In November 2019, one of the first approved human trials of the gene-editing technology "CRISPR” reported dramatically positive results in each of the two initial patients given a single round of therapy [1]. Both patients suffered from a painful, life-threatening, inherited blood disease and had received an infusion of billions of their own hematopoietic stem cells. These precursor blood cells had been altered with CRISPR to produce disease-free blood cells.
One patient with transfusion-dependent beta thalassemia, who required blood transfusions more than once a month to survive had not needed any transfusions for nine months since the treatment. The other patient, a woman in her 30s with severe sickle-cell anemia stricken by attacks of the misshapen red blood cells clogging her blood vessels an average of seven times per year, had stopped having attacks altogether in the four months since her infusion.
"To be able to take this new technology and give those people a chance for a new life, which it really would be, is a dream come true,” the director of the US National Institutes of Health Francis Collins said in an interview at the time the results were announced [2].
《1. A breakthrough tool》
1. A breakthrough tool
Renowned worldwide as a breakthrough tool for altering the genomes of nearly all living creatures, CRISPR makes gene-editing easier, more precise, quicker, cheaper, and more versatile than prior gene-manipulation techniques (Fig. 1) [3]. Since CRISPR first burst onto the world stage in 2012, the global news media, many scientists and engineers involved in its discovery and abundant applications, plus droves of other commentators, have told the world to expect marvels from the technology, particularly improvements in human health.
《Fig. 1》

Fig. 1. In the late 1990s, the sheep Dolly, her preserved body shown here in a museum in Edinburgh, Scotland, caused a sensation and controversy as a demonstration of cutting-edge applied biology. Today, way beyond simple cloning, CRISPR provides a powerful genetic engineering tool that can be used to create an enhanced Dolly—or human being—raising profound ethical concerns. Credit: Wikimedia Commons (CC BY 2.0).
The acronym CRISPR refers to ancient genetic-code sequences in bacteria that biologists first puzzled upon in the late 1980s. It stands for ‘‘clustered, regularly interspaced, short, palindromic repeats.” Although cryptic to someone not familiar with molecular genetics, the phrase neatly sums up a pattern of genetic coding in bacterial chromosomes that, after years of study, was found to comprise snippets of genetic codes of viruses that had previously attacked the cell [4].
Viruses reproduce by injecting their genetic material—either ribonucleic acid (RNA) or deoxyribonucleic acid (DNA)—into the cells they invade [5]. Instead of manufacturing the cell’s own components, an infected cell’s internal machinery then replicates the attacker’s proteins and genome. With many viruses, those viral subunits self-assemble into so many new viral particles that the host cell bursts, spilling out viruses to infect more cells and hosts.
Not far from the CRISPR regions on bacterial genomes reside genes for proteins that have evolved to patrol a bacterium’s interior. Like explosive-sniffing hounds with powerful jaws, these molecular complexes, Cas (CRISPR associated systems) proteins coupled to viral snippets copied from a CRISPR chain, check genetic strands they encounter for matching enemy virus sequences [6]. If the complex finds a match, it dispatches the hostile genome by cleaving it into inactive pieces. By the mid-2000s, molecular biologists realized that networks of CRISPR/Cas agents served as a rudimentary, adaptive immune system in bacteria, and they began deconstructing and tinkering with the elaborate choreography of this bacterial defense system.
By 2012, some of these CRISPR/Cas agents, particularly those involving the then little-studied Cas9 protein had dazzled researchers with the ability, targeted by their guide RNAs, to home in on exact locations along DNA. From then onward, researchers recognized that they could engineer such constructs to target precise locations in a genome [7]. By cutting DNA strands at pre-selected, exact positions, CRISPR/Cas9 complexes could disable or activate individual genes, as well as correct and precisely modify and regulate their function.
《2. Engine of innovation》
2. Engine of innovation
As an engine of innovation, CRISPR has not disappointed. The improved understanding of molecular biology gleaned in discovering the CRISPR/Cas machinery and the types of experiments and manipulations the technology enables now drive how research is performed throughout a broad swath of the life sciences and related industries [8].
Scientists now can "study organisms at a level that was never possible in the past,” said biochemist Jennifer Doudna, a CRISPR pioneer who heads the Innovative Genomics Institute of the University of California, an academic partnership of the statewide university system’s San Francisco and Berkeley (where the institute is located) campuses. Pre-CRISPR, "if you wanted to ask a question about the genetics of life and understand the genetics of a pathway, you typically had to work in model organisms,” she recalled in an October 2019 public lecture [9]. "But with CRISPR it suddenly became possible to alter the DNA of essentially any organism.”
The technology has spawned a global tidal wave of scientific and bioengineering insights, technical papers and applications, a host of start-up companies, and new divisions of established scientific, biotechnology, and biomedical firms. The kinds of novel organisms and advances that CRISPR has produced in agriculture, industry, energy, materials, and other fields include, to mention just a few: algae for making new biofuels richer in combustible fat [10], a beer-brewing process that uses less water because flavors come from yeasts engineered by CRISPR to include mint and basil genes rather than water-guzzling hops [11], woolier sheep [12], and white button mushrooms that stay white thanks to gene edits that stop them from browning with age [13].
"This is a really powerful technology. It is a whole toolbox that scientists now have for controlling genetics,” said Doudna. "We are living right in the midst of this transformative technology that is going to change our world—and it will change it in the very near future.” In human health, where these transformations are perhaps most eagerly awaited, the technology’s potential has now begun to be tested.
Anticipation runs particularly high among millions of people around the planet with inherited illnesses (Fig. 2), like beta thalassemia and sickle-cell anemia [14], as well as cystic fibrosis, spinal muscular atrophy [14], and thousands of other conditions that result from one or just a few coding errors in a gene or genes. CRISPR may also offer some hope for those suffering from cancers or other physical or mental illnesses governed by a much larger set of defective, interacting genes, first by helping to tease out the roles of contributing genes, and then perhaps by reducing a malady’s severity or its chances of affecting a particular individual.
《Fig. 2》
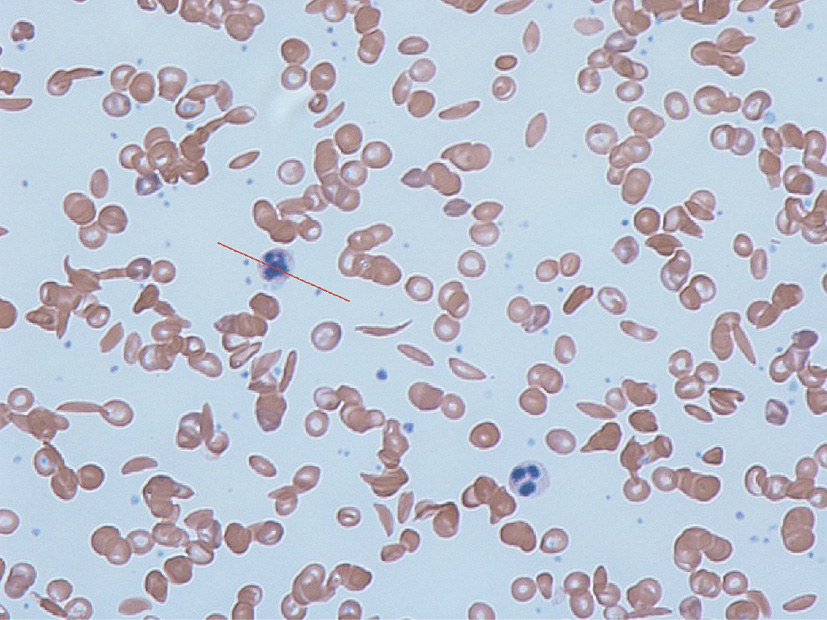
Fig. 2. Sickle-cell disease is a congenital blood disorder characterized by red blood cells that assume an abnormal, rigid, sickle shape, as shown in this blood smear. With initially promising results, clinical trials currently in progress are using CRISPR to manipulate genes in—and potentially cure—patients with congenital disorders like sickle cell. Credit: Graham Beards (Wikimedia Commons, CC BY-SA 3.0).
The technology is, however, not without known risks. Its editing machinery can sometimes alter an "off-target” gene, which can itself trigger cancer or cellular malfunctions. In addition, when introduced into patients, the molecular complexes that do the precise cutting and pasting of genetic code can trigger potentially dangerous immune system responses [3].
《3. First approved human trials》
3. First approved human trials
Shedding some light on safety, one of the early human trials of a CRISPR-editing-based therapy, which took place in China and was reported last fall in The New England Journal of Medicine [15], focused on human immunodeficiency virus (HIV)-infected subjects who also had a type of leukemia for which they were to receive bone-marrow transplants. Hoping for a "double” cure, the researchers planned to use CRISPR to introduce a gene known to cause HIV immunity into the donated bone marrow cells prior to their implantation in patients. However, because the trial was ultimately reduced to only one patient whose HIV infection did not improve, it provided only a welcome, but tentative, safety check. The procedure led to no apparent adverse consequences over 19 months during which the patient was monitored and their leukemia remained in remission [15]. Other efforts to use CRISPR specifically against cancer, including in three patients at the University of Pennsylvania in Philadelphia, also appear to have been well tolerated [2].
The results of further attempts to apply CRISPR in human health in trials that began last year were eagerly expected in 2020. In one study, the biotech firms Editas Medicine (Cambridge, United Kingdom) and Allergan (Parsippany-Troy Hills, NJ, USA) aimed to correct an inherited form of blindness called Leber congenital amaurosis 10. Among CRISPR human trials to date, this one stands out because the procedure injects its biomolecular repair machinery directly into patients’ eyes, first at a modest dose to test safety, then potentially at higher doses to evaluate efficacy. All other CRISPR trials have so far carried out the gene editing in harvested or donated cells outside the body, where they can be checked for off-target changes before being introduced into patients’ bodies [16].
However, due to the onset of the coronavirus disease 2019 (COVID-19) pandemic, some CRISPR-related clinical trials—and many others [17]—have stalled. At the end of March, CRISPR Therapeutics (Zug, Switzerland) reported that it and its partner firm Vertex Pharmaceuticals (Boston, MA, USA) had halted further dosing of subjects in their clinical trial, a follow up study to the earlier one reporting encouraging results in the two patients with beta thalassemia and sickle-cell anemia. A lack of beds and other hospital resources due to the pandemic prompted the suspension, according to a filing by the Swiss biotech firm with the US Securities and Exchange Commission on 31 March 2020 [18]. Closure of the company’s offices and a 20% decline in its stock price during the month of March increased uncertainty about when the trial might continue.
《4. Pivot to COVID-19》
4. Pivot to COVID-19
With at least one human trial now on hold, bioengineers have pivoted to the new challenge of applying CRISPR against the pandemic. A couple of notable examples include a group investigating whether CRISPR could provide a biological shield against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Fig. 3), and another that is leveraging CRISPR to improve COVID-19 diagnostic testing.
《Fig. 3》

Fig. 3. As it has with molecular biology research globally, CRISPR is expediting basic science investigation related to SARS-CoV-2, the COVID-19 coronavirus, shown here in a medical illustration, as well as the development of novel antivirals and rapid diagnostic tests. HE: hemagglutinin esterase. Credit: Scientific Animations (Wikimedia Commons, CC BY-SA 4.0).
In 2018, the US Defense Advanced Research Projects Agency contracted with the Stanford University laboratories led by assistant professor of bioengineering L. Stanley Qi [19] and professor of pediatrics David Lewis to marshal CRISPR’s natural virus-fighting weaponry against emerging and potentially catastrophic new influenza strains in humans. As the coronavirus outbreak rapidly expanded, the team ‘‘quickly pivoted and applied our approach to COVID-19,” said staff scientist Marie La Russa, who manages the group’s work on this project.
The researchers are developing a novel virucide that depends on CRISPR, but not its vaunted gene-editing capabilities. Nicknamed PAC-MAN by Qi (for prophylactic antiviral CRISPR in human cells), the molecular countermeasure is designed to target and destroy the genomes of virus particles and prevent them from replicating in people who have been exposed. The experiments might ultimately lead to "the first use of CRISPR as a clinically effective antiviral,” La Russa said.
Lacking laboratory strains of SARS-CoV-2 to work with at the time of their initial experiments, Qi, La Russa, and colleagues simulated SARS-CoV-2 infections by fabricating RNA copies of regions of the virus’s genome and using them to "infect” tissue cultures of human lung epithelial cells. To test the strategy’s protective potential, they treated the cells with Cas13d coupled with fabricated RNA sequences from the SARS-CoV-2 genome as guide molecules. As reported in the 29 April issue of Cell, the targeted counterattacks reduced replication of viral components by 49% to 90% [20]. Several other groups are also investigating a similar strategy [21,22].
The Stanford group has moved on to testing its approach against live SARS-CoV-2 infection in a Level 3 biohazard facility and to identifying an appropriate method for the delivery of the CRISPR/ Cas13d agents safely into upper and lower respiratory tracts of animals. The ultimate goal is to translate CRISPR/Cas13d delivery to the respiratory tract of humans as a means to prevent or treat viral respiratory infections, including those caused by coronaviruses or influenza viruses. Lewis, a medical doctor and viral immunology researcher, said that the project could potentially reach human trials by the spring of 2021, although two years to reach this goal is more likely.
Other teams of US-based CRISPR experts had been exploring using RNA-cleaving Cas proteins to create rapid and potentially low-cost diagnostic tests that are highly sensitive and specific for infectious pathogens, cancer DNA, and other targets of interest [23]. In early May, the US Food and Drug Administration granted the Cambridge, MA, USA, company Sherlock Biosciences an emergency use authorization for the high-volume version of its test designed for hospital laboratory use, the first such authorization issued for a CRISPR-based diagnostic test for COVID-19. Behind this effort is a group of Boston-based academic researchers at Massachusetts Institute of Technology, Harvard University, and associated institutes who formed the company to commercialize the technology.
Applying this work to COVID-19 has shown that their CRISPR system can generate a fluorescent signal when it detects SARSCoV-2. According to the company, the test produces results in about an hour and can detect as few as 100 viral particles on a swab or in saliva [24]. The company also claims that the device performed perfectly in 2000 tests, producing no false-negative or false-positive results. The company is also working to apply the technology in a simple pregnancy test-like device for rapid, point-of-care diagnosis of COVID-19.
《5. Ethical concerns》
5. Ethical concerns
In the long run, how the power of CRISPR is applied to human health and well-being, especially as an enabler of genetic engineering, is a focus of growing concern. In November 2018 it was discovered that a small research team in China had quietly used CRISPR to modify human embryos—a pair of twins and a third child from a different family—and then re-implanted them in their mothers, who later gave birth to the three children [25].
As this work demonstrated to much uproar, CRISPR provides the ability to, with relative ease, alter and control inherited traits by editing embryos or adult reproductive cells like eggs to produce genetic modifications that can be passed on to offspring. Because of this, many see the technology as an ethically- and morally-fraught threat to the human lineage. Germline edits such as those encoded in the ‘‘CRISPR babies” might carry unknown health risks that could also pass to their descendants. Taken to the extreme, however, critics warn that CRISPR could facilitate eugenics whereby children could be custom-engineered to have— or lack—specific traits.
In December 2019, China’s state news agency Xinhua reported that, following a criminal trial for illegal medical practice, a court had sentenced the three members of the team that had altered the embryos to jail terms and fines [25]. Jiankui He, a former associate professor at the Southern University of Science and Technology in Shenzhen and the team’s leader, received the stiffest sentence: three years in prison and a three million CNY fine (~425 000 USD). The court also banned the researchers for life from participating in humanassisted reproductive technology services and other activities.
CRISPR had already added urgency to discussions and debates by scientists, engineers, ethicists, religious leaders, policy makers, and others in conferences, scholarly articles, and institutional reports about the rights, wrongs, risks, and benefits of human germline editing. But, until November 2018, this dialogue took place against a backdrop of general agreement—more formally recognized in some countries than others [26]—that such editing should not be performed—if ever—until deep and thorough vetting of the matter had occurred globally and possibly an international body with authority over the matter was established.
Prompted by the 2018 incident, the World Health Organization set up a new committee to revisit the issue while the US National Academies of Sciences and Medicine teamed up with the UK Royal Society to establish an international commission to do the same [27]. Reports from those bodies are expected to appear mid2020; a spokesperson for the US National Academies said in early April that the release of its commission’s report was unlikely before June. Meanwhile, on 3 March, three ethics councils in France, Germany, and the United Kingdom issued a joint statement regarding their position about heritable genome editing in humans [28], which is prohibited by law in those three countries and most others in Europe, as well as in the Republic of Korea, Australia, Canada, Mexico, and Brazil [26].













 京公网安备 11010502051620号
京公网安备 11010502051620号




