Pentazole, the last discovered structural unit of the azole series of compounds, has received considerable attention from researchers [1,2]. The pentacyclic structure that is formed by the highenergy N–N and N=N bonds between the five nitrogen atoms in pentazole possesses aromaticity, which provides good thermal stability [3]. Consequently, most metallic and non-metallic salts synthesized from pentazole have decomposition temperatures exceeding 100 °C. Moreover, pentazole is the only all-nitrogen structure other than N2 and N3– that is stable at room temperature [4–7]. In contrast, the five-nitrogen-atom chain structure of N5+ , which was first synthesized in 1999 [6,8], has a decomposition temperature below 0 °C owing to a lack of aromaticity (Fig. 1) [6,7].
《Fig. 1》
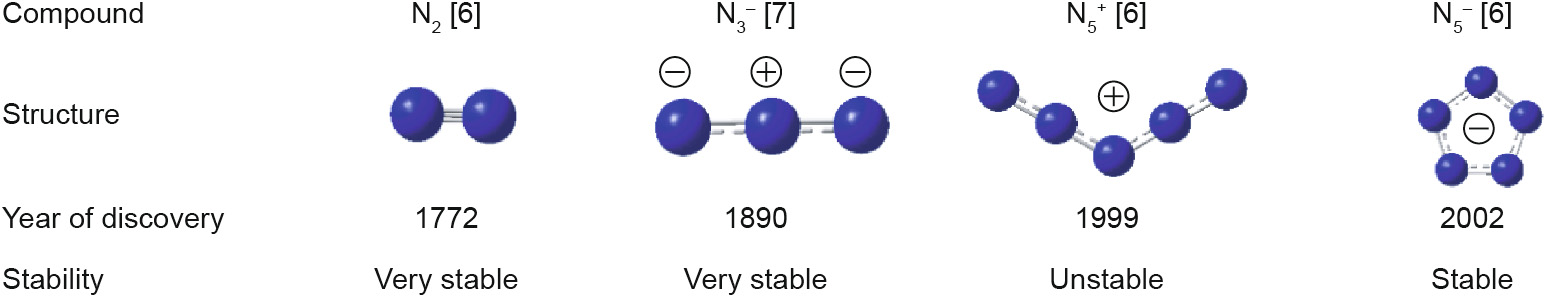
Fig. 1. Stability and year of discovery of all-nitrogen structures.
Azoles are widely applied in the synthesis of energetic materials. In particular, five-membered heterocycles, such as pyrazole, imidazole, triazole, and tetrazole, are commonly used as the backbone of energetic compounds, which enables tens of thousands of energetic materials with vastly different performances to be synthesized via modifications of energetic groups on the backbone. As the ultimate member of the azole series, pentazole is also expected to play a key role in the synthesis of novel energetic materials. To date, room-temperature-stable pentazolate compounds that have already been synthesized include metallic [9– 12] and non-metallic pentazolates [13–16], coordination polymers [17], and porous metal–inorganic frameworks (MIFs) [18–20] (Fig. 2).
《Fig. 2》

Fig. 2. Many pentazolate compounds have been synthesized.
However, in contrast to other pentacycles, functional group (e.g., –NO2, –NNO2, –NH2, and –N=N–)-substituted pentazolate compounds are yet to be synthesized. This may be due to the inherent instability of the ring structure of pentazole. Under certain strongly acidic or alkaline conditions, gradual ring opening occurs, which results in the decomposition of pentazole into N2 and N3–. Although this makes functional group modifications to the pentazole ring structure difficult, the synthesis of pentazolate compounds should not be considered impossible, and it is expected that functional-group-modified pentazolate compounds will be the key direction in pentazole chemistry.
In the field of energetic materials, various metallic and nonmetallic pentazolates have been synthesized. At certain temperatures, even minute amounts of metallic pentazolates can undergo thermal explosions, which clearly demonstrates the energetic characteristics of metallic pentazolates. Calculations have shown that the bond energy released by the pentazolate anion is 46.4 kJ·g-1 , which is 86.3% higher than that of N3– (24.9 kJ·g-1 ) [21]. Compared with non-metallic pentazolates, metallic pentazolates have higher densities. In particular, water-containing metallic pentazolates have a relatively low density, while heavy metal (e.g., silver, barium) pentazolates have a higher density because metal ions result in a substantial increase in density. However, owing to the small contribution of heavy metal ions to the energy level and the fact that certain metallic salts are hydrates, which leads to the absorption of thermal energy during the explosive reaction process, high-density heavy metal pentazolates do not possess higher energy than non-metallic pentazolates. Nevertheless, the energy of the pentazolate anion is still significantly higher than that of azide, which is another all-nitrogen anion. For example, the energies released by sodium azide and anhydrous sodium pentazolate are 16.1 and 34.9 kJ·g-1 , which means that the chemical energy has been theoretically increased by 116.8% with the pentazolate anion. Metallic azides are commonly used as primary explosives, with lead azide [Pb(N3)2] being the most typical primary explosive used in various applications [22,23]. Compared with [Pb(N3)2], anhydrous metallic pentazolates have a higher energy and do not contain environmentally hazardous heavy metals such as lead, indicating the prospect for wide application in the field of green, non-toxic, high-energy primary explosives (Table 1).
《Table 1》
Table 1 Physical and energetic performance of AgN5, KN5, and [Na8(N5)8(H2O)3]n compared with Pb(N3)2.
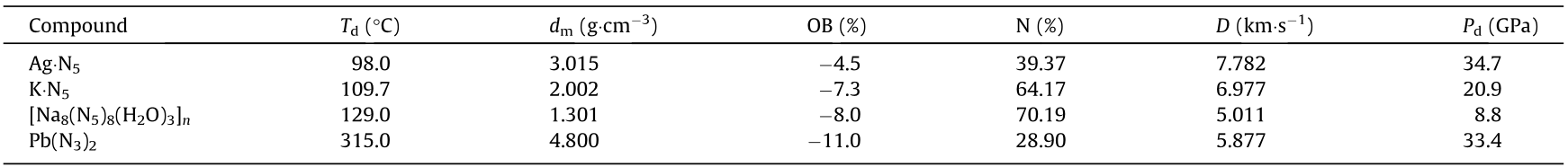
Td: decomposition temperature; dm: density; OB: CO oxygen balance, an index of the deficiency or excess of oxygen in a compound required to convert all C into CO and all H into H2O; N: nitrogen content; D: detonation velocity; Pd: detonation pressure. For a compound with the molecular formula of CaHbNcOd (without crystal water), OB = 1600[(d – a – b/2)/MW] (MW: molecular weight of salt).
Non-metallic pentazolates are inherently energetic materials, and more than ten non-metallic energetic salts based on pentazole have been synthesized. Their density and heat of formation were first calculated by Gaussian 09 software (Gaussian, Inc.; USA); the data was then imported into EXPLO5TM software (OZM Research s.r.o; Croatia) to obtain their detonation performances. As shown in Table 2 [24], the data indicate that these pentazolate compounds have low densities but higher heats of formation, resulting in good detonation performances. However, in general, there is no qualitative leap in detonation performance compared with traditional C– H–O–N energetic materials, which may be attributed to the low density of the pentazole ring structure. Nonetheless, experts have predicted that all-nitrogen materials with no other elements involved will be used as a new generation of ultra-high-energy energetic materials, which has led to attempts over several years by researchers of energetic materials to synthesize these target compounds. However, to implement the synthesis of a polynitrogen compound, lower temperature or high-pressure assembling studies in both experimental and theoretical aspects are required. Thus, no room-temperature-stable all-nitrogen compounds have been synthesized to date. Among the successfully synthesized non-metallic pentazolates, ammonium pentazolate (N2H5N5) has a nitrogen content of 95%, making it close to being all-nitrogen. In addition, hydroxylamine pentazolate (NH3OHN5), which has an N + O content greater than 96%, has a relatively high density due to the presence of oxygen atoms, which contributes to the good performance of the compound. At present, hydrazine pentazolate and hydroxylamine pentazolate are regarded as the nonmetallic pentazolates with the best performance and application prospects.
《Table 2》
Table 2 Physicochemical properties of NH4N5, N2H5, NH3OHN5, RDX, HMX, and CL-20.

RDX: 1,3,5-trinitro-1,3,5-triazacyco-hexane; HMX: 1,3,5,7-tetranitro-1,3,5,7-tetrazocane; CL-20: hexanitrohexaazaisowurtzitane;  : molar enthalpy of the formation of salt (kJ·mol-1 );
: molar enthalpy of the formation of salt (kJ·mol-1 ); 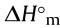 : enthalpy of the formation of ionic salts per gram (kJ·g-1 ); D: detonation velocity, where values after slashes are from Ref. [24]; Pd: detonation pressure, where values after slashes are from Ref. [24].
: enthalpy of the formation of ionic salts per gram (kJ·g-1 ); D: detonation velocity, where values after slashes are from Ref. [24]; Pd: detonation pressure, where values after slashes are from Ref. [24].
Calculated data has shown that ammonium pentazolate and hydrazine pentazolate possess good detonation performances approximately equivalent to that of hexanitrohexaazaisowurtzitane (CL-20), which has the best detonation characteristics according to current measured data. Yang et al. [24] and Christe et al. [25] from Nanjing University of Science and Technology and the University of Southern California has even reported a calculated detonation velocity of more than 10 km·s-1 (N2H5N5), which exceeds the detonation velocities of all synthesizable energetic materials. In addition, based on our theoretical calculations, the theoretical specific impulse achieved by the application of hydrazine pentazolate and hydroxylamine pentazolate as energetic components in the formulation of certain solid propellants may be more than 5 s higher than that of solid propellants containing CL-20, which possesses the highest energy among the currently available energetic materials. This data is of substantial significance because it may lead to new-generation solid propellant formulations that are superior to the CL-20-based formulation, and it also demonstrates that pentazolate energetic compounds have the potential to become new-generation energetic materials that surpass CL-20 in detonation performance.
It is notable that the data that has been obtained thus far mainly originates from theoretical calculations; it is expected that the true detonation performance of non-metallic pentazolates will be revealed through experiments and actual test data in the near future. At present, issues in the formulation of pentazolate compounds limit the feasibility of performance testing. The keys to resolving these issues are the optimization of synthesis processes and the scaling-up of preparation processes, which are the focal points of our current research efforts. At present, pentazolates are mainly synthesized by the oxidative cleavage of the aromatic pentazole group via chemical methods, which requires the use of the reducing agent ferrous bisglycinate (Gly-Fe) and the oxidizing agent meta-chloroperbenzoic acid (m-CPBA). Because a considerable amount of m-CPBA is used during the synthesis process, a few hundred grams of m-CPBA are required for the synthesis of merely 1 g of sodium pentazolate. Large amounts of unreacted oxidizing agent will remain in solution after the synthesis reaction has been completed, which may pose certain dangers during the experiment. Therefore, it is imperative to optimize the synthesis processes, reduce the required amount of m-CPBA, and enhance the yield of sodium pentazolate from the oxidative cleavage of the aromatic pentazole group. Once the yield has been effectively enhanced, the synthesis processes can then be de-bottlenecked for the mass production of pentazolate compounds, which will provide assurance for both applied and basic research on pentazolates, including actual energy measurements.
In conclusion, cyclo-N5– is a novel all-nitrogen group with broad prospects for further development. However, because the energy levels of pentazolate compounds are purely theoretical values obtained from calculations, actual energy measurements are first required in order to achieve a comprehensive understanding of the true performance of such compounds. Moreover, because cyclo-N5– has low density, greater enhancement of the detonation performance of energetic materials can only be achieved by the assembly of cyclo-N5– with all-nitrogen cations to obtain carbonfree and hydrogen-free compounds. The discovery of the cyclo-N5– anion provides a foundation for the formulation of all-nitrogen materials and further motivates the pursuit of all-nitrogen materials by researchers in the field of energetic materials.
《Acknowledgements》
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21805138 and 21975127) and the Natural Science Foundation of Jiangsu Province (BK20191291).














 京公网安备 11010502051620号
京公网安备 11010502051620号




