Medical devices designed to be implanted in the body have gotten impressively tiny. To a great extent, this miniaturization has also decreased the invasiveness of the devices, simplifying implantation and reducing patient risk. Now, the devices are also getting smarter. Engineered to both transmit and measure clinically relevant physiological signals, two new state-of-the-art devices—a pacemaker and a deep brain stimulator (DBS)—can use sophisticated algorithms to autonomously modulate the electrical stimulation they provide. In addition to improving patient outcomes, these smart features are also expanding the potential clinical utility of the devices.
The first implantable pacemaker, a handmade device built by Rune Elmqvist and Ake Senning, was surgically embedded in a patient in 1958 [1]. Two years later, the first commercially available implantable pacemaker, invented by Wilson Greatbatch, was licensed to Medtronic [1]. For their pioneering work in realizing the medical promise of the pacemaker, Greatbatch and Earl E. Bakken, co-founders of Medtronic and developers of the first wearable, battery-powered, external pacemaker, shared the first Russ Prize, 500 000 USD awarded biannually to recognize achievement in bioengineering, in 2001 [2]. Currently, more than a dozen companies build implantable pacemakers and more than one million of the devices are implanted annually around the world [3].
Placed under the skin just beneath the collarbone, the plumsized first pacemakers had wire leads running to the right side of the ventricle the heart’s bottom chamber. The devices provided electrical stimulation at fixed intervals, mimicking the average person’s heart rate. Later generations of pacemakers were designed with additional wires leading to the left ventricle and the atrium, allowing for more targeted stimulation and treatment of a larger variety of specific heart defects. However, each additional wire increased the odds of infection. And the wires are known to break due to mechanical stress.
To untether the pacemaker from its leads, medical device manufacturer Medtronic developed the Micra VR. The 1.75 g device—the size of a large vitamin pill—is a ‘‘leadless” pacemaker that attaches directly to the muscle wall of the right ventricle using small tines. As an added bonus, the Micra, approved for use by the US Federal Drug Administration (FDA) in 2016 [4], is delivered to the heart percutaneously—not surgically—via a catheter slid through the femoral vein. There is no surgery scar on the chest and no tell-tale lump under the collarbone, and patient recovery after the procedure is quicker and associated with fewer complications. Powered by a battery lasting an average of 12 years, the Micra is currently the only leadless pacemaker available commercially; its 14 500 USD price is roughly 50% more than the 9200 USD for a conventional unit [5]. One other leadless pacemaker, Abbott’s Nanostim, was approved for use in Europe, also in 2016, but was recalled later that year after reports of premature battery failure and remains off the market [6].
‘‘The fundamental idea behind the Micra is that removing the need for the subcutaneous pocket and the wired leads can substantially reduce the number of complications,” said Leonardo Rapallini, vice president of research and development, cardiac rhythm management business at Medtronic, citing a study showing a 63% reduction in complications with the leadless devices [7].
The Micra VR, like many modern leaded pacemakers, is outfitted with accelerometers that can infer when a patient is walking briskly or going up stairs and increase the patient’s heart rate to accommodate. For its second-generation leadless pacemaker, the Micra AV (Fig. 1), Medtronic’s engineers repurposed these accelerometers to sense movements of the atrium, when it is filling with blood and then pushing blood out to the ventricle en route to the body. With this information, the Micra AV, which is the exact same size and shape as the Micra VR, employs a series of algorithms to autonomously time, on a beat-by-beat basis, the ventricular beat to immediately follow the atrial beat, as occurs in normal hearts.
《Fig. 1》
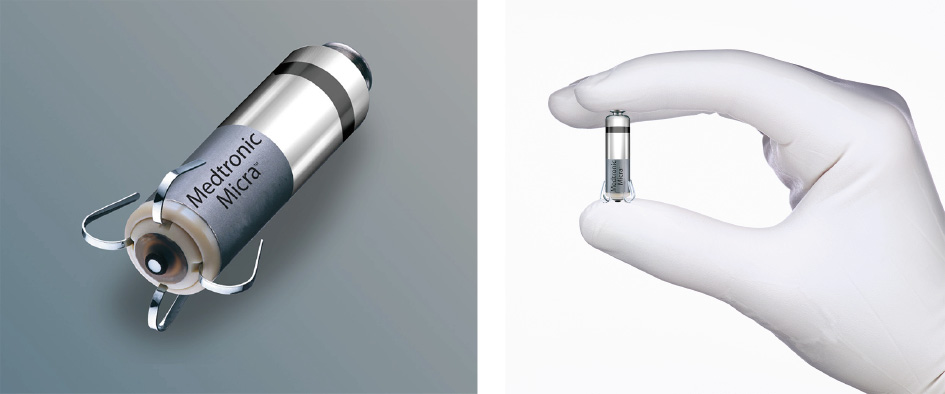
Fig. 1. Measuring 25.9 mm long with a diameter of 6.7 mm, Medtronic’s Micra AV ‘‘leadless” pacemaker is inserted into the wall of the heart’s right ventricle via a catheter. The novel device monitors the movements of the atria with onboard accelerometers, autonomously crunching the data in sophisticated algorithms to achieve atrioventricular synchrony, whereby the beating of the atria and ventricle are tightly coupled as in a normal heart. Credit: Medtronic (public domain).
This updated version of the Micra was approved by the FDA in January 2020 to treat atrioventricular (AV) block, which occurs when electrical signals between the atria and the ventricles become impaired [8]. While the Micra VR was appropriate for only about 15% of patients needing pacemakers, the ability of the Micra AV to tightly couple atrial and ventricular beats now makes it suitable to treat about half of all patients for whom pacemakers are indicated, Rapallini said.
‘‘With the Micra AV, we are basically expanding the population that might benefit from a leadless system to include patients with complete heart block,” said Mikhael El Chami, medical director of cardiac electrophysiology at Emory University Hospital Midtown in Atlanta, GA, USA. ‘‘While the Micra AV sensing algorithm is a very ingenious way to provide AV synchrony, it is by no means perfect. Younger patients with complete heart block will probably benefit more from a transvenous dual chamber pacemaker. In my practice, I mainly offer the Micra AV to elderly patients with complete heart block who I do not expect to be physically active enough to need AV synchrony with exercise.”
The Micra AV is not the only medical device Medtronic released this year that can fine-tune its stimulation parameters based on feedback signals from the body. In June, the company received FDA approval to sell the Percept PC, a DBS device indicated for the treatment of Parkinson’s disease, essential tremor, dystonia, epilepsy, and obsessive–compulsive disorder [9,10]. The Percept is a 61 g device with the shape of a standard pacemaker (Fig. 2). Implanted in the chest, the device sends electrical pulses via tiny wires snaked under the skin through the neck and skull to specific brain regions.
《Fig. 2》

Fig. 2. Medtronic’s Percept PC DBS device, measuring 68 mm × 58 mm, is designed to treat a variety of neurological disorders, including Parkinson’s disease. In addition to stimulating brain tissue, wires extending under the skin through the body from the chest-implanted device into the brain can also record brain signals that could be used to autonomously modulate stimulation parameters, potentially resulting in improved treatment. Credit: Medtronic (public domain).
‘‘One challenge with DBS is that it has always been open loop, meaning we can stimulate but we cannot measure the feedback from that stimulation, and we cannot adjust it over time,” said Steve Goetz, senior distinguished engineer at Medtronic. ‘‘It has always been our goal to develop a system with two-way communications that closes the loop.”
Medtronic engineers overcame this challenge by designing the Percept’s electrode wires to also record brain signals while therapy is delivered. Transmitted externally to patients and clinicians by wireless connectivity, these data can be correlated with patientreported events such as taking medications and experiencing symptoms and side effects. Ultimately, all this information can be used to precisely program, via a tablet or phone-based app, the Percept’s stimulation parameters.
‘‘For example, when Parkinson’s patients are on medications their symptoms go down and certain brain signals go down, but if those medications wear off before it is time for their next dose those signals come back and the symptoms come back,” Goetz said. ‘‘The Percept could monitor those signals and increase stimulation to make up for the waning medication dose. It is akin to cruise control keeping your car at a constant speed when driving uphill or downhill.”
In practice, individuals’ brain signals are extremely noisy, said Kip Ludwig, associate professor of biomedical engineering at the University of Wisconsin-Madison. This means it will be challenging, he said, to use them in real time to autonomously adjust treatment. At the moment, researchers are collecting data from patients with the Percept and using artificial intelligence to identify patterns that might be useful therapeutically. ‘‘Maybe we find that four specific electrodes firing in a certain pattern can predict the severity of tremor,” said Ludwig. ‘‘Knowing that, you can program the device to provide electrical stimulation only when those four electrodes are firing.”
Medtronic plans to begin a trial in 2021 to gather evidence in support of US FDA approval for an adaptive mode for the Percept, where the device’s stimulation settings can change automatically based on recorded neural signals. Being able to track neural signals will also mean the Percept will only need to stimulate when symptoms are occurring, therefore saving battery power. ‘‘Most patients don’t need all the stimulation they get and when we save energy that means replacement surgeries are less frequent or the patient has to recharge the device less often,” Goetz said. While not currently rechargeable like Medtronic’s Activa DBS device, future versions of Percept likely will be, Goetz said, possibly using a wireless inductive charger held in place just beneath the clavicle by a shoulder harness. Pacemakers, in contrast, are not currently designed to be rechargeable, primarily due to liability concerns related to relying on patients to take responsibility for the recharging process.
A potential concern with the Percept, or any medical device that transmits data to or accepts marching orders from external devices, is security. Any device with wireless capabilities is potentially vulnerable to being hacked, as Medtronic found out with a previous version of its user interface software [11]. A security flaw in the software’s update downloading process was identified that could allow connected devices to be hacked, though the company has not reported any successful attacks or harm to patients.
‘‘Security is a topic we take very seriously,” Goetz said. ‘‘We use the Medical Implant Communications System for wireless telemetry with our devices because it is more secure than other technologies like Bluetooth. We also make sure that the data that go between the device and the programmers are encrypted and properly authenticated.”
Whether the Percept’s advanced features will make DBS a more attractive option for patients remains to be seen. ‘‘With DBS for Parkinson’s disease, for example, we have seen that a patient’s symptoms have to be pretty severe before they will accept the risk of getting a burr hole and having a 1.2 mm set of wires stuck deep into their brain,” Ludwig said. ‘‘Are patients going to improve dramatically enough to really change the percentage of them willing to undergo the procedure? Realistically, unless the effects are huge, it could take a while to figure out how effective these devices can actually be.”













 京公网安备 11010502051620号
京公网安备 11010502051620号




