《1. Introduction 》
1. Introduction
Pressure drastically alters all materials and impacts on materials science researches. Pressure ranges of several hundred gigapascals (GPa) attainable in diamond-anvil cells (DACs) directly affect crystal structures, electronic structures, and the chemical bonds of condensed matter [1]. Thus, pressure is an ideal tool for pursuing breakthrough energetic materials with extremely favorable properties, such as the highest-efficiency chemical fuels and highestenergy explosives. However, a realistic, useful material must be preservable at ambient conditions for practical applications. Therefore, exploratory research on energetic materials at extreme pressures includes two steps: first, a purely curiosity-driven scientific adventure casting a wide net to discover novel materials and understand their fundamental physics and chemistry at high pressures; second, application-inspired recovery of the novel materials at ambient conditions, either by direct quenching or by chemical synthesis based on the knowledge gained at high pressures [2]. Such explorations may eventually lead to materials production and applications such as, the development of superhard diamonds, which were first synthesized at high pressures and successfully quenched as a metastable phase at ambient conditions for superabrasive industrial usage. Later, the metastable growth of gemquality diamonds also became practical at zero pressure by advancing the chemical vapor deposition (CVD) method [3] based on knowledge of sp3 diamond bonding.
Research on extreme energetic materials at ultrahigh pressures is still mostly in the discovery stage. Here, we discuss ultrahighpressure studies of pure elemental nitrogen and hydrogen, two extremely energetic materials. Starting as gases in one atmosphere, N2 and H2 are some of the most compressible materials, making it very challenging to carry out in situ studies at pressures of hundreds of gigapascals. Although studies of N2 and H2 have their own very different and fascinating scientific agendas, they share many similar technical challenges and are benefited by the same experimental developments, that is loading tenuous gases in DACs to be compressed into solids with thousand times higher density, monitoring the molecular bonding by Raman and infrared (IR) spectroscopy, determining the crystal structure and equations of state by X-ray diffraction (XRD), and probing electronic changes by electrical transport measurements. Relatively speaking, ultrahigh-pressure hydrogen is much harder to study than nitrogen. It is often prudent to study nitrogen as a pilot project before investigating hydrogen. For instance, high-density nitrogen can be easily loaded by clamping a DAC in a bucket of readily available liquid nitrogen, whereas loading high-density hydrogen requires a sophisticated compression system with numerous safety precautions. The XRD signal of a hydrogen atom is 49 times weaker than that of a nitrogen atom because the X-ray scattering power is proportional to the square of the atomic number [4]. The nonmolecular phase of nitrogen can be reached at a comfortable pressure range below 150 GPa, whereas that of hydrogen requires pressures beyond 500 GPa into the nonreproducible and poorly calibrated pressure range of a DAC. Consequently, new experimental probes have been developed to characterize specific properties of nitrogen and hydrogen for these high-risk–high-return quests.
《2. Nitrogen at ultrahigh pressures 》
2. Nitrogen at ultrahigh pressures
《2.1. Triple-bonded molecular nitrogen》
2.1. Triple-bonded molecular nitrogen
Advances of modern explosive materials are mostly based on the progress of high-nitrogen-content compounds [5]. Naturally, the ultimate material is pure single-bonded nitrogen. Under normal conditions, pure nitrogen consists of isolated triple-bonded N2 (N N) diatomic molecules, which solidify at low temperature or moderate pressure with weak van der Waals interactions among the molecules. Theory has predicted [6] that at sufficiently high pressures, molecular nitrogen will convert to a three-dimensional network structure of polymeric nitrogen, where each nitrogen atom has a single bond with three other nitrogen atoms and the conversion will store a tremendous amount of energy owing to the breakage of these very strong triple bonds of nitrogen (4.94 eV per atom). In comparison, the heat of explosion per unit mass of polymeric nitrogen is 2.9, 6.7, and 10.7 times higher than that of pentaerythritol tetranitrate (PETN, C5H8N4O12), trinitrotoluene (TNT, C6H2CH3(NO2)3), and lead azide (LA, Pb(N3)2), respectively. Searching for single-bonded nitrogen in the 1980s prompted the pioneering development of high-pressure DAC techniques using XRD to determine the crystal structure and Raman and IR vibrational spectroscopies to investigate the pressure-induced changes of nitrogen bonding [7]. Such studies over the past century have revealed a complicated high-pressure–temperature phase diagram of nitrogen and at least seven stable phases (α, β, γ, δ, ε, λ, and ζ). Each phase has a different crystal structure and various vibrational spectra up to 120 GPa, but all phases remain molecular with the basic building blocks of N2 (Fig. 1[8–12]).
N) diatomic molecules, which solidify at low temperature or moderate pressure with weak van der Waals interactions among the molecules. Theory has predicted [6] that at sufficiently high pressures, molecular nitrogen will convert to a three-dimensional network structure of polymeric nitrogen, where each nitrogen atom has a single bond with three other nitrogen atoms and the conversion will store a tremendous amount of energy owing to the breakage of these very strong triple bonds of nitrogen (4.94 eV per atom). In comparison, the heat of explosion per unit mass of polymeric nitrogen is 2.9, 6.7, and 10.7 times higher than that of pentaerythritol tetranitrate (PETN, C5H8N4O12), trinitrotoluene (TNT, C6H2CH3(NO2)3), and lead azide (LA, Pb(N3)2), respectively. Searching for single-bonded nitrogen in the 1980s prompted the pioneering development of high-pressure DAC techniques using XRD to determine the crystal structure and Raman and IR vibrational spectroscopies to investigate the pressure-induced changes of nitrogen bonding [7]. Such studies over the past century have revealed a complicated high-pressure–temperature phase diagram of nitrogen and at least seven stable phases (α, β, γ, δ, ε, λ, and ζ). Each phase has a different crystal structure and various vibrational spectra up to 120 GPa, but all phases remain molecular with the basic building blocks of N2 (Fig. 1[8–12]).
《Fig. 1》

Fig. 1. Phase and synthesis diagram of nitrogen. CG: cubic-gauche phase; BP: blackphosphorus phase; LP: layered polymeric. Adapted from Ji et al. [8].
《2.2. Single-bonded polymeric nitrogen》
2.2. Single-bonded polymeric nitrogen
Above 150 GPa at 300 K, nitrogen adopts amorphous nonmolecular state (η nitrogen) and turns opaque and electrically conductive [13]. The N2 molecular vibrational Raman and IR peaks disappear, and new broad peaks appear. Its crystalline XRD Bragg peaks also disappear [10], indicating the transition to a nonmolecular, amorphous, and polymeric phase. The pressure-induced amorphous η phase is metastable. Nevertheless, the N2 triple bonds are broken and replaced by polymeric single bonds in the η phase.
The transition to the η phase shows significant hysteresis. At 300 K, the polymeric phase forms as pressure exceeds 150 GPa, but the phase persists metastably as pressure is released to 50 GPa. The hysteresis increases at lower temperatures owing to the exponential decrease in the reaction kinetic with temperature, and polymeric nitrogen is recoverable at zero pressure at 100 K [14]. In general, using high temperatures to accelerate a transition to the desired phase and low temperatures to inhibit the back transition and preserve the metastable phase is a sound strategy for synthesizing novel materials [2].
《2.3. Cubic-gauche and other single-bonded nitrogen》
2.3. Cubic-gauche and other single-bonded nitrogen
Although room-temperature high-pressure synthesis and cryogenic recovery demonstrate the principle of bringing metastable high-pressure phases to ambient pressure, it is preferable to conduct high-temperature–high-pressure synthesis and roomtemperature recovery to obtain a useful end product at room temperature. Continuing the search for a stable phase at higher temperatures and pressures, Eremets et al. [9] studied N2 at 110 GPa and 2000 K using a laser-heated DAC, and they observed the XRD patterns of crystalline single-bonded nitrogen in the theoretically predicted [6] cubic-gauche (CG) structure. CG nitrogen (CG-N) has since been confirmed by a number of other high-pressure– high-temperature experiments [8,10–12,15]. In addition, various stable novel phases of polymeric nitrogen were theoretically predicted at higher pressures through an evolutionary structural search [16]. Experimentally, single-bonded crystalline nitrogen with a layered polymeric (LP) structure was synthesized at 120– 180 GPa [12], and one with a hexagonal layered polymeric (HLP) structure was synthesized above 240 GPa using a laser-heated DAC and XRD and Raman spectroscopic diagnostics. The coexistence of multiple stable and metastable phases of nitrogen in the same pressure–temperature range indicates these phases having small differences in their free energy formation but large energy barriers among them. This is a promising situation for recovering novel materials with favorable properties beyond the thermodynamically stable phases.
《2.4. Black-phosphorus structured nitrogen》
2.4. Black-phosphorus structured nitrogen
An analogy can be drawn between nitrogen and elemental phosphorus at ambient conditions, which has four allotropes: white phosphorus, red phosphorus, violet phosphorus, and black phosphorus (BP). All four allotropes can be preserved indefinitely at ambient conditions, but they exhibit strikingly diverse properties and possible usages. Nitrogen and phosphorus are Group V elements in the first and second rows of the Periodic Table. A general rule in high-pressure science states that ‘‘increasing pressure causes an element to behave like the next-row element of the same group." The single-bonded BP allotrope is particularly interesting because it represents the archetypal two-dimensional (2D) materials of Group V elements, with highly anisotropic layers ‘‘puckered” in one direction and smooth in the other direction. This structure is found in all Group V elements heavier than nitrogen, including phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). They exhibit a number of unusual electronic, mechanical, optical, and transport properties and have exciting potential application as next-generation 2D materials [17] beyond the flat 2D-layer materials of the Group IV elements following graphene. However, the leading element of this group, nitrogen, was not known in the BP structure. Enthalpy comparison rules out the low-temperature stability of the black-phosphorus nitrogen (BP-N) phase at high pressures [16], but this does not mean that BP-N cannot be synthesized at high temperatures and preserved metastably at low temperatures. We have searched a wide range of pressures and temperatures and found that BP-structured nitrogen [8] can be synthesized and quenched in a pressure–temperature region overlapping that of CG-N and layered polymetric nitrogen (LP-N) (Fig. 1). The XRD and Raman spectroscopy analyses show that BP-N has single-bonded nitrogen in a puckered 2D-layered structure, and thus it is both an extremely energetic material and a new-generation 2D material in one. Similar to CG-N, BP-N shows significant hysteresis once formed, and it could be metastable over a wide pressure range. However, it reverts to N2 when the pressure is released below 48 GPa at 300 K.
《2.5. Prospect of polymeric nitrogen recovery as an energetic material》
2.5. Prospect of polymeric nitrogen recovery as an energetic material
The hystereses of the crystalline and amorphous polymeric nitrogen phases reflect the large energy barrier between the single- and triple-bonded nitrogen, which is advantageous for the possible recovery of the novel phases for materials applications. Recently, CG-N was successfully synthesized under near-ambient conditions without pressure from the radio-frequency plasma reaction of β-sodium azide on multiwall carbon nanotubes [18], thus substantially increasing its potential for energetic materials applications. One successful strategy for extending the metastability for materials design and synthesis is by chemically doping compatible impurities. Nitrogen is at a favorable crossroads in the periodic table, where its neighboring elements, boron and carbon [19] in the same row and phosphorus in the same group, all exhibit strong metastability and rich allotropes. Nitrogen can form numerous compounds at high pressures [20], and polymeric nitrogen has been synthesized through various types of chemical doping [21,22]. Future application-inspired basic research may proceed to design and synthesize BP-N or other superb single-bonded nitrogen materials at near-ambient conditions through a chemical path, possibly using the neighboring elements [22] as chemical dopants or as substrates for the epitaxial growth of polymeric nitrogen.
《3. Hydrogen at ultrahigh pressures》
3. Hydrogen at ultrahigh pressures
《3.1. Hydrogen as an extreme energetic material》
3.1. Hydrogen as an extreme energetic material
Low-density hydrogen gas or liquid has already been used extensively as an important energetic material with a wide range of applications, from rocket propellants to automotive fuel cells. Ultradense "metallic hydrogen” at ultrahigh pressures, however, is sometimes regarded as the ultimate energetic material other than nuclear energy materials. It could even be considered as the most efficient nuclear fusion fuel owing to the extremely high initial density of hydrogen. Its potential as an above-roomtemperature superconductor has also been demonstrated by the very high critical temperatures (Tc) of hydrogen-rich hydrides of lanthanum (La) and yttrium (Y) [23]. However, the feasibility of its materials application is unsupportable at present. First, even if metallic hydrogen could be synthesized, the quantity that could be obtained with the currently known technology would be mere picograms, insignificant even as a nuclear fusion fuel. Secondly, and more importantly, the transitions in hydrogen are spontaneous without hysteresis. Theoretical calculations indicate that the lifetime for metastable metallic hydrogen is less than a picosecond, even if it could be quenched [24]. Thirdly, although the heat of formation per unit mass of metallic hydrogen at 500 GPa is 76 times that of TNT, the extra energy comes from the enormous elastic compressibility of hydrogen, which is non-preservable. Overall, hydrogen research at ultrahigh pressures is in its infancy in terms of practical material applications, but it has great potential for curiosity-driven basic science with a plethora of unexpected discoveries.
《3.2. The quest for "metallic hydrogen”》
3.2. The quest for "metallic hydrogen”
Indeed, the century-old quest for "metallic hydrogen” is one of the key 21st-century problems in physics and astrophysics, as accentuated by Ginzburg [25]. It is a multifaceted problem carrying many different, important meanings to multiple disciplines for their individual interests. To astrophysicists, metallic hydrogen is the most abundant materials in the universe, hidden under the extreme pressure conditions in the deep interiors of celestial bodies (Fig. 2, liquid metallic hydrogen). To condensed-matter physicists, it is potentially the highest-temperature superconductor and highest-energetic material. To theoreticians, it is an exotic novel state of dense-matter physics, possibly consisting of twocomponent (electron and proton) superfluids at zero temperature [26,27]. To experimentalists, it is the "Holy Grail” of highpressure physics, dangling beyond the limit of achievable pressure and probing capabilities. Scientists’ interest in the anticipated "metallic hydrogen” has been evolving and coinciding with contemporary hot topics of the time. The alkali metal-like freeelectron prediction of "metallic hydrogen” was emphasized in 1935 [28], when the electronic band structure theory was firmly established as a cornerstone of solid-state physics. In the 1960s, when the electron–phonon coupling of the Bardeen–Cooper– Schrieffer (BCS) theory became popular, "metallic hydrogen” was predicted to be a high-temperature superconductor owing to its extremely high phonon frequency [29]. At the turn of the century, when Bose–Einstein condensation took center stage of physics, a two-component superfluid was discovered [26,27] owing to the strong quantum behavior of the protons and electrons. Now, as 2D graphene rises as a wonder material, the high-pressure hydrogen structure has been postulated to be composed of alternating layers of graphene and bromine gas (Br2) [30]. In hindsight, "metallic hydrogen” appears to be an archaic name. In 1935, pressureinduced metallization was exciting and novel, but it has since become a very ordinary and expected phenomenon that has been observed in more than one thousand materials. The true meaning of "metallic hydrogen” is, therefore, to understand the pressureinduced evolution of hydrogen from diatomic molecular crystals to an atomic end state, possibly consisting of two-component superfluids or other novel states. The process may involve many structural phase transitions and a series of continuous or discrete electronic band changes, from a low-density insulator with a very wide electronic band gap to a semiconductor with a narrow gap to a dense molecular metal with a closed gap and finally to an alkalilike atomic metal or other exotic states.
《Fig. 2》
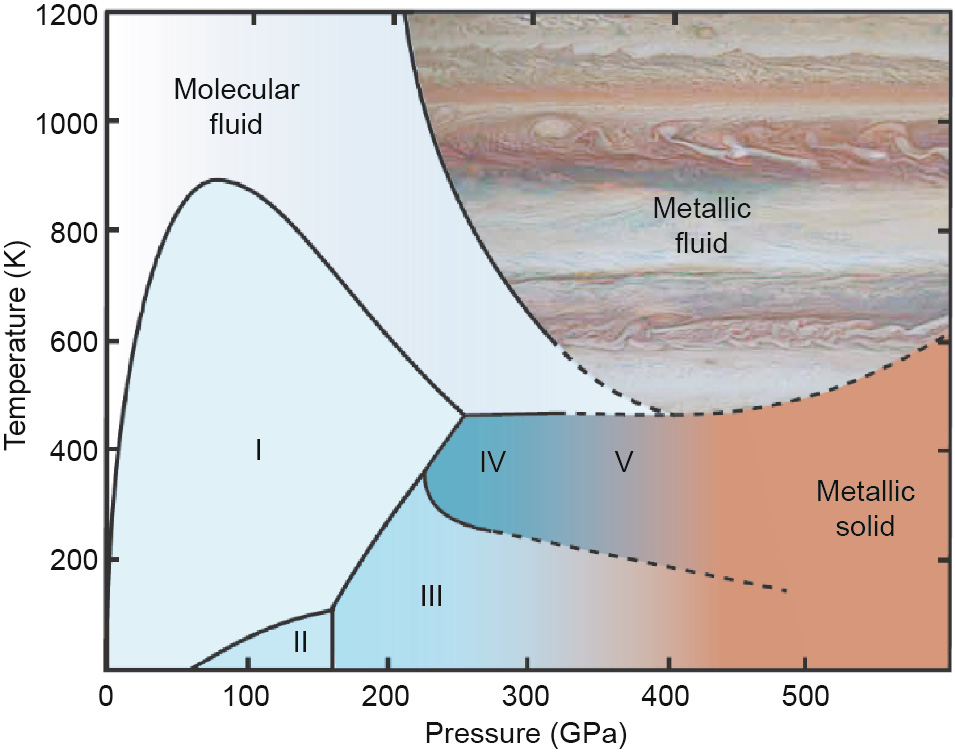
Fig. 2. Phase diagram of hydrogen. Reproduced from Gregoryanz, et al. [31].
《3.3. The current experimental frontier》
3.3. The current experimental frontier
Observations of metallic behavior have been reported in numerous static and dynamic studies of solid and liquid hydrogen [32– 34], but the mission is far from being accomplished. The key problem is the lack of experimental means to investigate hydrogen and reveal its fundamental physics in situ under pressure, especially at pressures beyond the pressure limit of DACs, where results are often controversial [31]. If confirmed, observation of the longexpected metallization owing to an incremental reduction of the band gap would be a technical milestone, but it would reveal very little new physics. The true mission of "metallic hydrogen” would be better defined as exploration of the rich physics and unusual, unexpected behavior displayed by the extraordinary changes of elemental hydrogen over an extensive range of pressures and temperatures. A prerequisite of such a mission would be to probe the fundamental properties of the hydrogen sample to reveal its crystal structure and electronic band structures, two pillars of condensedmatter physics. The most robust probes are optical Raman [35] and IR [36] spectroscopies, which can be used to study changes in the vibrational characteristics of H2 molecules up to the limit where the sample becomes opaque. These spectroscopic techniques have been used to map out the hydrogen phase diagram and to define melting and the four high-pressure solid phases: II, III, IV, and V up to 400 GPa [35], in addition to the low-pressure solid phase I (Fig. 2). The vibrational spectroscopies probe individual molecules and only provide indirect information about the crystal and electronic structures that are essential for condensed matter.
Using high-brilliance, high-energy synchrotron radiation, we have been developing a set of comprehensive X-ray probes for studying the fundamental physics of hydrogen in DACs. We improved the high-pressure XRD technique to monitor the crystal structure of hydrogen up to 245 GPa. We found that high-pressure hydrogen phases III and IV remained in the hexagonal close-packed (HCP) structure with a decreasing  lattice parameter ratio, indicating possible electronic topological transitions [37] and thus defining the frontier limit of our understanding of hydrogen as condensed matter. However, the electronic band structure of solid hydrogen has not been measured at any pressure other than that which is indirectly revealed by optical probes.
lattice parameter ratio, indicating possible electronic topological transitions [37] and thus defining the frontier limit of our understanding of hydrogen as condensed matter. However, the electronic band structure of solid hydrogen has not been measured at any pressure other than that which is indirectly revealed by optical probes.
《3.4. Beyond the frontier》
3.4. Beyond the frontier
With the scientific target being to discover the pressureinduced rich physics of hydrogen from a molecular insulator to an atomic metal, experimentalists still have a great deal of knowledge gaps to fill. Some of the most fundamental questions beyond the current knowledge frontier are: What is the crystal structure of phase V before the molecular dissociation? What are the electronic band structures of all five phases? How do molecules interact with one another in the crystal to create new structures and lead to molecular dissociation? The answers rely on further technological advancement, so we can benefit from the rapid development of synchrotron technology and capability. With the optimization of the high-pressure XRD method [4], crystal structures could be investigated across the phases IV–V transition to the upper limit of molecular hydrogen. In principle, the incremental development of high-pressure medium-resolution (eV) inelastic X-ray probe can provide the first direct measurement of a hydrogen electronic band structure similar to the determination of the electronic band structure of crystalline helium at high pressures [38].
All transitions during the metallization process of hydrogen are the result of pressure-induced interactions among the compressed hydrogen molecules. The optical Raman spectrum measures the in-phase vibration of the hydrogen vibron, and the IR spectrum measures the out-of-phase vibration; their difference gives the intermolecular interaction [39]. However, owing to the very limited momentum transfer at the optical energy, the information from optical Raman and IR is limited to the Brillouin zone center. The limitation could also be overcome using high-energy synchrotron X-rays, which cover the momentum transfer of the entire Brillouin zone. Further development of the high-pressure–highresolution (meV) inelastic X-ray probe, which determines the dispersions of hydrogen vibron along all major Brillouin zone branches, could yield rich information about the intermolecular interaction in the three-dimensional crystal lattice. This could be a vital new direction for advancing the frontier toward our fundamental understanding of the ultradense molecular hydrogen system.
Further into the atomic hydrogen regime, experimentalists are facing a completely new set of challenges; without molecular bonding or a crystal lattice, Raman spectroscopy and XRD are no longer applicable. The key questions will be: How does one contain the superfluid in the DAC? What is the diagnostic evidence and probes for the superfluid, two-component superfluids, or another exotic state of matter? Answering these will be the true experimental quest for "metallic hydrogen.”
《Acknowledgements 》
Acknowledgements
The authors acknowledge the support of National Natural Science Foundation of China (U1930401). We thank Dr. Ross Howie for the artistic drawing of Fig. 2 and Ms. Freyja O’Toole for professional editing.
《Compliance with ethics guidelines 》
Compliance with ethics guidelines
Ho-Kwang Mao, Cheng Ji, Bing Li, Gang Liu, and Eugene Gregoryanz declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




