《1. Introduction》
1. Introduction
Signet ring cell carcinoma (SRCC) is a poorly cohesive carcinoma composed predominantly of tumor cells with prominent cytoplasmic mucin and a crescent-shaped, eccentrically located nucleus [1]. According to the World Health Organization (WHO) Classification of Tumors of the Digestive System, the histological type of SRCC is defined by the presence of more than 50% neoplastic cells with intracellular mucin and displaced nuclei [2]. SRCC is considered to be one of the most aggressive forms of carcinoma associated with invasive biological behavior, a younger age at presentation, higher local lymphatic spread, and peritoneal metastasis [3,4]. A majority of tumors originate in the stomach, but tumors in the breast, bladder, pancreas, lungs, gallbladder, and colon have also been reported previously [5]. Gastric and colorectal SRCC are the two most common forms of SRCC in the digestive system. According to previous studies, the incidence rates of both gastric and colorectal SRCC have increased slightly over the past few years [3,6]. The outcome of SRCC in these two sites is extremely dismal and is impacted by many factors including patients’ age, disease stage, comorbidities, and contextual, social, or cultural barriers [4,6].
Different metastatic patterns translated into distinct clinical outcomes and distant metastases are tightly linked to a poor prognosis. Pathologic features have been shown to be significantly connected with patterns of metastatic spread in both gastric SRCC and colorectal SRCC [7]. Thus, it is crucial to elucidate the metastatic distribution for better treatments and survival benefits. Due to the low incidence of SRCC, most analyses of the metastatic patterns of gastric and colorectal SRCC are based on limited sample sizes. Furthermore, few detailed studies have compared the metastatic patterns of gastric SRCC and colorectal SRCC. In this work, we utilized the Surveillance, Epidemiology, and End Results (SEER) database to systematically compare the clinical characteristics and prognosis of patients with metastatic disease with those of patients without metastatic disease.
《2. Methods》
2. Methods
《2.1. Data source》
2.1. Data source
The SEER Program is a publicly available, large, and populationbased cancer registry database that covers approximately 27.8% of the population of the United States [8]. This database not only includes the incidences and prevalence of various cancers, but also contains high-quality demographic information. Since 2010, the SEER Program has collected detailed information on the sites of distant metastasis among patients with cancer in the database. Our current study focused on the Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973–2014 varying) using the SEER*Stat software version 8.3.5.
《2.2. Study population》
2.2. Study population
The study participants had a primary diagnosis of gastric SRCC or colorectal SRCC in the registry and were diagnosed between 2010 and 2012 (SEER histology code: 8490 (SRCC)). In order to ensure an adequate follow-up duration, patients diagnosed after 2012 were excluded (Fig. 1). The mean follow-up period was 17.6 months (range, 0–59 months). Individual data for each eligible patient, such as gender, age, grade, stage, marital status, and definitive therapies, was retrieved from the SEER database. We grouped races into White, Black, and Others (e.g., Asian, Pacific Islander, American Indian/Alaska Native). Patients whose metastatic status was unknown and those who were diagnosed without histological examinations were excluded from this study.
《Fig. 1》
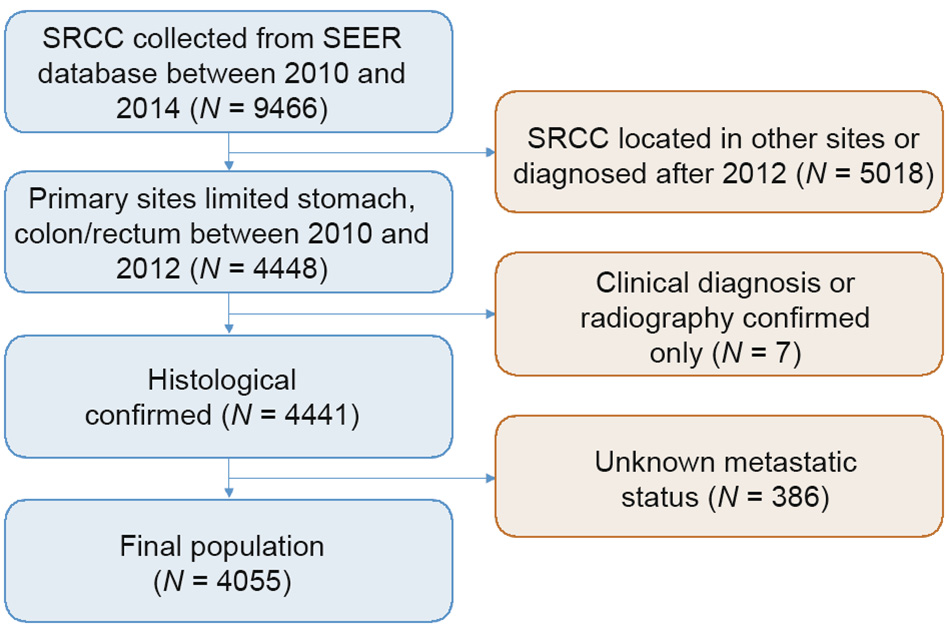
Fig. 1. Flowchart of included population in this study.
《2.3. Ethics and consent statement》
2.3. Ethics and consent statement
Data obtained from the SEER database is freely available, and no private information was used in the study. Approval from an institutional review board or ethics committee was therefore not required.
《2.4. Statistical analysis》
2.4. Statistical analysis
The baseline patient demographic, clinical, and treatment variables among patients with metastatic disease and those without metastatic disease were compared by the chi-square test. Survival differences and the association with variables among the different metastatic sites were analyzed by Kaplan–Meier curves and the log-rank test. Other variables that may potentially influence the prognosis of SRCC were determined by univariate and multivariate Cox proportional hazards models. Statistical significance was set at a two-tailed P < 0.05.
《3. Results》
3. Results
《3.1. Patient characteristics and metastatic patterns》
3.1. Patient characteristics and metastatic patterns
A total of 4055 patients with gastric SRCC or colorectal SRCC were identified between 2010 and 2012. Male patients comprised 52.2% of the study population. Of this total, 2936 patients died and 1119 patients were alive at the end of the follow-up. Among them, 2905 patients aged 12–99 were diagnosed with gastric SRCC, and the other 1150 patients aged 17–103 were diagnosed with colorectal SRCC. The mean age was 62.7 years and 64.5 years old in patients with gastric SRCC and colorectal SRCC, respectively. The baseline clinical characteristics of the patients are shown in Table 1. For both sites, most patients were white (70.6%; 82.3%) and had poorly differentiated/undifferentiated carcinoma (79.5%; 79.0%). Overall, gender and marital status were not significantly different between patients with metastatic disease and those without metastatic disease in both gastric and colorectal SRCC.
《Table 1》
Table 1 Patient sociodemographic and clinical characteristics by distant metastasis, SEER 2010–2012.
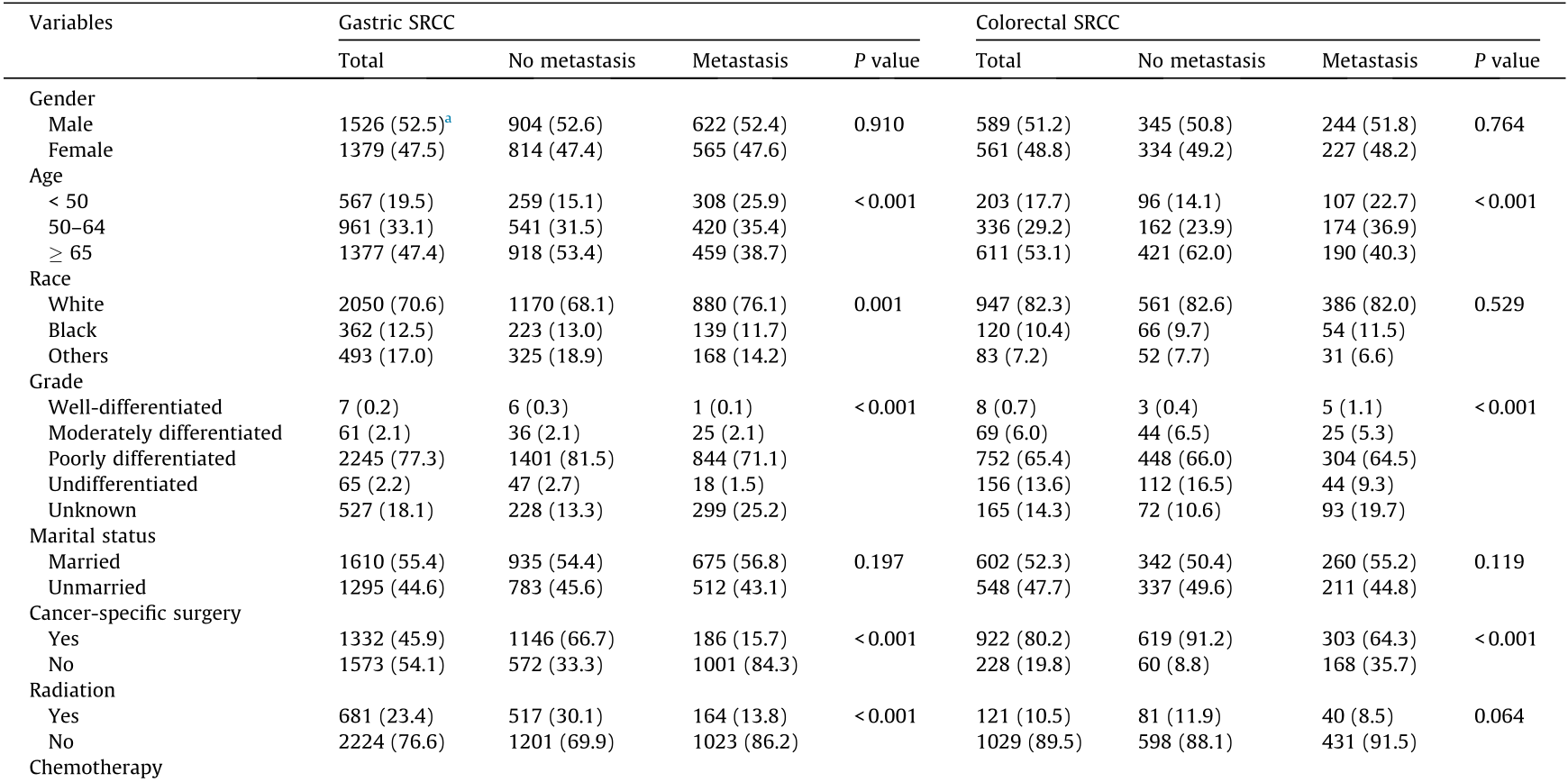

a The number in the parentheses is the percentage of this category.
In this retrospectively analyzed cohort, there were 1187 patients with gastric SRCC (40.9%) and 471 patients with colorectal SRCC (41.0%) who had advanced-stage disease at diagnosis. Since only information about bone, brain, liver, lung, and distant lymph node metastasis was recorded in the SEER database, we focused on these metastatic sites in this study. These five metastatic sites accounted for 47.9% (568/1187) and 36.5% (172/471) of metastatic sites in all advanced cases of gastric and colorectal SRCC, respectively. Among these metastatic sites, the brain was the least common metastatic site in gastric and colorectal SRCC, accounting for 1.7% (20/1187) and 1.5% (7/471) of metastatic sites, respectively. Distant lymph nodes were the most common metastatic sites (24.8%; 294/1187) in patients with gastric SRCC. On the other hand, in patients with colorectal SRCC, the liver was the most common metastatic site (18.7%; 88/471). In terms of the metastatic pattern, several differences were found between patients with gastric and colorectal SRCC (Fig. 2). The incidences of bone metastasis (23.8% vs 13.1%, P < 0.05), lung metastasis (14.5% vs 12.7%, P < 0.05), and distant lymph node metastasis (37.4% vs 31.2%, P < 0.05) were higher in gastric SRCC patients than in colorectal SRCC patients, while the incidence of liver metastasis (21.8% vs 39.8%, P < 0.05) was lower in gastric SRCC patients than in colorectal SRCC patients. No difference was found in the incidence of brain metastasis in patients with gastric and colorectal SRCC.
《Fig. 2》

Fig. 2. Distribution of distant metastatic sites in gastric SRCC and colorectal SRCC. *: P < 0.05 (chi-square test); DL: distant lymph node.
《3.2. Survival analysis》
3.2. Survival analysis
Because of the short duration of the follow-up, only the oneyear overall survival (OS) and cancer-specific survival (CSS) of patients with any distant metastases were calculated. Among patients with gastric SRCC, the one-year OS was 12.3%, 5.0%, 14.6%, 13.3%, and 21.8% in patients with bone, brain, liver, lung, and distant lymph node metastasis, respectively. In addition, the one-year CSS among patients with gastric SRCC was 21.4%, 5.0%, 25.1%, 23.7%, and 29.6% in patients with bone, brain, liver, lung, and distant lymph node metastasis, respectively. However, among patients with colorectal SRCC, the one-year OS was 6.9%, 14.3%, 31.8%, 25.0%, and 24.6%, and the one-year CSS was 13.8%, 14.3%, 42.0%, 32.1%, and 34.8% in patients with bone, brain, liver, lung, and distant lymph node metastasis, respectively.
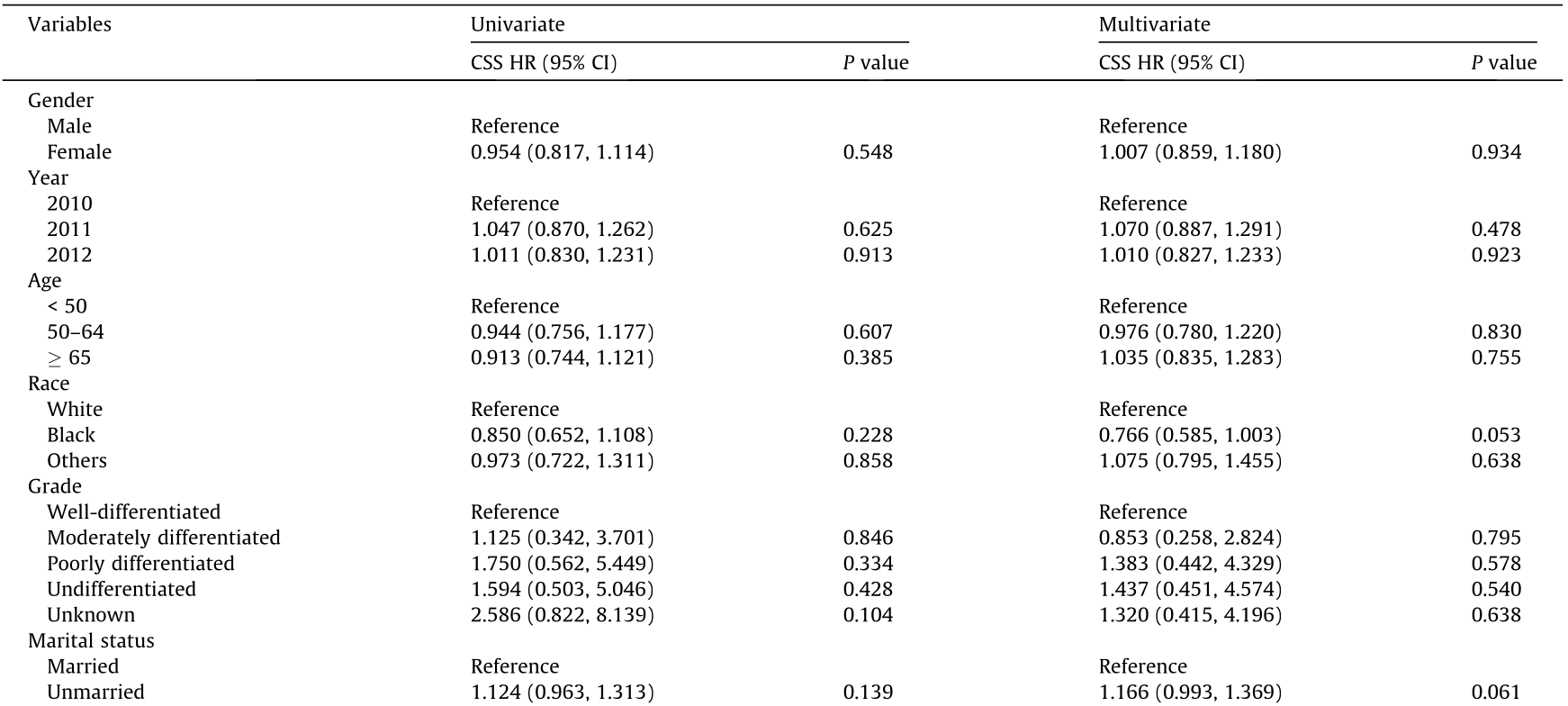
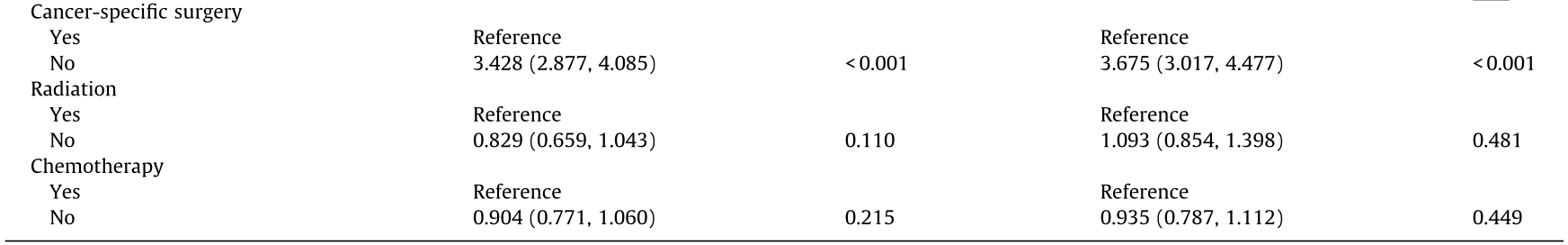
To determine whether other factors may influence survival, we conducted univariate and multivariate Cox proportional hazards regression analyses. The variables affecting survival were not identical in gastric and colorectal SRCC. In gastric SRCC, race and all treatment modalities influenced the CSS (Table 2). In contrast, in colorectal SRCC, only cancer-specific surgery had an impact on the CSS (Table 3). Furthermore, different metastatic sites were associated with a long-term risk of mortality (Table 4 and Fig. 3). In gastric SRCC, patients with brain metastases had a worse prognosis than patients without metastasis (CSS hazard ratio (HR), 1.705; 95% confidence interval (CI), 1.068–2.742; P = 0.025). In addition, in colorectal SRCC, patients who had distant lymph node metastasis at the time of diagnosis had the worst prognosis compared with patients with metastases at any other sites (CSS HR, 1.997; 95% CI, 1.493–2.670; P < 0.001).
《Table 2》
Table 2 Univariate and multivariate analyses of CSS in patients with gastric SRCC in the SEER database.
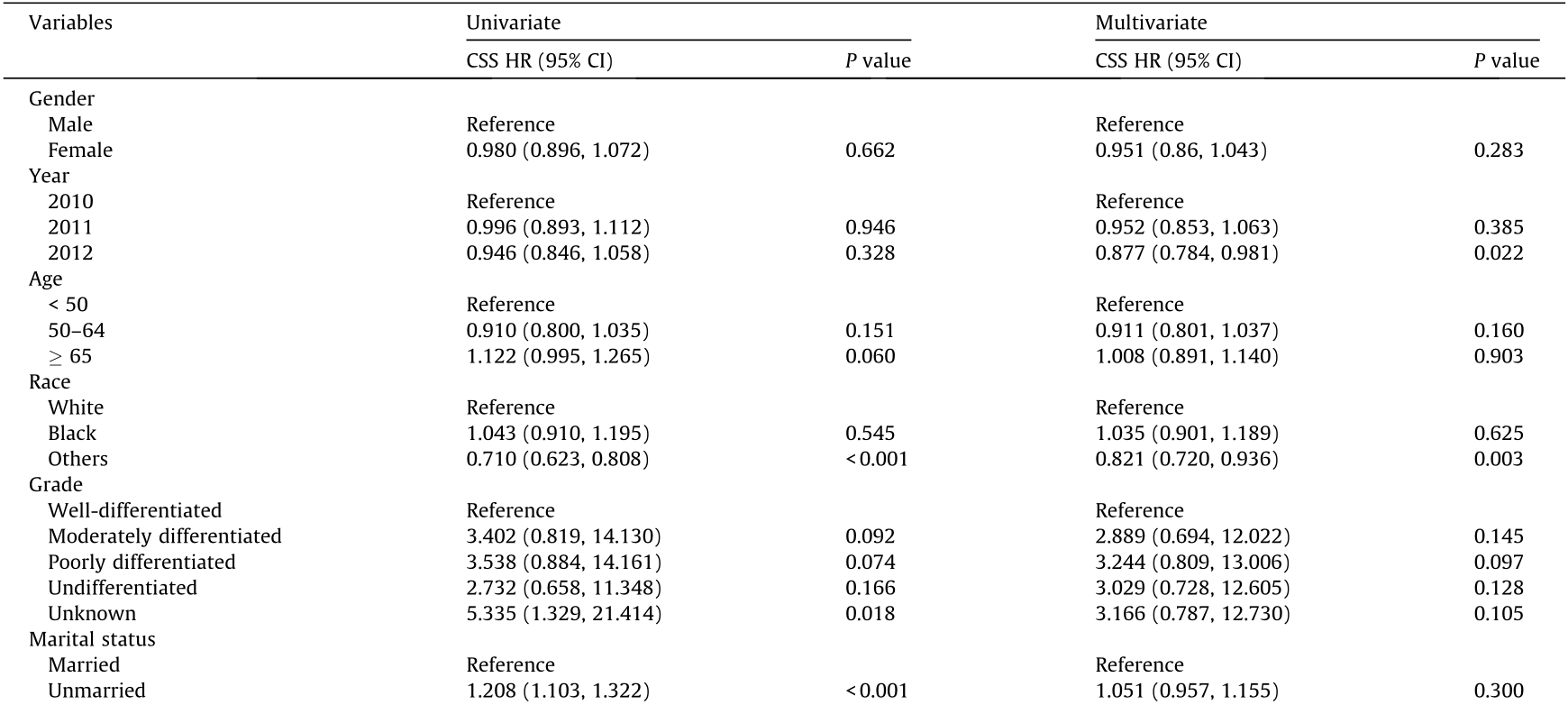
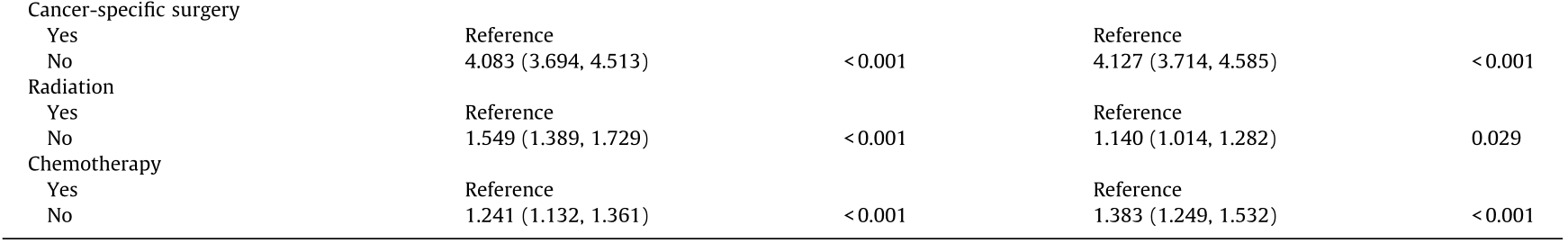
HR: hazard ratio; CI: confidence interval.
《Table 3》
Table 3 Univariate and multivariate analyses of CSS in patients with colorectal SRCC in the SEER database.
《Table 4》
Table 4 Multivariate analysis of CSS in patients with gastric and colorectal SRCC stratified by metastatic site.

Note: Adjusted for race, gender, age, marital status, grade, and therapies.
《3.3. Relationship between metastatic status and survival in gastric and colorectal SRCC by race, gender, and marital status》
3.3. Relationship between metastatic status and survival in gastric and colorectal SRCC by race, gender, and marital status
Studies have demonstrated that race, gender, and marital status impact the prognosis of multiple cancers [9–11]. We further examined the effect of these factors on the metastasis of gastric and colorectal SRCC (Table 5 and Appendix A. Table S1). For different races, the HR was smaller in white patients than in their black counterparts for both gastric SRCC (CSS HR, 2.271; 95% CI, 1.987– 2.595; P < 0.001) and colorectal SRCC (CSS HR, 3.181; 95% CI, 2.598–3.894; P < 0.001). For gastric SRCC, the influence of metastasis on CSS was greater in female patients (CSS HR, 2.500; 95% CI, 2.112–2.958; P < 0.001) and married patients (CSS HR, 2.604; 95% CI, 2.219–3.056; P < 0.001) than in male patients (CSS HR, 2.334; 95% CI, 1.991–2.735; P < 0.001) and unmarried patients (CSS HR, 2.213; 95% CI, 1.873–2.615; P < 0.001), respectively. In patients diagnosed with colorectal SRCC, the association between metastasis and CSS was stronger in male (CSS HR, 3.751; 95% CI, 2.901–4.852; P < 0.001) and unmarried patients (CSS HR, 4.448; 95% CI, 3.371–5.870; P < 0.001).
《Table 5》
Table 5 Relationship between metastatic status and CSS in gastric and colorectal SRCC by race, gender, and marital status (with non-metastatic status as the reference).
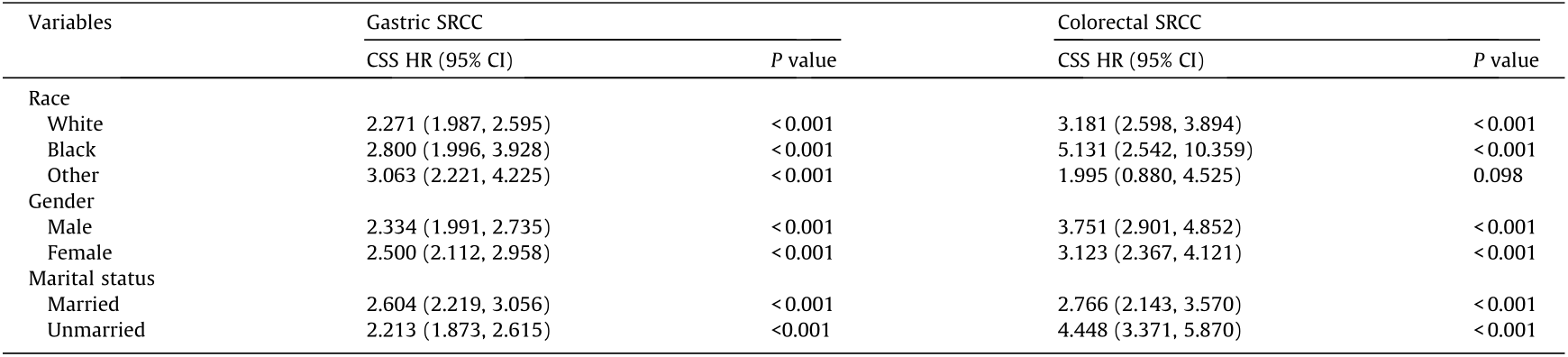
Adjusted for age, grade, and therapies.
《Fig. 3》

Fig. 3. Kaplan–Meier analysis of CSS in gastric SRCC and colorectal SRCC patients with and without metastasis. (a) Gastric and (b) colorectal SRCC patients with and without bone metastasis (log-rank P < 0.0001); (c) gastric and (d) colorectal SRCC patients with and without brain metastasis (log-rank P < 0.0001); (e) gastric and (f) colorectal SRCC patients with and without liver metastasis (log-rank P < 0.0001); (g) gastric and (h) colorectal SRCC patients with and without lung metastasis (log-rank P < 0.0001); (i) gastric and (j) colorectal SRCC patients with and without distant lymph node metastasis (log-rank P < 0.0001).
《4. Discussion》
4. Discussion
SRCC is a descriptive term denoting a carcinoma cell that has retained abundant intracytoplasmic mucin, causing the nucleus to be displaced to the periphery. Considering the aggressive biological behavior and extremely poor oncologic outcomes of SRCC, the clinical outcome is dismal. Until now, the prognostic determinants of SRCC have remained largely undefined. Thus, it is crucial to establish a clear understanding of the clinical features and metastatic patterns of SRCC.
Some large population-based studies have noted that SRCC commonly presents at a young age [12]. However, it remains controversial, as Psathakis et al. [13] reported a much older age at diagnosis, of 67.5 years old. Consistent with the latter study, SRCC was frequently diagnosed in older patients in this cohort, both for patients with gastric SRCC and those with colorectal SRCC. The pooled results of OS in patients with gastric and colorectal SRCC indicated that SRCC had a better prognosis in the early stage than in the advanced stage [14]. However, many patients with gastric or colorectal SRCC were confirmed as having advanced-stage disease at diagnosis. We found that nearly 40.9% and 41.0% of patients with gastric SRCC and colorectal SRCC, respectively, had advanced-stage disease at presentation. It is possible that delays in diagnosis occurred, with late manifestation of symptoms and the aggressive behavior of the tumor cells as the main reasons. A majority of patients with gastric or colorectal SRCC had tumors with diffusely infiltrative growth that did not penetrate the mucosa. In addition, no serious symptoms—such as hematochezia or severe abdominal pain—were present during the early stage of the disease. Some studies have suggested that SRCC is insensitive to chemotherapy, regardless of whether the treatment is administered pre- or postoperatively [15,16]. During the last several decades, the mismatch repair/microsatellite instability (MMR/MSI) status of RAS (KRAS and NRAS) and BRAF have been reported to affect the survival of patients with colorectal SRCC and may be linked to chemoradiotherapy efficacy [17–19]. In our study, we found that in gastric SRCC, chemotherapy was associated with decreased mortality in the multivariate analysis of CSS. A retrospective study also found that chemotherapy was an independent favorable prognostic factor for survival [20]. Among patients with colorectal SRCC, no significant difference was found between those who received chemotherapy and those who did not.
Clinically, colorectal SRCC most commonly metastasizes to the liver and peritoneum, although various other sites including bone, brain, spleen, and breast have also been described [21]. In contrast, gastric SRCC presents with a high incidence of peritoneal metastasis and lymphatic invasion at diagnosis [22]. The underlying mech anism for this metastatic pattern can be explained by the presence of loose clusters of cells and disruption of cell–cell adhesion driven by the absent expression of adhesive molecules such as E-cadherin and β-catenin [20,23]. These features are probably associated with the molecular etiology of colorectal SRCC. Moreover, we found that different metastatic sites had an influence on both OS and CSS (Appendix A. Tables S2–S4). In patients with gastric SRCC, the survival rates for patients with brain metastasis were worse than those for patients with any other metastatic sites. In patients with colorectal SRCC, patients with distant lymph node metastasis had the worst prognosis. In addition, we observed variations in the association of metastasis and prognosis across gender. For gastric SRCC, the association between metastasis and survival was stronger in female patients, which indicated that female patients with metastatic disease were at a higher risk of mortality. For colorectal SRCC, the influence of metastasis on CSS was attenuated in females. Unlike most tumors, gastric SRCC was frequent, poorly differentiated, and associated with a higher risk of metastasis in female patients [24]. Most likely, this gender difference can be attributed to the association between gastric SRCC and sex hormones such as estrogen. A study observed that 80% of gastric SRCC expressed estrogen receptors that influence tumor growth and invasion by estrogen [25]. Marital status has long been recognized as a prognostic factor in many cancers. Undertreatment and lack of social support in unmarried patients with colorectal SRCC partially explained this phenomenon. It has been suggested that the favorable effect of marriage is stronger in men than in women [26]. We found that the influence of metastasis on CSS was lesser in unmarried gastric SRCC patients but greater in unmarried colorectal SRCC patients. It may be necessary for other important factors such as socioeconomic, reproductive, hormonal, lifestyle, and other environmental factors to be taken into consideration in order to further clarify the role of marital status on cancer mortality.
These findings verify the association of the biological impacts of specific factors on the prognosis of gastric and colorectal SRCC, although the details remain unclear. We have summarized the metastatic patterns of SRCC among the metastatic sites recorded in the SEER database. Due to the nonrandomized nature of the SEER database, there are some limitations to this study. First, a high incidence of peritoneal metastasis in SRCC was not recorded in the SEER database, on which this study was based. In addition, metastases to the liver, lungs, bone, and brain were relatively low. Second, detailed information such as chemotherapeutic treatment, comorbidities, nutritional status, and recurrence were lacking in the SEER database. Thus, this study could not be adjusted for these potentially confounding factors.
In conclusion, metastasis is a significant risk factor for cancer mortality among patients with gastric SRCC or colorectal SRCC. Gastric SRCC patients with brain metastases were found to have the worst prognosis, while patients with distant lymph node metastases at the time of diagnosis of colorectal SRCC were found to have the highest mortality.
《Acknowledgements》
Acknowledgements
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program registries in providing high-quality open resources for researchers. This study was supported by the National Science Foundation of China (81330011 and 81790631) and the National Basic Research Program of China (2013CB531401).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jingjing Wu, Daiqiong Fang, Da Man, Wenrui Wu, Qing Wang, Yating Li, Jianzhong Ye, and Lanjuan Li declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2020.06.007.














 京公网安备 11010502051620号
京公网安备 11010502051620号




