A recent clinical study by Chen et al. [1] reported that the transplantation of allogeneic menstrual-blood-derived mesenchymal stem cells (MSCs) significantly reduced the mortality of influenza A (H7N9)-virus-induced acute respiratory distress syndrome (ARDS) without harmful side effects. This work may simultaneously provide a new insight into the treatment of ARDS caused by coronavirus disease 2019 (COVID-19).
ARDS is characterized by progressive arterial hypoxemia and dyspnea—a severe form of acute lung injury (ALI)—and may even cause multi-organ failure [2]. Effective therapies for ARDS have proven to be clinically challenging because no specific pharmacotherapies have been identified for lung-protective ventilation [3]. Multiple studies have shown that fulminant pneumonia and ARDS can be induced by various viral infections, such as severe acute respiratory syndrome coronavirus (SARS-CoV) [4], Middle East respiratory syndrome coronavirus (MERS-CoV) [5], and H7N9 virus [6].
Most H7N9 patients primarily had viral pneumonia, and some progressed to ARDS [7]. ARDS, lung failure, and fulminant pneumonia are major lung diseases, and H7N9 virus causes extrapulmonary diseases through cytokine storms [8]. COVID-19 is caused by SARS-CoV-2, which displays high similarity to SARSCoV. An early report on 24 January 2020 found that ARDS was present in 29% of COVID-19 patients, requiring intensive care unit (ICU) admission and oxygen therapy, and that respiratory failure from ARDS was the leading cause of mortality [9]. Virally triggered acute pro-inflammatory cytokines (interferon (IFN)-a, IFN-c, interleukin (IL)-1b, IL-6, IL-12, etc.) released by immune effector cells can result in pulmonary edema, dysfunction of air exchange, and ARDS, similar to the cytokines involved in H7N9 infection [9–11]. Thus, similar complications (e.g., ARDS and lung failure) and corresponding multi-organ dysfunction with lung inflammatory lesions and structural damage are shared by H7N9 and COVID-19. Early identification and early management of COVID-19 patients might lower the occurrence of ARDS and the corresponding mortality; however, the incidence rate of ARDS in severe patients is still high and their prognosis is poor [12]. This finding is in line with a prior study demonstrating that the quality of life of survivors with ARDS induced by H7N9 infection was worse than that of those without ARDS [6]. Hence, it is urgent to develop effective therapies for ARDS.
MSCs are key players in stem-cell-based therapeutics in regenerative medicine and immunoregulation [13]. They can be isolated from a multitude of sources, such as bone marrow, adipose tissues, fetal tissues, menstrual blood, and most types of mesenchymal tissues [14]. MSCs are pluripotential non-hematopoietic cells, which are capable of differentiating into various cell types, including myocytes, osteocytes, and adipocytes [15]. Due to the safe, non-immunogenic characterization, and great therapeutic potential of MSCs, their transplantation has been widely studied as innovative drugs to treat multiple pathologies including ALI/ARDS. MSCs can reduce the production of pro-inflammatory cytokines in ARDS patients [16], and facilitate lung tissue regeneration and repair by releasing a large amount of paracrine soluble factors, such as vascular endothelial growth factor (VEGF) and transforming growth factor-b1 (TGF-b1) [17]. MSCs can also preserve the vascular endothelial[18] and alveolar epithelial barrier function [19] in ARDS animal models. In addition, MSCs can enhance alveolar fluid clearance and increase the phagocytic activity of host immune cells to improve antimicrobial effects [20]. The general potential therapeutic mechanisms of MSCs in the treatment of ARDS are shown in Fig. 1. It has been demonstrated thatMSCs have considerable advantages over other immunosuppressive agents (i.e., monoclonal antibody-based drugs, which have a high cost, or glucocorticoid drugs, which carry a high risk of side effects) for ARDS therapy in clinical practice. These advantages include availability and ease of harvesting, multi-lineal differentiation potential, and safety with no possibility of malignancy [21–23].
《Fig. 1》
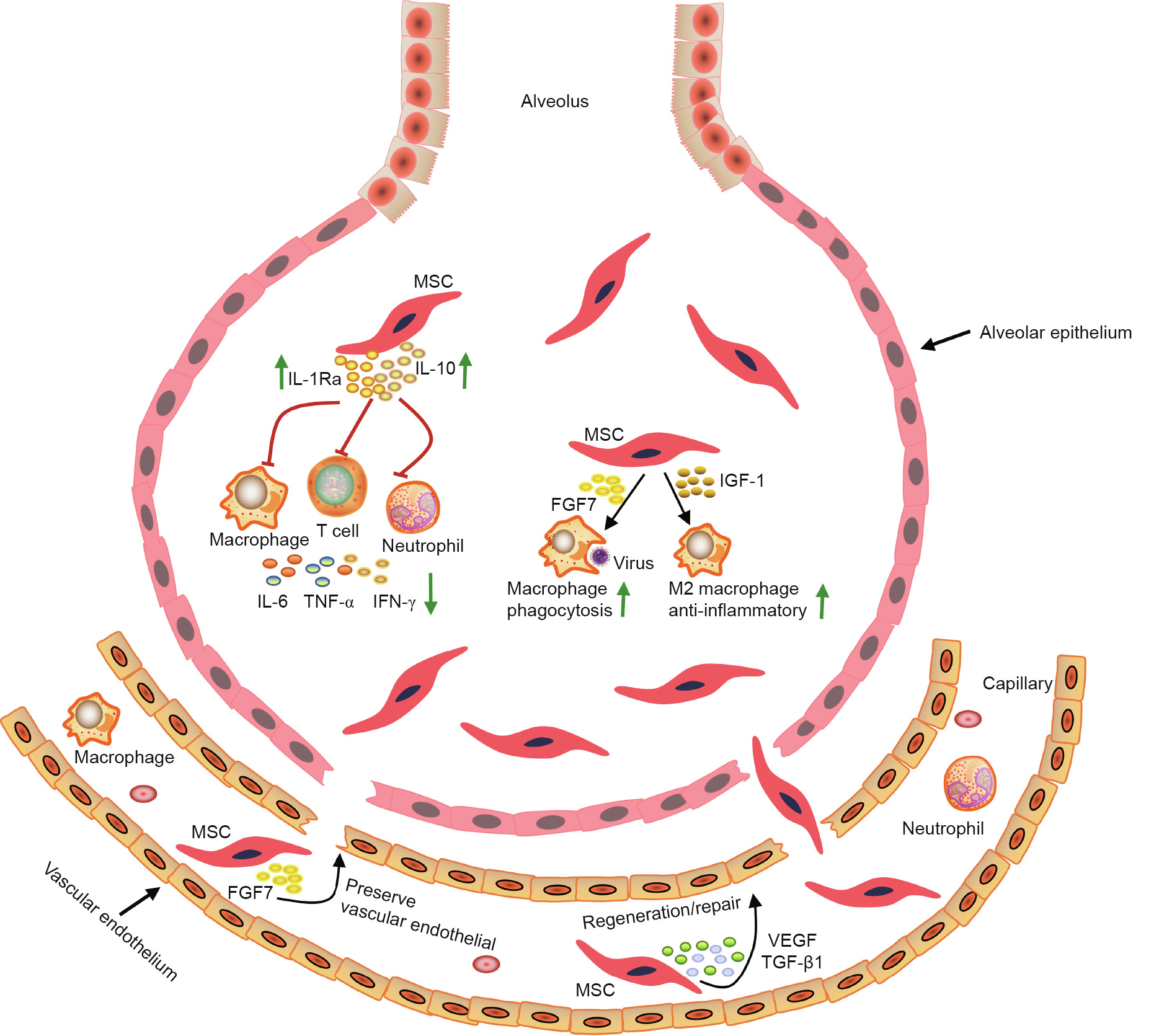
Fig. 1. Potential mechanisms involved in MSC therapy for ARDS. MSCs exert therapeutic effects for the treatment of ARDS through different mechanisms. MSCs can secrete a number of factors including IL-10, IL-1 receptor antagonist (Ra), fibroblast growth factor 7 (FGF7), insulin-like growth factor 1 (IGF-1), VEGF, and TGF-β1, reduce the production of pro-inflammatory cytokines including IL-6, tumor necrosis factor (TNF)-α, and IFN-γ, and improve the activity of macrophages. Once recruited and/or entrapped in the local microcirculation and/or pulmonary vasculature, activating factors released from local cells (both endothelium and locally recruited inflammatory cells) are able to stimulate MSCs to release various paracrine mediators, enhancing their ability to regenerate and repair damaged lung tissue. M2 macrophage: alternatively activated macrophage.
Chen et al. [1] proposed a new strategy to treat H7N9-induced ARDS using MSC transplantation. The major lung diseases found in H7N9 patients include ARDS, lung failure, and fulminant pneumonia. In the work of Chen et al., the mortality of the MSC transplantation group was remarkably reduced compared with the control group (17.6% vs 54.5%), and no infusion-related toxicities or seriously adverse events were found in these moderate-tosevere H7N9-induced ARDS patients. During the five years of follow-up, computed tomography checks showed that the radiological changes, including linear fibrosis, bronchiectasia, and isolated areas of pleural thickening, were improved by MSC transplantation. Furthermore, MSC infusion showed no harmful effects in patients during long-term follow-up. This is the first meaningful report demonstrating both the short- and long-term effectiveness of MSC transplantation to treat ARDS caused by virus infection.
Recently, another report on MSC transplantation for the treatment of patients with COVID-19 pneumonia showed that MSC transplantation is safe and effective, especially for patients in a severe condition; however, this treatment requires long-term observation to establish its clinical efficacy [24]. Due to the similarity of ARDS caused by H7N9 and that caused by SARS-CoV-2, the work of Chen et al. [1] may provide the most promising option for the treatment of ARDS caused by SARS-CoV-2. However, further investigation is needed on how MSCs affect the host and how host microenvironments affect MSC function. MSCs will be a promising therapeutic strategy for the treatment of ARDS caused by virus infection in future.













 京公网安备 11010502051620号
京公网安备 11010502051620号




