《1. Introduction》
1. Introduction
Sn-2 palmitate refers to a group of structured triacylglycerols (TAGs) that are used as a human milk fat substitute. A structured TAG is defined as an oil or fat based on a natural TAG that has been modified by artificial methods [1]. Sn-2 palmitate has been developed to mimic the fat and oil content of human milk.
Human milk is regarded as the best food for infants, and fats are an important component of it. Although human milk fat (HMF) only accounts for 3%–5% of human milk, it supplies about 50% of the energy for breastfeeding infants. HMF is one of the most complex natural lipids. It contains 98%–99% TAGs, 0.26%–0.80% phospholipids, 0.25%–0.34% sterols (mainly cholesterols), and various minor components including monoacylglycerols (MAGs), diacylglycerols (DAGs), free fatty acids (FFAs), and other substances [2]. More than 400 different TAGs have been identified in HMF [3].
It is well known that the functional and nutritional properties of fats and oils are directly related to the type of TAGs they contain. A TAG molecule consists of a glycerol backbone esterified with three fatty acids (Fig. 1). Types of TAG are determined not only according to their fatty acid composition but also based on the positional distribution of fatty acids on an individual glycerol backbone.
《Fig.1》
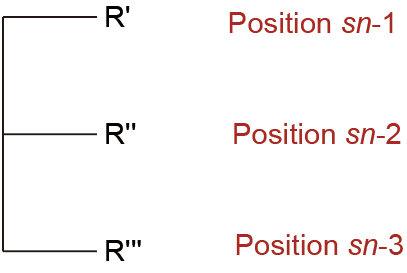
Fig. 1. A triacyl-sn-glycerol structure. R', R'', and R''' are the fatty acids acylated to the sn-1, sn-2, and sn-3 positions, respectively.
Positions on the glycerol backbone are numbered using the stereospecific numbering (sn) system, where R', R'', and R''' are the fatty acids acylated to the sn-1, sn-2, and sn-3 positions, respectively. HMF is a natural fat with a unique TAG composition, which is characterized by the distribution of fatty acids on the glycerol backbone. The three primary fatty acids of HMF are oleic acid (OA; 18:1n-9, about 33%), palmitic acid (PA; 16:0, about 24%), and linoleic acid (LA; 18:2n-6, about 15%) [4]. In human milk, most PA (about 70%) is esterified at the sn-2 position [5]. The sn-1 and sn-3 positions are mainly occupied by unsaturated fatty acids (USFAs), such as those with 18:1 and 18:2 [6,7]. Long-chain polyunsaturated fatty acids (LCPUFAs), such as eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), are mostly esterified to the sn-2 position [8]. The most abundant TAGs in HMF are 1,3- dioleoyl-2-palmitoyl-glycerol (OPO; 16%–29%) and 1-oleoyl-2-pal mitoyl-3-linoleoylglycerol (OPL; 13%–20%) [3,6,9,10].
In contrast to HMF, vegetable oils, which are generally used in infant formulas, have different fatty acid distribution. In vegetable oils, PA is predominantly (>80%) esterified to the sn-1 and sn-3 positions. Therefore, natural vegetable oils with classic processing of edible oil, physical modification (e.g., blending, fractionation), and chemical interesterification cannot achieve the same TAG structure as HMF.
The unique TAG structure in HMF plays a distinctive and important function in infant growth. The primary digestion of TAGs occurs in the stomach and small intestine, hydrolyzed by lipases. Since the stomach and pancreatic lipases are sn-1,3 regioselective, the hydrolysis of TAGs results in the formation of sn-2 MAG and FFAs [11]. The long saturated fatty acids (mainly PA) in HMF are generally absorbed as sn-2 MAG. Free PA forms insoluble soaps with calcium and magnesium, which will be lost through the feces [12–14]. Thus, modification of the TAG structure (by adding sn-2 palmitate) in infant formula fat could increase the efficiency of fatty acid absorption and reduce the symptoms caused by calcium palmitate [4,15,16].
The lipid absorption difference due to the positional variation of PA has led to interest being taken in the development and commercialization of the sources of sn-2 palmitate. Modification of oils and fats with the aim of improving their nutritional quality may take the form of changes in the fatty acyls esterified on the glycerol backbone [17]. The development of enzyme technology for lipid modification makes this possible. The sn-1,3-selective lipasecatalyzed interesterification of TAGs and acyl donors enables the modification of sn-1 and sn-3 fatty acids on the glycerol backbone to produce TAGs with specific structures. In addition to sn-2 palmitate, cocoa butter equivalents (CBE), medium/long-chain triglycerides (MLCT), and other structured TAGs are being studied.
During the last 20 years, numerous studies and patents on the lipase-catalyzed synthesis of sn-2 palmitate have been reported. Several commercial sn-2 palmitate products are now incorporated into infant formulas and follow-up formula as nutritional supplements. Researchers have published several reviews on regioselective lipases and their application to sn-2 palmitate [1,4,18–20]. However, few of these reviews have provided detailed information on TAGs analysis, which is critical to the evaluation of TAG structure. This review discusses the progress that has been made over the past 20 years on lipase-catalyzed sn-2 palmitate, with a particular focus on TAG analysis. We further provide an integrated view of the perspectives on sn-2 palmitate development in the laboratory and on an industrial scale.
《2. Lipases used in the synthesis of sn-2 palmitate》
2. Lipases used in the synthesis of sn-2 palmitate
Lipases (EC 3.1.1.3, triacylglycerol hydrolases) are one of the most-used biocatalysts in lipid modification [21]. Fats and oils are the natural substrates to lipases. Enzymatic reactions possess several recognized advantages, which include mild reaction conditions, reduced environmental pollution, and utility in the production of "natural” products. However, there is a particular need for the lipase-catalyzed synthesis of structured TAGs—namely, the opportunity for regioselective modification of TAGs [1]. Using specific lipases, lipids with a specific molecular structure can be produced, which are unobtainable by chemical interesterification methods, especially for food processing in large-scale production [1,22].
The three-dimensional structure and catalytic mechanism of lipases began to attract interest in the 1990s, and since then, lipases have been well understood as serine proteases. The serine hydroxyl group of the lipase makes a nucleophilic attack on the carbonyl carbon of the substrate, which leads to the formation of an acyl-enzyme as an intermediate [17]. As the nucleophile, the acyl-enzyme is hydrolyzed by water in an aqueous solution [1,23].
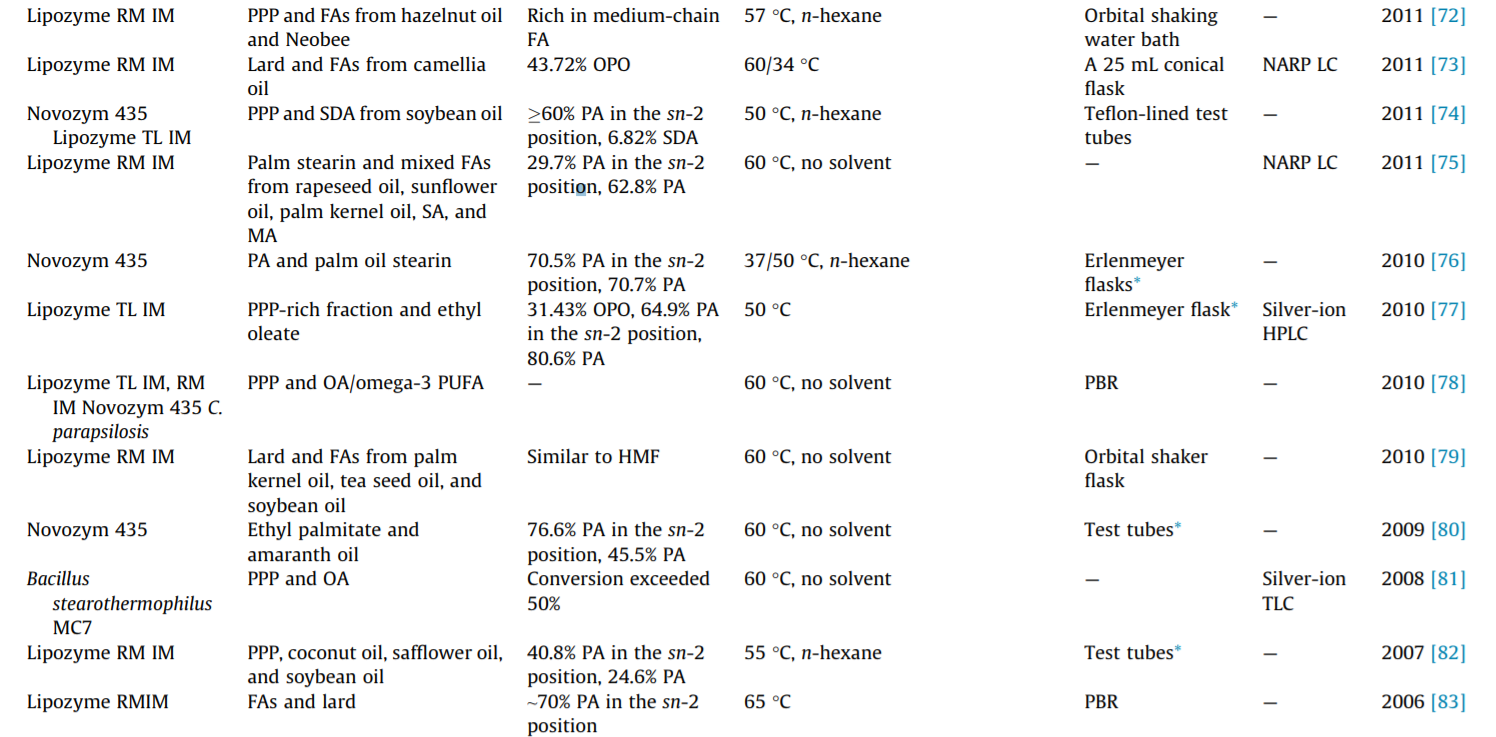
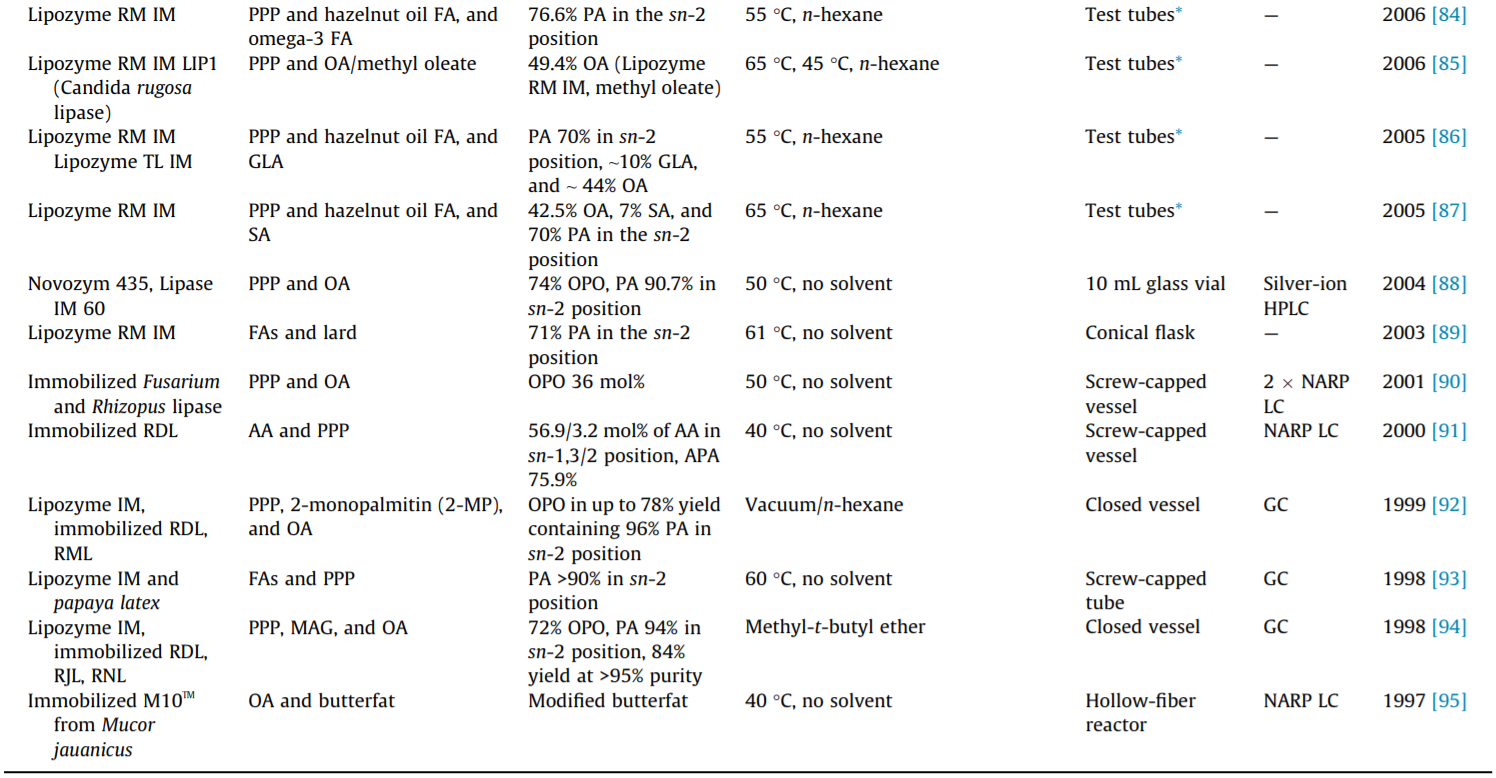
Although increasing numbers of microbial lipases have been successfully used as biocatalysts in the laboratory, only a limited number of lipases have been commercially developed. The commercialized lipases that are commonly used in the production of structured TAGs are listed in Table 1. To date, the most widely used lipases in the synthesis of sn-2 palmitate are Lipozyme TL IM and Lipozyme RM IM. More detailed information on the lipases used in sn-2 palmitate synthesis is also provided in Table 1.
《Table 1》
Table 1 Commonly used commercialized sn-1,3 regioselective lipases.

The regioselectivity of lipases is of particular importance in the lipase-catalyzed steric modification of TAGs. However, the general mechanism of lipases’ regioselectivity is still unclear. Most lipases exhibit regioselectivity to the sn-1 and sn-3 positions of the glycerol backbone and are thus known as sn-1,3-specific lipases. Lipases that have been confirmed to have sn-1,3 regioselectivity, including pancreatic lipase, pre-gastric lipase, and microbial lipases, are Penicillium camembertii, Rhizopus arrhizus, Penicillium roquefortii, Rhizomucor miehei lipase (RML), and so forth. The regioselectivity toward the glycerol backbone depends on both the substrate and the type of lipase [24,25]. The sn-1,3 regioselectivity of RML is explained by the docking of the substrate to the binding site of the lipases [26]. Only a few lipases are reported to show sn-3 regioselectivity (e.g., rabbit gastric lipase) and sn-2 regioselectivity (e.g., Candida antarctica A lipase) [27]. However, it should be noted that the regioselectivity of lipase may be altered by the reaction conditions [28]. The regioselectivity of some known lipases has been well documented in publications by Xu [1], Bornscheuer [29], and Adlercreutz [23], among others; therefore, these lipases will not be covered in detail here.
The stability of lipases (i.e., long-term process stability, reusability, tolerance of organic solvents, etc.) is another critical property due to their high cost in industrial applications. Increased stability, especially thermostability, is also beneficial for storage and shipping. An effective and frequently used method to increase lipase stability is immobilization, which causes lipases to be more stable at high reaction temperatures than free enzymes while allowing them to be reused [30]. Most studies on the lipasecatalyzed synthesis of structured TAGs involve immobilized lipases—either commercialized products or lipases that have been immobilized in the laboratory. Protein engineering by means of directed evolution is a more effective method to increase enzyme stability [31,32]. Other processing methods, such as high hydrostatic pressure [33] and modification of the hydrophobicity of the oxyanion residue [34], can stabilize and increase the activity of some lipases.
《3. Analysis of TAGs》
3. Analysis of TAGs
The composition of TAGs in natural fats and oils is usually given as a list of the fatty acids present (i.e., the fatty acid amount and fatty acid composition) [2]. There has been increasing interest in TAG composition (i.e., fatty acids distribution on the glycerol backbone) due to the remarkable influence it has on lipid digestion [11], metabolism [35], and improving overall health. However, the analysis of TAGs is challenging work. TAGs are extremely complex mixtures containing glycerol molecules linked to three of the same or different fatty acids [36]. Nevertheless, studies on TAG analysis have significantly increased in recent decades [36], mainly due to improvements in state-of-the art analysis technologies—especially mass spectrometry (MS). When investigating sn-2 palmitate, TAG analysis is extremely important, because the different position distribution of the products will have a direct influence on their functional and nutritional properties.
The difference in the positional distribution of the fatty acids of the glycerol backbonewas first demonstrated bymeans of enzymatic (mainly pancreatic lipase) hydrolysis, which can separate the fatty acids at the sn-2 position (regioselective analysis). The results reflect the fatty acid composition on the sn-1,3 and sn-2 positions, which cannot yield information on the TAG structure.However, thismethod is still used in some studies on sn-2 palmitate synthesis, due to its less expensive and simple calculation process. This method is often used at the cost of real information on TAG composition.
The most widely used and powerful methods are chromatographic methods, such as gas chromatography (GC) and liquid chromatography (LC), which are sometimes combined with MS [37]. The use of chromatographic methods in TAG profiling has been described in a number of excellent books and reviews, such as the review on chromatographic techniques by Buchgraber et al. [38], the review on thin-layer chromatography (TLC) by Fuchs et al. [39], the review on high-temperature GC by Ruiz-Samblás et al. [40], the review by Indelicato et al. [36], and the book by Christie et al. [41]. The present review summarizes the analysis methods that are generally used—especially in the study of structured TAG synthesis—with an emphasis on the quantitative determination of positional isomers.
The generally used TAG analysis methods for structured TAGs are high-temperature GC, non-aqueous reversed-phase LC/evaporative light-scattering detector (NARP LC-ELSD), and silver-ionLC-ELSD. High-temperature GC is GC in which the capillary columns are maintained at high temperatures (>350℃). TAGs are separated based on their degree of unsaturation. However, a commonly used detector—the flame ionization detector—cannot provide information about TAG isomers. Thus, the identification of TAGs is based on the retention times compared with standards. For the analysis of structured TAGs, this method is generally combined with the analysis of sn-2-position fatty acids composition.
The LC that is widely used in TAG analysis can be divided into two kinds, depending on the relative polarity of the two chromatographic phases; namely, normal-phase LC (NP-LC) and nonaqueous reversed-phase LC (NARP LC). NARP LC uses a gradient elution and various mobile-phase systems [36]. The elution order depends on both the carbon numbers (CN) and the double bonds (DB), which depend on the effective carbon number (ECN): CN–2 × DB. Under optimized chromatographic conditions, TAGs with the same ECN can be separated [42–44]. The sample preparation is simple, and the equipment is relatively cheap. Therefore, this method is the most widely used [42,43,45–95]. However, this method cannot separate TAG-region isomers.
The most-used NP-LC in TAG analysis is silver-ion normal-phase LC. Silver-ion LC can be applied for the separation of TAG isomers due to the weak complexes that form between silver ions of the stationary phase and the π electrons of double bonds [96]. The separated TAGs are divided into groups that differ in DB number. Using this method, the exact content of each TAG isomer can be measured [44,45].
Another method that involves high-resolution 13C nuclear magnetic resonance (NMR) can provide information on the fatty acid composition attached to the TAG and at the specific sn-2 position of the TAG, which can be used for the regioselective analysis of the fatty acids attached on the TAG [44]. Recently reported LC methods, including ultra-high-performance liquid chromatography equipped with quadrupole time-of-flight MS [3], twodimensional GC [97], and silver-ion atmospheric pressure chemical ionization (APCI) MS [98], can effectively separate the TAGs of the complex lipids matrix in natural fats and oils, as well as perform stereospecific analysis of TAGs, and should therefore be applied to structured TAG analysis in the future.
《4. Reaction schemes》
4. Reaction schemes
This review summarizes the studies that have been published on the lipase-synthesis of sn-2 palmitate between 1997 and 2018, as shown in Table 2. In general, there are three reaction schemes: acidolysis, transesterification, and alcoholysis. The acidolysis reaction is the most common method, followed by the transesterification reaction.
《Table 2》
Table 2 A 25-year literature survey of the lipase-catalysis of sn-2 palmitate.



《4.1. Acidolysis reaction》
4.1. Acidolysis reaction
The typical scheme for an sn-1,3 regioselective lipase-catalyzed acidolysis reaction is shown in Fig. 2. The acidolysis reaction is generally carried out by TAGs rich in PA at the sn-2 position with an FFA or FFA mixture by the use of sn-1,3 regioselective lipases. Due to its high purity, tripalmitoylglycerol (PPP) is generally used as the substrate in the laboratory; however, since its price is high, cheap and natural oils (e.g., palm stearin, palm oil, butterfat, lard, etc.) are often considered as substitutes for PPP. FFA sources are usually OA, LA, gamma-linolenic acid (GLA), or an FFA mixture of vegetable oils (e.g., soybean oil, rapeseed oil, sunflower oil, palm kernel oil, coconut oil, hazelnut oil, etc.), fish oil, or single-cell oils rich in LCPUFAs.
《Fig.2》

Fig. 2. Scheme for the sn-1,3 regioselective lipase-catalyzed acidolysis reaction.
As shown in Table 2, the acidolysis reaction was used in most of the studies over the past two decades, in which the contents of PA in the sn-2 position usually reached over 60%. Esteban et al. [72] used several sn-1,3 regioselective lipases (including lipase DF, Lipozyme RM IM, Palatse 20000L, Lipozyme TL IM, and lipase QLC) to catalyze the acidolysis reaction of PA-enriched TAGs with OA. The results showed that lipase DF could achieve relatively high incorporation of OA (50.4%) with a short reaction time (1 h) to maintain a high PA content in the sn-2 position (68.6%). After the optimization of various factors, a final structured TAG containing 67.2% OA and 67.8% PA at the sn-1,3 and sn-2 positions, respectively, was obtained.
Recently, our group also carried out the synthesis of sn-2 palmitate. Wei et al. [59] synthesized high-purity sn-OPO catalyzed by different sn-1,3 regioselective lipases (Lipozyme RM IM and Lipozyme TL IM) in both solvent (n-hexane) and solvent-free systems with a high purity of PPP and high OA, prepared from camellia seed oil. Lipozyme RM IM was found to be suitable for n-hexane, and Lipozyme TL IM was found to be suitable for solvent-free systems. The relative contents of PA in the sn-2 position reached 92.92% and 86.62%, while the contents of sn-OPO were 32.34% and 40.23%, respectively. Zou et al. [48] produced sn-2 palmitate from basa catfish oil and sesame oil fatty acids using a Lipozyme RM IMcatalyzed acidolysis reaction. The enzymatic product contained a 67.7% distribution proportion of sn-2 PA out of the total PA under optimal conditions. This approach is characterized by simple steps and fewer byproducts. The main byproduct of the acidolysis reaction is FFA, which can be effectively eliminated by molecular distillation. However, due to the presence of acyl migration, the fatty acid at the sn-2 position migrates to the sn-1,3 position, which affects the yield of the target structured TAGs. Therefore, the yield of pure structured TAGs obtained by the acidolysis reaction is relatively lower than that obtained by the alcoholysis reaction.
《4.2. Transesterification reaction》
4.2. Transesterification reaction
The transesterification reaction is performed with TAGs rich in PA in the sn-2 position with FFA esters or oils, using sn-1,3 regioselective lipases. Fig. 3 shows the scheme for the sn-1,3 regioselective lipase-catalyzed transesterification reaction. The FFA esters mainly include ethyl and methyl esters, while natural oils rich in OA/PUFA are usually used as the acyl donors. In this process, the selected materials are generally cheap and widely distributed, making it quite popular for the industrial production of structured TAGs. However, the final product is a mixture of different TAGs with similar physical properties, which makes it difficult to purify and obtain the structured TAGs. Therefore, it is essential to choose the appropriate kinds and proportions of oils.
《Fig.3》
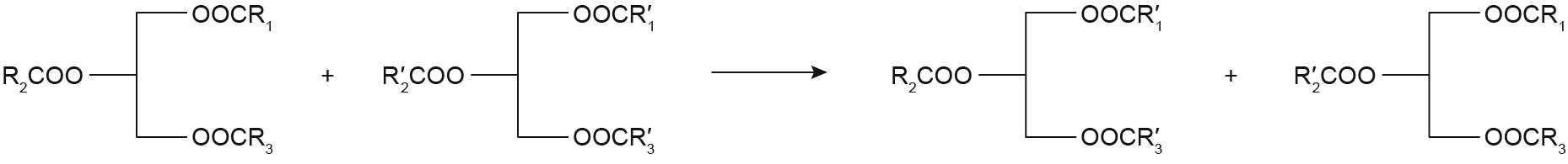
Fig. 3. Scheme for the sn-1,3 regioselective lipase-catalyzed transesterification reaction.
With the aim of low cost, some studies selected lard as the substrate [47,70]. Zou et al. [99] produced sn-2 palmitate by means of the Lipozyme RM IM-catalyzed transesterification of lard with a vegetable oil mixture in a packed-bed reactor. The vegetable oil was a blend of high oleic acid oils (e.g., sunflower oil and canola oil), microbial source oils (e.g., algal oil and microbial oil), palm kernel oil, and palm oil. The final product contained 39.2% PA in the sn-2 position and showed high degrees of similarity in its fatty acid profile with HMF. Srivastava et al. [85] synthesized structured TAGs by the transesterification of PPP with either OA or OA methyl ester using LIP1 or Lipozyme RM IM as the biocatalyst. The results showed that higher proportion incorporation of OA was observed with the OA methyl ester (transesterification) than with OA (acidolysis) in both lipases and that Lipozyme RM IM might be more suitable than LIP1 for preparing sn-2 palmitate.
《4.3. Alcoholysis and esterification reaction》
4.3. Alcoholysis and esterification reaction
To overcome the drawbacks of the reaction schemes described above, and to obtain a higher yield of structured TAGs, the alcoholysis reaction has been proposed. The scheme for the sn-1,3 regioselective lipase-catalyzed alcoholysis reaction is shown in Fig. 4. This method is a two-step process, and sn-1,3 regioselective lipases are needed for both reactions. First, natural oil or fat is selected to react with alcohols, resulting in the formation of 2-MAG rich in PA. Second, the purified 2-MAG is esterified with FFA to obtain a higher yield of the target structured TAGs. Schmid et al. [94] produced sn-OPO by means of the alcoholysis reaction of PPP; in the first step, these researchers investigated the influence of different lipases (Lipozyme RM IM, Rhizopus delemar, and Rhizopus javanicus) on the yield and purity of 2-MAG enriched in PA. The results showed that Rhizopus delemar immobilized on celite gave the best yield, which was 95% 2-MAG after crystallization in methyl-t-butyl ether.
《Fig.4》
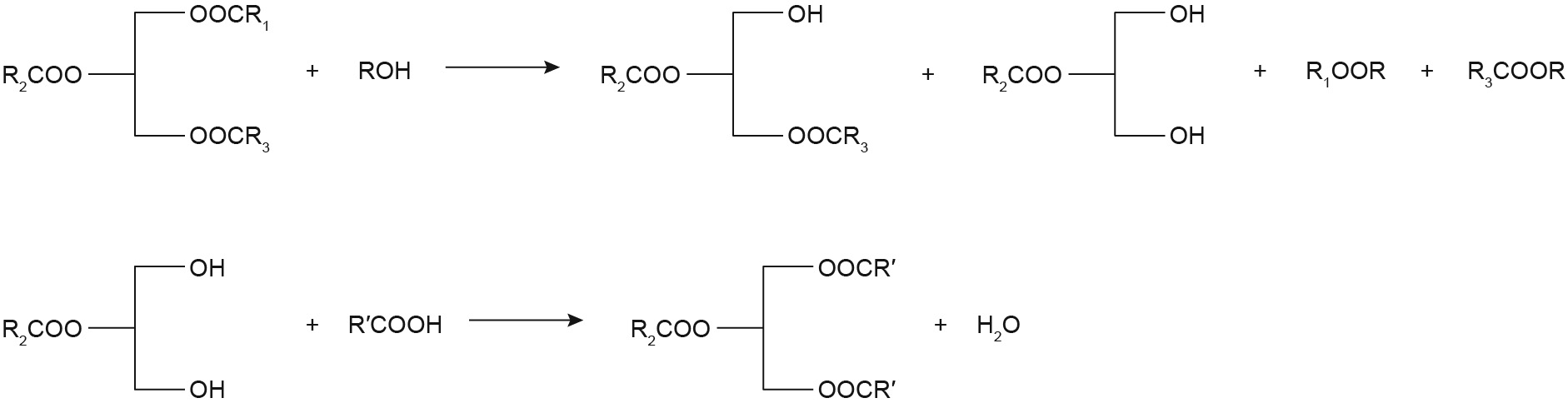
Fig. 4. Scheme for the sn-1,3 regioselective lipase-catalyzed alcoholysis reaction
In the second step, the purified 2-MAG was esterified with OA in n-hexane using Lipozyme RM IM or Rhizopus delemar immobilized on celite. The final product contained 92%–94% PA in the sn-2 position and 83%–89% OA in the sn-1,3 positions, while the yield of sn-OPO reached 70%–72%. As shown in Table 2, the alcoholysis reaction has been used in only a few studies, in which an sn-OPO content greater than 70% was achieved, and in which the PA contents in the sn-2 position usually reached greater than 90%. This process avoids acyl migration and obtains pure structured TAGs. However, the complexity of the steps leads to an increase in cost. Therefore, it is not commonly used in industrial production.
《5. Marketed products 》
5. Marketed products
Structured TAGs are designed to have desired nutritional, textural, or physicochemical properties for multipurpose applications in food products and in products for special medical purposes. Many studies have focused on the commercialization of structured TAGs. Today, commercialized products of sn-2 palmitate have been manufactured, and are commonly designed to mimic the composition and distribution of HMF. These products have been practically applied to infant formulas as a nutrient fortifier.
The production of sn-2 palmitate was first developed by Loders Croklaan [100]. In 1995, the product was approved as an infant formula ingredient in Europe and began to be produced and commercialized as Betapol®. Betapol® is produced by the acidolysis of PPPrich fats (palm stearin) with OA (FFAs obtained from high oleic sunflower oil) using immobilized sn-1,3 regioselectivity lipase (Rhizomucor miehei). The production is a two-stage process in two packed-bed reactors filled with the enzyme in order to increase the fatty acid conversion [101]. The TAG molecular weight distribution of Betapol® is mainly composed of an ECN:DB of 52:2 (33.4 mol%) and an acyl carbon number (ACN):DB of 52:3 (10.5 mol %), the main regio-isomers of which are sn-OPO (82.2 mol%) and sn-OPL/LPO (82.0 mol%), respectively [102].
At present, there are several commercial versions of sn-2 palmitates, such as INFAT®, which is manufactured by Advanced Lipids (Sweden, Karlshamn). INFAT® contains 70%–75% PA esterified on the sn-2 position of the glycerol backbone. In the Chinese market, sn-2 palmitate products are being manufactured by Wilmar International (Singapore) and Zhejiang Beijia Bio-Technology (Hangzhou, China), among others. Other commercial sn-2 palmitates include Bonamil (Wyeth Ayerst), Alsoy (Nestlé), and Cow & Gate Premium (Nutricia). For more information, readers are referred to the book chapters by Ferreira et al. [101] and Happe et al. [103].
《6. Conclusions》
6. Conclusions
In the past two decades, researchers have developed several structured TAGs using lipase catalyst technologies, such as CBEs, sn-2 palmitate, sn-BOB (where B refers to behenic and O refers to oleic acids), and more [1]. Among these, sn-2 palmitate is a successfully produced structured TAG that has been added to infant formula as a nutritional supplement by many manufacturers.
In addition to PA, it is necessary to study and modify the minor fatty acids to achieve a similar positional distribution to that of HMF. Some LCPUFAs (e.g., arachidonic acid (AA) and EPA) are reported to be mainly attached to the sn-1,3 position, while a group of minor fatty acids, such as the branched fatty acids, have shown much higher content (up to 60%) at the sn-2 position [104]. A better understanding of the composition and structure of HMF will provide detailed information for the investigation of novel structured TAGs.
Lipase applications for the production of structured TAGs will have a broad market. Although lipases have obvious advantages compared with chemical catalysts for structured TAG synthesis, the industrial application has been relatively slow, mainly due to the high cost. Screening for cheaper sn-1,3 regioselectivity lipases and methods for improving their stability will be promising technologies for future research.
《Acknowledgements》
Acknowledgements
This work was financially supported by a National Natural Science Foundation of China grant (31701558), the Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), the Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B90719028), and the national first-class discipline program of Food Science and Technology (JUFSTR20180202).
《Conflict of interest statement 》
Conflict of interest statement
Wei Wei, Cong Sun, Xiaosan Wang, Qingzhe Jin, Xuebing Xu, Casimir C. Akoh, and Xingguo Wang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




