《1. Introduction》
1. Introduction
Coronavirus disease 2019 (COVID-19) is an emergency public health incident happening in nearly 190 countries and regions and affecting more than 1.2 million patients worldwide, with 72 614 deaths as of 7 April 2020 [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) causes symptoms in the majority of cases, the most common being fever, cough, shortness of breath, fatigue, and muscle pain [2]. High-resolution computed tomography (CT) allows accurate evaluation of lung lesions, thus enabling us to better understand the pathogenesis of the disease [3]. Acute respiratory distress syndrome (ARDS), respiratory failure, sepsis, acute cardiac injury, and heart failure have been the most common critical complications during the exacerbation of COVID-19 [4].
A growing body of evidence suggests the notable impact of comorbidities of chronic diseases on the clinical outcomes in patients with COVID-19. According to coronavirus reports from the Centers for Disease Control and Prevention of the US Department of Health and Human Services, patients with type 2 diabetes mellitus and metabolic syndrome may have up to 10 times greater risk of death when they get COVID-19 [5]. In 1590 laboratory-confirmed hospitalized patients from 575 hospitals, 399 (25.1%) reported having at least one comorbidity [6]. The most prevalent comorbidity of COVID-19 was hypertension (16.9%), followed by diabetes (8.2%). This report has drawn considerable attention to on COVID-19 coexisting disorders patients. The presence of coexisting illness was found to be more common among severe patients [7]. COVID-19 patients have underlying risk factors associated with mortality, including male gender, advanced age, and the presence of comorbidities including hypertension, diabetes mellitus, cardiovascular diseases, and cerebrovascular diseases [8]. The major comorbidities in fatality cases include hypertension, diabetes, coronary heart disease, cerebral infarction, and chronic bronchitis [9]. It is notable that diabetes has been demonstrated as a potential risk factor in close association with mortality [10].
COVID-19 places a huge burden on healthcare facilities, especially regarding patients with comorbidities. The intensive care was required for approximately 20% of polymorbid COVID-19 patients, and hospitalization was associated with a case fatality rate (CFR) greater than 13% [11]. Special attention and efforts to protect or reduce transmission should be applied in susceptible populations including children, healthcare providers, and elderly people [12]. Given the higher mortality and higher proportion of critically ill adult COVID-19 patients with diabetes, good in-patient glycemic control is particularly important in the comprehensive treatment of COVID-19. Individualized blood glucose target goals and treatment strategies should be made according to the specific circumstances of COVID-19 patients with diabetes [13,14].
In our recent meta-analysis, which included 1936 COVID-19 patients in nine studies, COVID-19 was significantly correlated with several metabolic diseases, indicating that hypertension, diabetes, and coronary heart disease may exert a profound effect on the progression of COVID-19 [15]. The adverse effects of glucose and lipid metabolism disorders on the immune system make patients more vulnerable to various infections. However, more evidence for the worsening of COVID-19 patients with diabetes compared with non-diabetes is required for these conclusions to be consolidated.
To elucidate the risk and severity of comorbidity in diabetes patients with COVID-19, a retrospective study was carried out to investigate the clinical features, radiographic and laboratory tests, complications, treatments, and clinical outcomes in COVID-19 patients with or without diabetes.
《2. Methods》
2. Methods
《2.1. Study design and data sources》
2.1. Study design and data sources
We included 208 hospitalized patients (≥ 45 years old) with laboratory-confirmed COVID-19 from the Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine between 12 January and 25 March 2020. COVID-19 was diagnosed on the basis of the World Health Organization (WHO)’s interim guidance. A confirmed case of COVID-19 was defined as a positive result on high-throughput sequencing or real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swab specimens. A total of 96 diabetic and 112 non-diabetic patients were randomly selected.
Information on recent exposure history, clinical symptoms or signs, and laboratory findings on admission was extracted from electronic medical records. We determined the presence of radiologic abnormality on the basis of the documentation or description in medical charts. A major disagreement between two reviewers was resolved by consultation with a third reviewer. Laboratory assessments consisted of the following: a complete blood count; blood glucose and lipids; coagulation testing; assessment of liver and renal function; measures of electrolytes, C-reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase (LDH), and creatine kinase; blood gas analysis; and detection of inflammation markers.
We divided the cases into two groups: a group for patients with COVID-19 and type 2 diabetes, based on the Guidelines for the prevention and control of type 2 diabetes in China (2017 edition) [16]; and a group for COVID-19 patients without diabetes. All medical data were processed by a team of experienced clinicians, who reviewed and abstracted the data. Data were entered into a computerized database and checked. If core data were missing, requests for clarification were sent to the coordinators, who subsequently contacted the attending clinicians.
《2.2. Laboratory confirmation》
2.2. Laboratory confirmation
Laboratory confirmation of SARS-CoV-2 was performed at the Hubei Provincial Hospital of Integrated Chinese and Western Medicine. RT-PCR assays were performed in accordance with the protocol established by the WHO.
《2.3. Statistical analysis》
2.3. Statistical analysis
Categorical variables were described as frequency rates and percentages, and continuous variables were described using mean, median, and interquartile range (IQR) values. The means for continuous variables were compared using independent group t-tests when the data were normally distributed; otherwise, the Mann–Whitney U test was used. Proportions for categorical variables were compared using the χ2 test, although the Fisher’s exact test was used when the data were limited. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 13.0 software (SPSS Inc., USA). For unadjusted comparisons, a two-sided P value of less than 0.05 was considered to be statistically significant. The analyses have not been adjusted for multiple comparisons and, given the potential for type I error, the findings should be interpreted as exploratory and descriptive.
《3. Results》
3. Results
《3.1. Demographic and clinical characteristics》
3.1. Demographic and clinical characteristics
As shown in Table 1, 96 (46.2%) patients with type 2 diabetes and 112 (53.8%) patients without diabetes were enrolled. The median age of the patients was 64 years (IQR: 55–69). A total of 51.4% was female. Fever (body temperature ≥ 37.5 °C) was present in 9.1% of the patients on admission but developed in 51.9% during hospitalization. The other most common symptoms were cough (59.1%), fatigue (52.4%), shortness of breath (34.6%), sputum (28.4%), myalgia or arthralgia (15.9%), chills (7.7%), and diarrhea (7.2%). Among the overall population, 66.3% had at least one coexisting glucose-lipid metabolism disorder such as diabetes, hypertension, coronary heart disease, or cerebrovascular disease.
《Table 1》
Table 1 Clinical characteristics of study patients with COVID-19.

1 mmHg = 133.3 Pa.
a Included in this category is any type of cancer.
b The presence of hepatitis B infection was defined as a positive result on testing for hepatitis B surface antigen with or without elevated levels of alanine or aspartate aminotransferase.
On admission and during hospitalization, the body temperature of the diabetic patients was lower than that of the non-diabetic patients (36.7 °C (IQR: 36.4–37.0) vs 36.9 °C (IQR: 36.5–37.0), P = 0.038), (37.0 °C (IQR: 36.8–38.0) vs 38.0 °C (IQR: 37.4–38.6), P < 0.001) [17]. This profile may be attributed to the impaired immune response in diabetic patients. Furthermore, fatigue (44.8% vs 58.9%, P = 0.042) and chills (14.6% vs 1.8%, P < 0.001) were the symptoms that differed most among diabetic and non-diabetic patients. Since the diabetic patients had been prone to fatigue for a long time due to energy metabolism disorders, after infection with SARS-CoV-2, they may have become less sensitive to the symptoms of fatigue. Diabetes promotes a detrimental pro-inflammatory state [18] and may cause chill symptoms to be more pronounced.
The present study showed significant differences in comorbidities for diabetic COVID-19 patients versus non-diabetic patients, including hypertension (58.3% vs 31.3%, P < 0.001), coronary heart disease (17.7% vs 8.0%, P = 0.035), and chronic renal disease (6.3% vs 0%, P = 0.007). It has been well established that hypertension, coronary heart disease, and chronic renal disease coexist with diabetes, leading to a higher risk of morbidity and mortality for COVID-19. In diabetic patients and patients with related glucose and lipid dysfunctions, the angiotensin-converting enzyme 2 (ACE2) receptor overexpresses in multiple organs such as the lung, kidney, and liver, which facilitates the receptor-binding domain of the spike protein of SARS-CoV-2 bound to the cell receptor ACE2 [19]. Several lines of evidence suggest that diabetes is a risk factor for the progression and prognosis of COVID-19 [17,20]. Our study enhanced this hypothesis. Nonetheless, the prevalence of cerebrovascular disease, hepatitis B infection, cancer, and chronic obstructive pulmonary disease (COPD) history was comparable between the two groups.
《3.2. Radiologic and laboratory findings》
3.2. Radiologic and laboratory findings
The radiologic data on admission is shown in Table 2. In the 201 CT scans that were performed at the time of admission, 90.4% showed abnormal results. No CT abnormality was found in seven of the 111 patients (6.3%) without diabetes and in six of the 90 patients (6.7%) with diabetes. The most common patterns in the chest CTs were ground-glass opacity (85.6% vs 64.9%, P < 0.001) and bilateral patchy shadowing (76.7% vs 37.8%, P < 0.001) among diabetic patients in comparison with non-diabetic patients, which is consistent with other recent reports [2] that suggest more severe lung injury in diabetic COVID-19 patients.
Table 3 shows the laboratory findings on admission. Blood glucose (7.23 mmol·L-1 (IQR: 5.80–9.29) vs 5.46 mmol·L-1 (IQR: 5.00– 6.46)), blood low-density lipoprotein cholesterol (LDL-C) (2.21 mmol·L-1 (IQR: 1.67–2.76) vs 1.75 mmol·L-1 (IQR: 1.27– 2.01)), and systolic pressure (130 mmHg (IQR: 120–142) vs 122 mmHg (IQR: 110–137), P < 0.001, Table 1) (1 mmHg = 133.3 Pa) in diabetic COVID-19 patients were significantly higher than in non-diabetic COVID-19 patients (P < 0.001). This result shows that diabetic patients have disorders in glucose and lipid metabolism.
《Table 2》
Table 2 Radiographic examination.

《Table 3》
Table 3 Laboratory findings.

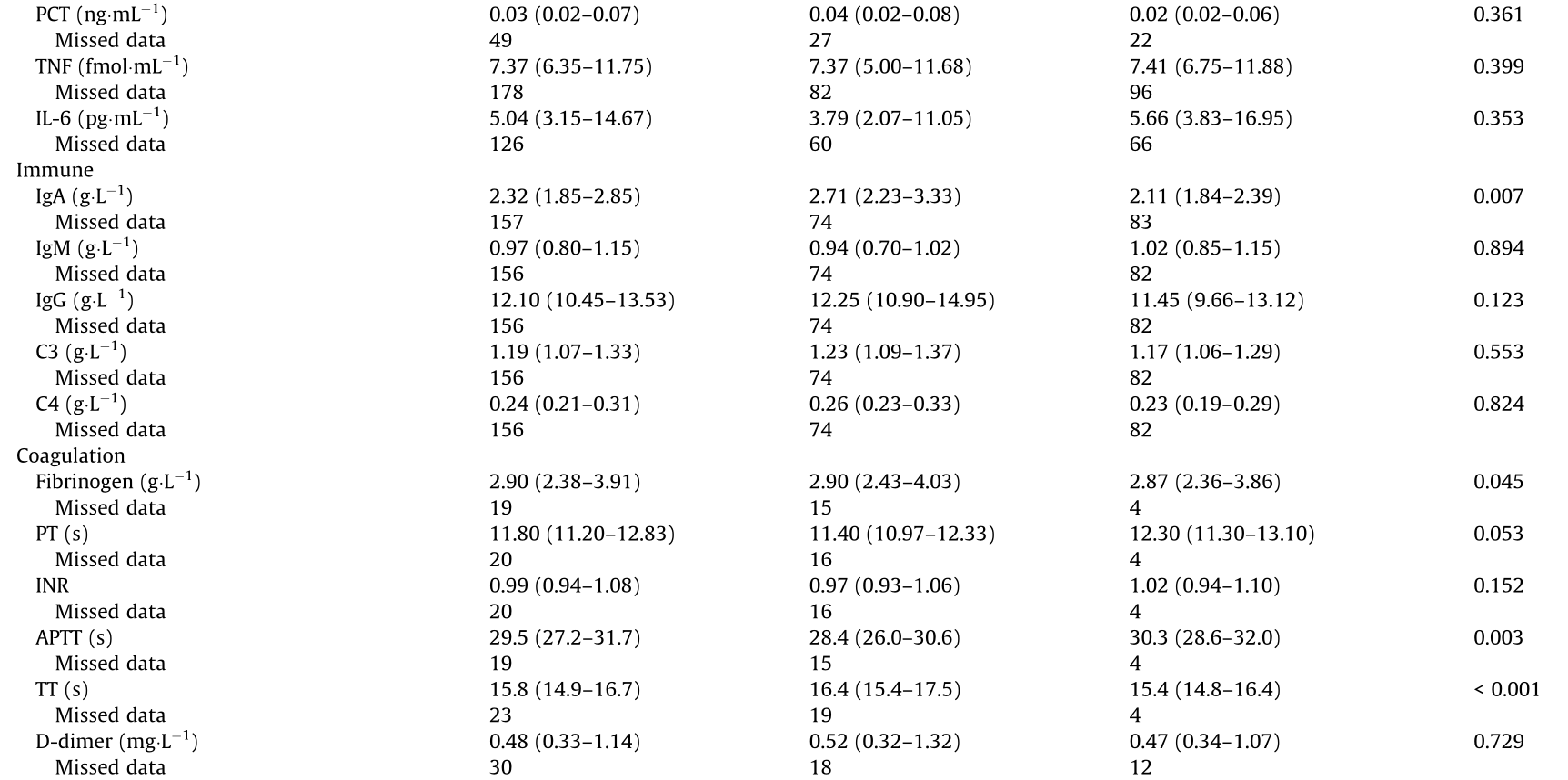


HbA1c: glycosylated hemoglobin A1c; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; ESR: erythrocyte sedimentation rate; TNF: tumor necrosis factor; IL: interleukin; Ig: immunoglobulin; C3: complement 3; C4: complement 4; PT: prothrombin time; INR: international normalized ratio; APTT: activated partial thromboplastin time; TT: thrombin time; AST: aspartate aminotransferase; ALT: alanine aminotransferase; BUN: blood urea nitrogen; Po2: partial pressure of O2; Pco2: partial pressure of CO2.
Regarding hematological parameters, on admission, lymphocytopenia was present in 19.7% of the patients, thrombocytopenia was present in 1.4%, and leukopenia was present in 5.7%. The presence of lymphocytopenia suggested viral infection in the patients. There were no differences in the hematological parameters of the diabetic and non-diabetic COVID-19 groups.
Levels of the inflammatory parameters erythrocyte sedimentation rate (ESR) (31.7%) and CRP (21.2%) were elevated, but there were no difference between the two COVID-19 groups with and without diabetes. There were no difference in the proinflammatory cytokines PCT, tumor necrosis factor (TNF), interleukins (ILs), and so forth in the two COVID-19 groups.
The blood biochemical parameters alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine, and so forth showed no laboratory abnormalities, suggesting less abnormal liver, renal, and myocyte functions. The levels of immunity parameters showed similar negative results, although immunoglobulin A (IgA) had a positive change. Serum IgA has an important role in the protection of the mucociliary areas, and acts as a barrier against pathogenic organisms, antigens, and even allergens [21]. The combination of COVID-19 and diabetes has a different effect on immune status than COVID-19 without diabetes, as glucose and lipid metabolism disorders may increase the immune response.
The COVID-19 group with diabetes had the following changes in comparison with the COVID-19 group without diabetes: D-dimer (0.52 mg·L-1 (IQR: 0.32–1.32) vs 0.47 mg·L-1 (IQR: 0.34–1.07), P = 0.729); activated partial thromboplastin time (APTT) (28.4 s (IQR: 26.0–30.6) vs 30.3 s (IQR: 28.6–32.0), P = 0.003); fibrinogen (2.90 g·L-1 (IQR: 2.43–4.03) vs 2.87 g·L-1 (IQR: 2.36–3.86), P = 0.045); and thrombin time (TT) (16.4 s (IQR: 15.4–17.5) vs 15.4 s (IQR: 14.8–16.4), P < 0.001). These findings suggest that diabetic patients with COVID-19 are more likely to show abnormal blood coagulation function in clinical practice. During the inflammatory storm, blood coagulation was abnormal. In the early stage, this is the result of inflammation-activating plasmin. Subsequently, hypoxia-induced molecules can activate thrombin directly, with progressive inflammation, and the activation of monocytemacrophages also secretes a number of tissue factors, activating the exogenous coagulation pathway, which leads to an overall hypercoagulable state [17].
Compared with the non-diabetic cases, the diabetic cases had much lower partial pressure of O2 (Po2) (85 mmHg (IQR: 53–97) vs 110 mmHg (IQR: 93–164), P = 0.029) and plasma levels of LDH (189 U·L-1 (IQR: 159–227) vs 220 U·L-1 (IQR: 181–270), P = 0.195), globulin (27.2 g·L-1 (IQR: 22.6–30.3) vs 25.4 g·L-1 (IQR: 22.4–28.1), P = 0.041), cystatin C (0.90 mg·L-1 (IQR: 0.75– 1.05) vs 0.73 mg·L-1 (IQR: 0.64–0.93), P = 0.006), and potassium (4.03 mmol·L-1 (IQR: 3.66–4.38) vs 3.81 mmol·L-1 (IQR: 3.61– 4.10), P = 0.001). Lower Po2 indicates hypoxia, which activates the oxidative stress and inflammatory reaction, leading to the need for mechanical ventilation later on. The elevated globulin and cystatin C levels suggested the presence of damage affecting the renal function. Chronically damaged kidney function may slow down the potassium metabolism and promote a higher blood level.
《3.3. Clinical outcomes》
3.3. Clinical outcomes
Table 4 shows that a primary composite end point event occurred in 18 (8.7%) of the 208 patients, including eight patients (3.8%) who were admitted to the ICU, eight patients (3.8%) who underwent mechanical ventilation (one patient experienced both invasive and noninvasive mechanical ventilation), and nine patients (4.3%) who died. The median duration of hospitalization was 14 d (IQR: 9–20).
Among the 96 patients with diabetes, a tendency toward severe prognosis was observed, even though there was no statistical significance in the clinical outcomes between the diabetic and nondiabetic groups, including the primary composite end point event (10.4% vs 7.1%, P = 0.402), median duration of hospitalization (12 d vs 15 d, P = 0.307), incidence of admission to ICU (5.2% vs 2.8%, P = 0.344), discharge from hospital (84.8% vs 88.4%, P = 0.397), death (6.3% vs 2.7%, P = 0.307), and hospitalization (17.7% vs 18.8%, P = 0.846).
《3.4. Treatment and complications》
3.4. Treatment and complications
The majority of the patients (62.9%) received intravenous antibiotic therapy, 85.1% received antiviral therapy, 30.4% received systemic glucocorticoids, and 0.6% received antifungal medication. 4.3% received mechanical ventilation, 80.4% were treated with traditional Chinese medicine, and 11.3% were treated with acupuncture. Noninvasive mechanical ventilation was initiated in more diabetic patients than non-diabetic patients (4.2% vs 1.8%, P = 0.306) (Table 4).
《Table 4 》
Table 4 Complications, treatments, and clinical outcomes.


DIC: disseminated intravascular coagulation.
a One of them used both invasive mechanical ventilation and noninvasive mechanical ventilation.
During hospital admission, some patients received a diagnosis of ARDS (2.0%), acute kidney injury (1.5%), septic shock (1.0%), or heart failure (1.0%) from a physician. No patient entered the disseminated intravascular coagulation (DIC) stage.
In our clinical data, 17 diabetic patients (17.7%) were given glucocorticoids, accounting for 23.9% of all diabetic patients, and 35 non-diabetic patients were given glucocorticoids, accounting for 35.0% of all non-diabetic patients (P = 0.121). According to a retrospective study, the patients with ARDS from COVID-19 showed lower mortality among those receiving glucocorticoids [22]. COVID-19 patients can benefit from glucocorticoids treatment to reduce autoimmunity and cytokine toxicity [23].
In this retrospective study, blood glucose was not monitored in every COVID-19 patient after treatment with glucocorticoids, due to outbreak isolation management. Therefore, we could not analyze the impact of glucocorticoids on the glucose level of COVID19 patients with and without diabetes due to insufficient clinical data. This is a limitation of the study. Although glucocorticoids unavoidably affect the blood glucose level, there is no evidence that the administration of glucocorticoids worsens COVID-19. In the future, the influence of glucocorticoids on COVID-19 patients with diabetes will be further investigated.
《4. Conclusions and perspectives》
4. Conclusions and perspectives
This retrospective study provides the first direct evidence supporting the high frequency of the coexistence of type 2 diabetes and hypertension, coronary heart disease, and chronic renal disease in patients with COVID-19. This new finding strongly supports our proposed novel concept of glucolipid metabolic disease (GLMD) and an integrated strategy for the treatment of the whole spectrum of metabolic diseases underlying SARS-CoV-2 infection [24]. It has been recognized that disorders in the metabolism of glucose and lipids act as the initial trigger and as a potent driving force in the development and progress of various metabolic diseases, including type 2 diabetes mellitus, dyslipidemia, nonalcoholic fatty liver disease, hypertension, atherosclerosis, and cardiovascular complications. As the metabolism of lipids and glucose is a highly coordinated process under both physiological and diseased conditions, impairment in the signals corresponding to the metabolism of either lipids or glucose is not only a common mechanism, but also the key mechanism underlying the pathogenesis of GLMD [25].
Another novel finding in the present study is the potentiated severity in COVID-19 patients with metabolic comorbidities. Although several previous studies have reported on diabetes as one of the most common comorbidities in patients with SARS-CoV-2 infection [7,26,27], the pathophysiological consequence of the close association between diabetes and COVID19 progression still remains elusive. Our analysis highlights the markedly promoted morbidity in diabetes patients with COVID-19. The frequency and degree of abnormalities in radiologic examinations of diabetic COVID-19 patients were notably higher than those in the control group without diabetes. In addition, disruption in the homeostatic control of glucose metabolism and blood pressure was more severe in diabetic COVID19 patients. Diabetes and its related metabolic disorders have consistently been documented as one of the key risk factors for severe outcomes in patients infected with other types of coronavirus, such as Middle East respiratory syndrome coronavirus (MERS-CoV) [28,29] and SARS-associated coronavirus (SARS-CoV) [30]. Thus, our data in the present study demonstrate that diabetes and its associated metabolic complications are a crucial contributor to enhanced morbidity and mortality in COVID-19 patients.
The conceptional construction of GLMD has great relevance to the clinical management of COVID-19. On the one hand, our data suggest that maintenance of the homeostasis in glucose and lipid metabolism via optimal clinical management in COVID-19 patients is mandatory. Furthermore, evidence from infection with another member of the coronavirus family—namely, SARS-CoV— has demonstrated that the binding of SARS-CoV to its receptor ACE2 in the pancreas results in islet damage and a notable decrease in the release of insulin, thereby causing short-term hyperglycemia, and even the onset of diabetes [31]. Furthermore, SARS-CoV-evoked diabetes-like traits markedly potentiate the progression to multiple-organ failure via a positive feedback loop. Therefore, tight control of the parameter set of glucose and lipid metabolism may be the key to optimal clinical outcomes in COVID-19 patients without a history of diabetes and/or other metabolic diseases. On the other hand, diabetes has attained epidemic proportions worldwide. According to estimated data from the International Diabetes Federation, 415 million people have diabetes mellitus, with 90% of these individuals having type 2 diabetes mellitus [32]. Hence, powerful and integrated approaches should be launched for the comprehensive prevention of GLMD, which is of significant importance for marked reduction in the morbidity and mortality caused by COVID-19 and other emerging infectious diseases.
The present study may possess limitations. First, this retrospective study was conducted on historical cohorts, with all recorded events having already occurred. Thus, information on relevant risk factors with potentially profound influence on the progression of COVID-19 may not be included in the database and subsequent analysis. Therefore, the measurement of the impact of diabetes and its complications on the clinical outcomes would not have been as accurate as in a prospective study. Second, the sample size is relatively small. Therefore, the findings observed in the present study warrant further validation through future study with a large sampling size.
In conclusion, diabetes and other coexisting metabolic disorders within the spectrum of GLMD significantly boost the morbidity in COVID-19 patients. Therefore, these findings deliver at least two important messages for the clinical management of COVID-19. First, optimal management of the metabolic hemostasis of glucose and lipids is key in ensuring better clinical outcomes. Second, increased clinical vigilance is warranted for COVID-19 patients with GLMD in the form of fundamental and chronic conditions.
《Acknowledgements》
Acknowledgements
The authors are grateful to the Hubei Provincial Integrated Chinese and Western Medicine Hospital for its assistance. We are grateful to Qian Cai, Yuwan Wu, Xiaohui Bai, Yongshi Ni, Mingkai Guo, Haili Zhu, Kaixuan Yan, Yiqi Yang, Guizhi Yang, Zhiquan Chen, Bowei Ma, Yuzhen Ye, and Zhujian Lin for data extraction, and grateful to Tian Lan for suggestions for the paper. This work was supported by the National Support Project for Leading Talents of Chinese Medicine; the Guangdong Provincial Inheritance Studio of Famous Traditional Chinese Medicine; the Department of Education of Guangdong Province (2020KZDZX1054); the National Key Research and Development Plan of China (2018YFC1704200 and 2020YFC0845300); and the Natural Science Foundation of Guangdong Province (2018A030313391).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Yingyu Chen, Jiankun Chen, Xiao Gong, Xianglu Rong, Dewei Ye, Yinghua Jin, Zhongde Zhang, Jiqiang Li, and Jiao Guo declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




