly(L-lactic acid)-modified halloysite nanotubes (m-HNTs)/PCL were prepared by direct solvent evaporation of m-HNT-stabilized W/O Pickering emulsion templates containing PCL in the oil phase. The porous microstructure could be adjusted by simply modifying the template preparation conditions (e.g., mHNT concentration and water-to-oil volume ratio). Moreover, in vitro release studies in which the ENR antibacterial drug was loaded into the scaffolds suggested that m-HNTs/PCL porous scaffolds are also suitable material for the delivery of therapeutic compounds [123]. Macro-porous composite hydrogels with a hierarchical structure were prepared by the reversible assembly of GO/polyvinyl alcohol hybrid-stabilized Pickering emulsions. After polymerization of the aqueous phase, the hydrogels were tested for the targeted delivery of doxorubicin hydrochloride, a chemotherapeutic agent used for tumor treatment [124].
Pickering emulsions are a very versatile template for the preparation of microcapsules. Jiang et al. [129] presented a method for the preparation of silica-colloidosomes with an impermeable shell that enable the long-term encapsulation of small tracer molecules. They studied the influence of Pickering emulsifiers on the structure and size of colloidosomes and their release properties.
Pickering emulsions have also been used as microcontainers or encapsulation systems. For example, Cong et al. [130] proposed a method to fabricate dual-stimuli-responsive hybrid microcontainers with the pre-programmed sequential release of two payloads in different storage spaces through the UV-initiated polymerization of Pickering emulsions. The release of the payloads could be selectively triggered by temperature or pH changes. This strategy can be extended to the fabrication of other hybrid microcontainers to respond to different stimuli, such as a magnetic field.
Xia et al. [131] designed Pickering emulsions to act as elastic adjuvants in order to address the challenge in vaccine formulations of the stimulation of humoral and cellular responses for welldefined antigens. These particle-stabilized emulsions are able to retain the force-dependent deformability and lateral mobility of antigens while displaying high biosafety and antigen-loading capabilities. Compared with solid particles and conventional surfactant-stabilized emulsions, the optimized Pickering emulsions enhance the recruitment, antigen uptake, and activation of antigen-presenting cells, potently stimulating both humoral and cellular adaptive responses. This may be a facile and effective strategy to enhance adaptive immunity against infections and diseases. Gobbo et al. [132] proposed the use of Pickering emulsions to address the challenges emerging from bottom-up synthetic biology and bioinspired engineering in the fabrication of artificial tissuelike materials. For this purpose, they synthesized two new types of thermally responsive protein–polymer nanoconjugates and used these conjugates to prepare two separate populations of reactive proteinosomes. Mixed populations of the proteinosomes were spatially confined using a W/O/W Pickering emulsion procedure, and then structurally linked in situ via an interfacial cycloaddition reaction in order to yield synthetic tissue-like spheroids upon the removal of the oil phase.
《5. Future trends in Pickering emulsions 》
5. Future trends in Pickering emulsions
In the last 20 years, a great deal of research has been focused on Pickering emulsions. This global trend has inspired scientists to use particles-stabilized emulsions to engineer functional interfaces and new materials. The particles placed at the interfaces can form well-defined structures; they can also be employed as templates for fabricating novel materials and as a reaction site for specific targeting. For example, to obtain a stable O/W emulsion, orthogonally functionalized Fe–Pt nanoparticles were first self-assembled at the oil–water interface and then cross-linked by dithiocarbamate chemistry [133].
The major challenge for the scientific community is now to further improve the emulsion stability using nanoparticles that can be used directly for the chosen application. There are still many underexplored potential organic and inorganic particles with interesting properties. For example, 2D compounds (e.g., tungsten diselenide and molybdenum disulfide) could be candidate stabilizers to prepare Pickering emulsions, due to their high atomic efficiency and barrier properties. In addition, molybdenum disulfide displays good catalytic activity and good thermal and chemical stability [134,135]. These features make them suitable particles for designing materials for different applications.
Although the stabilization mechanisms of spherical solid particles are well described, the stabilizing effects of non-spherical particles and the destabilization mechanisms are still poorly known. More research on the physical properties of non-spherical particles adsorbed at the liquid–liquid interface is needed.
Concerns about the risks and acceptability of hazardous solvents have been raised. Although a considerable amount of research has focused on the suitability of organic and bio-based particles to physically stabilize emulsions—such as in food applications—some issues need to be addressed prior to the wide industrial application of Pickering emulsions. Some bio-particles (e.g., starch particles) display better physical stability than conventional emulsifiers, which could be linked to major differences in the underlying stabilization mechanisms. However, easy and lowcost preparing methods still need to be explored.
Pickering emulsions could also be used for electrochemical applications [136,137]. The assembly of nanostructures between fluids to reduce the area in contact due to the interfacial tension between two immiscible liquids brings a significant energetic benefit. It would be possible to control heterogeneous redox reactions at the Pickering emulsion surface, such as for photocatalysis reactions. The nanoparticle surface in Pickering emulsions could have the capacity to transfer electrons from the electrode surface through an electron-hopping mechanism. Pickering emulsions could be an innovative way to produce ordered nanoparticles assembled in 3D building blocks at the interface of two liquids that cannot be mixed. Suitable nanoparticles, such as noble metal sols, are likely to show remarkable advantages for reaction monitoring or detection. Appropriate surfactants and polymers could be added as emulsifiers, to help stabilize the emulsions prepared with these nanoparticles by lowering the interfacial tension. These nanoparticle-stabilized emulsion substrates have been used in surface-enhanced Raman spectroscopy for trace detection in both single and multiple phases [138,139].
In addition, Pickering emulsions could be used for biorecognition and bioseparation by molecular imprinting. This technique traditionally uses polymeric matrices to design materials (i.e., molecularly imprinted polymers, (MIPs)) that display a strong binding affinity and high selectivity toward a targeted (bio)– molecule. Harman et al. [140] developed MIPs immobilized in cryogels for the capture and purification of hemoglobin. These researchers demonstrated that compared with traditional immobilization strategies, Pickering emulsion-formed MIPs display higher binding capacity and enhanced selectivity toward hemoglobin, as a result of the excellent accessibility of the active MIP groups.
《6. Conclusions 》
6. Conclusions
Pickering emulsions have become an important tool for the preparation of new materials. These emulsions are excellent templates for fabricating porous materials, or for direct use as a system to carry out reactions. These particle-stabilized emulsions are attractive because of their easy production, high stability, and non-toxicity, as well as the possibility of tailoring the droplet size. Moreover, the synthesis steps for obtaining materials can be reduced, and the production of these emulsions is environmentally friendly compared with materials obtained from classical surfactant-based emulsions that cause environmental pollution (e.g., in rivers or lakes).
In this review, we briefly discussed different factors that can affect the emulsion stability, such as particle concentration, wettability or oil/water volume fraction, and the destabilization mechanisms. Moreover, we described the stabilization and the type of emulsions obtained as a function of the nanoparticle morphologies, along with the current applications of Pickering emulsions.
2D nanoparticles are acquiring increasing importance in materials science and in the preparation of Pickering emulsions and related materials. These 2D structures provide more valuable features than spherical nanoparticles due to their higher atomic effi- ciency and multilayer tiling. The possibility of forming more than one layer on the liquid–liquid interface can enhance the stability of 2D emulsions in comparison with conventional emulsions. Moreover, the properties of 2D emulsions, such as thermal conductivity and mechanical strength, can enhance the material performance. More attention should be paid to Pickering emulsions stabilized by 2D nanostructures as new systems for the preparation of materials.
Given the progress that has been made in the development of Pickering emulsions, studies should now focus particularly on stabilization by non-spherical particles and on destabilization mechanisms. It is our belief that increased knowledge of Pickering emulsions production and mechanisms of action will further promote their use.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Danae Gonzalez Ortiz, Celine Pochat-Bohatier, Julien Cambedouzou, Mikhael Bechelany, and Philippe Miele declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




