《1. Introduction》
1. Introduction
Irritable bowel syndrome (IBS) is a chronic intestinal disease that is usually accompanied by intestinal dysfunction but does not cause organic lesions [1]. The global prevalence of IBS currently exceeds 10% of the population [2]. Due to this high prevalence and the lack of a cure, IBS has placed an enormous burden on the healthcare system in various countries. Based on the characteristics of a patient’s stool (hard lumps, loose, or watery stools), IBS can be divided into four subtypes, IBS with diarrhea (IBS-D), IBS with constipation, mixed IBS, and untyped IBS [3]. IBS-D is a subtype of IBS, whose main symptoms include severe diarrhea, recurrent abdominal pain and bloating, and changes in defecation habits [4]. Because the clinical symptoms of IBS-D are more urgent than those of other IBS subtypes, IBS-D patients are more restricted in their diets and daily activities [5]. Moreover, among patients with various IBS subtypes, those with IBS-D must usually undergo more than ten times the number of examinations to determine the diagnosis, so the diagnosis cost is usually expensive [6]. With the development of society, environmental pollution and mental pressure are also increasing [7,8]. This social situation further leads to the popularity of IBS-D. Based on these circumstances, IBS-D patients experience more significant mental and economic stress. It is therefore essential to find an effective treatment for IBS-D as soon as possible.
Multiple studies have indicated that many physiological characteristics of IBS-D patients undergo significant changes. The protein expression levels of cyclooxygenase-2 and prostaglandin E2 were significantly higher in the colons of IBS-D patients than in those of healthy control subjects, which can lead to visceral hypersensitivity symptoms and aggravated functional intestinal disorders [9]. The activation of mast cells in IBS-D patients results in a large amount of tryptase and histamine release, which exacerbates the degradation of occludin and increases intestinal permeability [10]. Moreover, IBS-D patients also show severe gut microbiota disorders. There was a significant increase in the abundance of the bacterial phyla Firmicutes and Proteobacteria and a decrease in the abundance of Bacteroidetes in the microbiota of IBS-D patients [11]. At the genus level, the abundance of several genera, like Bifidobacterium, Faecalibacterium, and Ruminococcus, also showed significant changes [12].
Probiotics are living microorganisms that colonize and play a beneficial role in the human body and are often used to alleviate various diseases because of their general recognition as being safe to consume [13]. Multiple animal models have proven that probiotics can alleviate gut microbiota dysbiosis, regulate the immune response, enhance intestinal barrier function, and inhibit visceral hypersensitivity [14–17]. In several clinical trials, some probiotics have been shown to be effective in alleviating IBS. Clostridium butyricum was reported could decrease the defecation frequency of IBS patients and improve their fatty acid metabolism [18]. The intervention of Bifidobacterium bifidum for eight weeks could significantly alleviate the abdominal pain and reduce the severity of IBS [19]. Supplementation of Bifidobacterium longum significantly reduces depression of IBS patients and improve their quality of life [20]. After four weeks of ingestion of Lactobacillus acidophilus, the bloating symptom of IBS patients decreased significantly, accompanied by a normalization of bowel habits [21]. These pieces of evidence indicate that probiotics are now receiving attention as a potential treatment for IBS.
Lactobacillus plantarum (L. plantarum) CCFM8610 is a probiotic strain previously isolated from fermented vegetables with superior physiological characteristics. In vitro experiments indicated that L. plantarum CCFM8610 has a survival rate of 83.18% ± 0.34% after being treated with the simulated gastrointestinal environment, an oligosaccharide fermentability with inulin, fructooligosaccharides, and galactooligosaccharides, a generation time of (124.2 ± 4.33) min, and a conjugated linoleic acid conversion rate of 57.39% ± 1.08% [22]. Compared with other Lactobacillus strains, L. plantarum CCFM8610 has significant advantages in these physiological characteristics. These excellent physiological characteristics are helpful for the strain to play a health-promoting role in vivo. In animal experiments, L. plantarum CCFM8610 has been demonstrated to significantly increase the expression of zonula occludens (ZO)-1, occludin, and claudin-1, thereby restoring intestinal barrier function [23]. In a colitis model, L. plantarum CCFM8610 reduced the expression of pro-inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α in the colon and inhibited the activation of the nuclear factor (NF)- jB-signaling pathway to relieve intestinal inflammation [22]. Clinical trials have also indicated that supplementation with L. plantarum CCFM8610 significantly restores gut microbiota diversity and improves gut microbiota composition [24]. Therefore, we believe that this strain has the potential to alleviate the clinical symptoms of IBS-D patients. To test this hypothesis, we designed a randomized, double-blind, placebo-controlled, pilot clinical study to investigate the efficacy of L. plantarum CCFM8610 in alleviating IBS-D. The study design and results provide new evidence for probiotics as a treatment to alleviate IBS-D symptoms and lay a foundation to explore the mechanism of probiotic treatment to alleviate IBS more broadly.
《2. Materials and methods》
2. Materials and methods
《2.1. Study design and ethics statement》
2.1. Study design and ethics statement
The study was a 12-week, randomized, double-blind, placebocontrolled, pilot clinical study conducted in the Tinghu People’s Hospital of Yancheng, Jiangsu, China. The study design and ethics statement were approved by the Ethics Committee of Tinghu People’s Hospital of Yancheng (Ethical No. ET2017015) and registered at Chinese Clinical Trial Registry (ChiCTR)↑ (↑ http://www.chictr.org.cn/index.aspx)(Registration No.: ChiCTR1800014886). The study was conducted in accordance with the Helsinki Declaration, and informed consent was obtained from each participant.
《2.2. Study population》
2.2. Study population
Patient recruitment for this study was conducted in February and March 2018. According to the research of Niv et al. [25], we have formulated the inclusion and exclusion criteria for this study. Patients at least 18 years of age with a diagnosis of IBS-D were recruited in this study. Two digestive physicians evaluated patients for IBS-D symptoms based on the Rome III criteria. Moreover, the patient needs to agree to provide an informed consent form. However, the following groups were excluded: patients with infectious diseases or gastrointestinal cancer; patients with preexisting inflammatory bowel disease or other intestinal diseases; patients who had taken anti-diarrheal drugs or antibiotics within the past month; patients who had taken microecologics, dietary supplements or probiotics within the past month; patients who regularly drank yogurt or probiotic beverages; patients with significant recent changes in their eating habits; and women who were or hoped to become pregnant. During the study, any patient who failed to take the probiotics on time, failed to retain their fecal samples, or failed to complete the questionnaire were considered to be automatically withdrawn.
《2.3. Study products》
2.3. Study products
The products used in this study included placebo products, oligosaccharide products, and L. plantarum CCFM8610 products. All products were in the form of powder and had the same appearance and the same packaging (2 g·pack-1 ). The ingredient of the placebo product was maltodextrin. Oligosaccharides product consisted of 5% maltodextrin and oligosaccharides (7.8% inulin, 15.6% galactooligosaccharides, and 71.6% fructooligosaccharides). L. plantarum CCFM8610 product contained L. plantarum CCFM8610 (5%, > 5 × 109 colony-forming units (CFU) per gram) and oligosaccharides with an identical amount to oligosaccharides product. All products need to be stored at 4 °C. The viable bacterial amount of L. plantarum CCFM8610 in the product remains over 5 × 109 CFU·g-1 within three months.
《2.4. Randomization and intervention》
2.4. Randomization and intervention
Initially, a total of 96 patients were recruited in this study. After a physical examination, 21 patients were excluded because they did not meet the study requirements. According to the computer-generated random number table, the remaining 75 patients were divided into three groups (placebo group, oligosaccharides group, and L. plantarum CCFM8610 group), with 25 patients in each group.
The total length of the study was 12 weeks, with a run-in period of two weeks (weeks 1 and 2), an intervention period of eight weeks (weeks 3–10), and a 2-week follow-up observation period (weeks 11 and 12). The purpose of the run-in period was to allow patients to check their IBS-D symptoms. Each group of patients was required to take the corresponding product (2 g) daily during the intervention period. During the follow-up observation period, the patients stopped taking the probiotics but were asked to report any adverse health conditions.
Moreover, throughout the study, the patients were required to refrain from taking antibiotics; any drugs related to gastrointestinal problems; or other probiotics, probiotic drinks, yogurts, microecological agents, or dietary supplements. The patients were also asked not to make significant changes in their eating habits and not to participate in other clinical trials.
《2.5. Questionnaire survey》
2.5. Questionnaire survey
According to the research of Niv et al. [25], the IBS symptom severity scale (IBS-SSS) and IBS quality of life scale (IBS-QOL) were used in this study to investigate the clinical symptoms and quality of life of IBS-D patients. With the help of a physician, patients reported their IBS-SSS score and IBS-QOL score in weeks 3, 7, and 11.
《2.6. Feces sample collection and fecal microbiota analysis》
2.6. Feces sample collection and fecal microbiota analysis
One feces sample collection was arranged in this study at week 11. Fresh fecal samples were collected and stored at –80 °C. Fecal DNA was extracted according to the instructions of the FastDNA® Spin kit (MP Biomedicals Ltd., USA). Polymerase chain reaction (PCR) and sequencing of gut microbiota were performed according to the method described by Wang et al. [26]. Principal component analysis (PCA) and linear discriminant analysis effect size (LEfSe) analysis were used to analyze the species in the gut microbiota.
《2.7. Statistical analysis》
2.7. Statistical analysis
The measurement data that fit the normal distribution in this study were expressed as mean ± standard deviation (SD). Oneway analysis of variance (ANOVA) was used to analyze the results, followed by Tukey’s multiple comparison test to determine statistical significance.
The measurement data in this study that did not meet the normal distribution were expressed as medians (interquartile range). A nonparametric test was used to analyze the results. The Friedman rank-sum test was used to determine the statistical significance of the IBS-SSS and IBS-QOL questionnaire data due to the pairing properties of their samples. The Kruskal–Wallis ANOVA was used to determine the statistical significance of the gut microbiota data due to the independent properties of their samples.
The enumeration data were directly expressed as a number. A chi-square test was used to determine the statistical significance of the primary data of patients, and the Mann–Whitney U test was used to determine the statistical significance of the severity of IBS in different groups. p-values less than 0.05 were considered to be statistically significant.
《3. Results》
3. Results
《3.1. Patients and baseline characteristics》
3.1. Patients and baseline characteristics
Sixty-three patients completed the study. Twelve patients were lost to follow-up and were excluded from the study (Fig. 1). The primary data of patients in each group are shown in Table 1. No significant differences were seen in the baseline characteristics of each group.
《Fig. 1》

Fig. 1. Flowchart of patients.
《Table 1》
Table 1 Baseline characteristics of patients in each group.
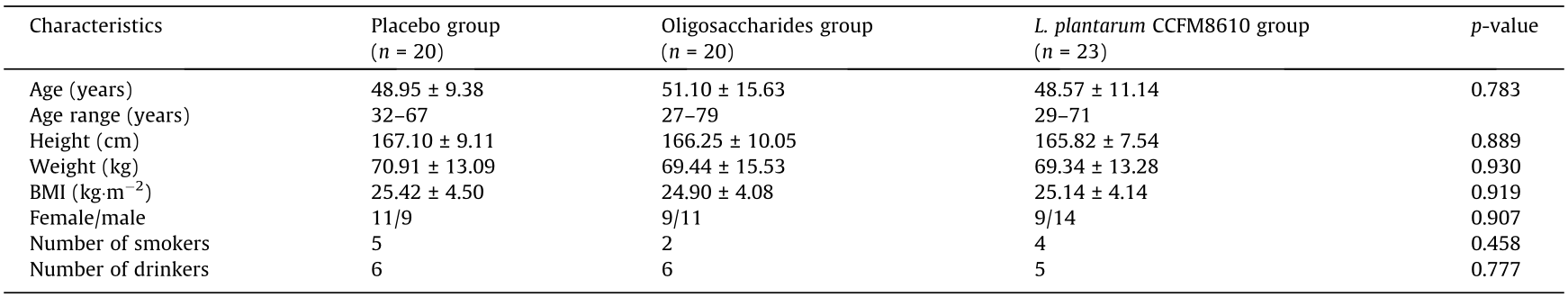
Age, height, weight, and body mass index (BMI) data were analyzed by one-way ANOVA; gender, smoking, and drinking data were analyzed by the chi-square test.
《3.2. Effects of L. plantarum CCFM8610 on IBS-D clinical symptoms and IBS-D severity》
3.2. Effects of L. plantarum CCFM8610 on IBS-D clinical symptoms and IBS-D severity
The IBS-SSS questionnaire scores changed significantly after the 8-week intervention period (Table 2). Patients who took the placebo reported a significant improvement in their bowel habit satisfaction (p < 0.05), but this improvement occurred only in the first four weeks of the intervention period, and no improvement was seen in the last four weeks. Moreover, during the 8-week intervention period, no significant change was seen in the total IBS-SSS scores of this group (p > 0.05). The patients in the L. plantarum CCFM8610 group indicated improvements in their bloating symptoms, bowel habit satisfaction, and life interference scores after supplementation with L. plantarum CCFM8610 and continued to improve over eight weeks (p < 0.05). After eight weeks of treatment, the total IBS-SSS scores of L. plantarum CCFM8610 group patients decreased significantly (p < 0.05). The patients in the oligosaccharides group did not show any significant improvement in IBS symptoms (p > 0.05).
The number of patients with severe IBS symptoms in the placebo group increased after eight weeks of intervention (Table 2). No significant difference was seen in the number of patients with different symptoms between week 3 and week 11 (p > 0.05). Similarly, no significant changes in the number of patients with different symptoms were observed in the oligosaccharides group (p > 0.05). However, after eight weeks of treatment, many patients with moderate symptoms who ingested L. plantarum CCFM8610 reported only mild symptoms. Furthermore, all patients with severe symptoms in the L. plantarum CCFM8610 group saw improvement to moderate symptoms. This indicates L. plantarum CCFM8610 effectively alleviated overall IBS symptoms (p < 0.05).
《3.3. Effects of L. plantarum CCFM8610 on quality of life of IBS-D patients》
3.3. Effects of L. plantarum CCFM8610 on quality of life of IBS-D patients
The results of the IBS-QOL survey show that the effects of the different products were quite distinct (Table 2). The patients in the placebo group did not report any significant changes in their total IBS-QOL scores. However, they reported fewer concerns about their health after taking the placebo (p < 0.05). The same conclusions were also reported by the oligosaccharides group (p < 0.05), and the patients in the oligosaccharides group also reported a significant reduction in their concerns about interpersonal relationships (p < 0.05). However, the total IBS-QOL score for this group of patients, including the emotion score, activity interference score, and food avoidance score, showed a significant increase in week 7 (p < 0.05). Similar results also appeared in some sub-scoring items of the placebo group (such as emotion, body image, and interpersonal relationships), but the changes were not statistically significant. The scores returned to baseline by week 11. This indicates that IBS-D symptoms significantly affected quality of life during the intervention period. Compared with placebo and oligosaccharides, L. plantarum CCFM8610 significantly reduced the impact of IBS on the patients’ quality of life. The patients in the L. plantarum CCFM8610 group reported significant improvements in emotion control, diet control, social reaction, and overall quality of life (p < 0.05).
《Table 2》
Table 2 IBS-SSS questionnaire scores and IBS-QOL questionnaire scores at weeks 3, 7, and 11.


The severity of IBS was determined by the IBS-SSS total score. Mild symptoms: 75–175 points; moderate symptoms: 176–300 points; severe symptoms: > 300 points. The number in parentheses indicated the interquartile range of the data.
《3.4. Effects of L. plantarum CCFM8610 on gut microbiota diversity and composition of IBS-D patients》
3.4. Effects of L. plantarum CCFM8610 on gut microbiota diversity and composition of IBS-D patients
The Simpson index and Shannon index of patients in the L. plantarum CCFM8610 group were significantly higher than those in the placebo group (Figs. 2(a) and (b)). This indicates that the intervention of L. plantarum CCFM8610 increased the diversity of gut microbiota. Treatment with oligosaccharides had no significant effect.
The relative abundance of the gut microbiota at the genus level in each group differed significantly (Fig. 2(c)). The relative abundance of the genus Prevotella in the placebo group was the highest of all genera in all groups. However, treatment with probiotics or oligosaccharides significantly altered the gut microbiota composition. Compared with other groups, the oligosaccharides group experienced a significant increase in the relative abundance of several genera. The oral administration of L. plantarum CCFM8610 resulted in a higher relative abundance of Bifidobacterium.
《Fig. 2》

Fig. 2. Gut microbiota alpha diversity and main bacterial genera relative abundance in different groups. (a) Simpson index; (b) Shannon index; (c) heat map of genera percentage in bacteria with relative abundances > 0.001. All data were analyzed by one-way ANOVA and Tukey’s multiple comparison test. Letters a and b indicate statistically significant differences (p < 0.05).
The results of PCA demonstrate that supplementation with oligosaccharides or probiotics could change the composition of the gut microbiota (Fig. 3(a)). However, after eight weeks of intervention, the changes in gut microbiota varied among groups. Compared to the placebo group, the ingestion of probiotics and oligosaccharides significantly increased the relative abundance of the genera Bacteroides and decreased the relative abundance of Prevotella (Figs. 3(b)–(d)). In the L. plantarum CCFM8610 group, a significant increase was seen in the abundance of several genera, such as Ruminococcus and Parabacteroides. We also found that the relative abundance of a number of butyric acid-producing species changed significantly in the L. plantarum CCFM8610 group (Fig. 3(c)).
《Fig. 3》
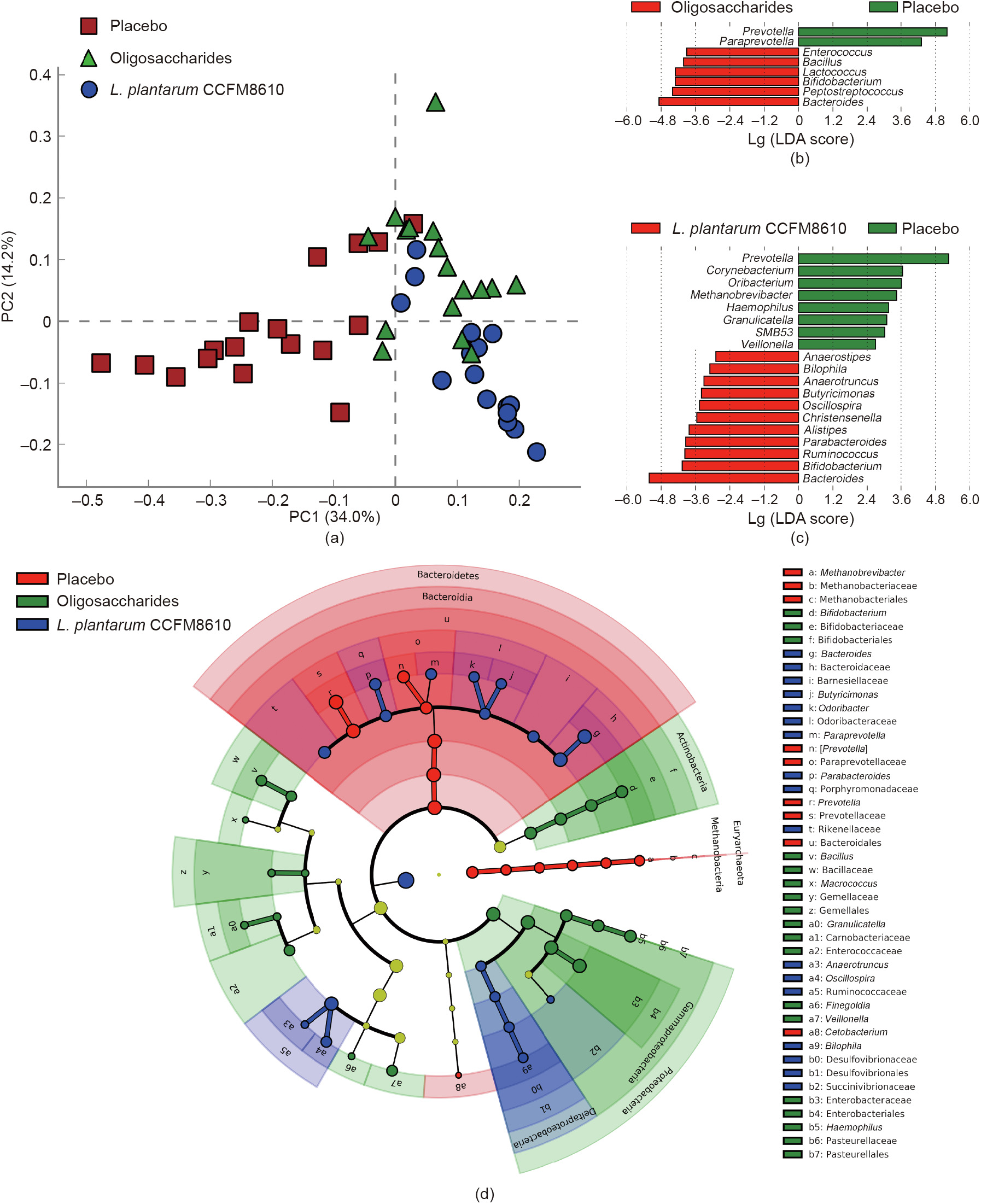
Fig. 3. Principal component analysis and LEfSe analysis of gut microbiota composition in different groups. (a) Principal component (PC) analysis of gut microbiota; (b) least discriminant analysis (LDA) scores at the genus level of the placebo group and oligosaccharides group; (c) LDA scores at the genus level of the placebo group and L. plantarum CCFM8610 group; (d) taxonomic cladogram.
《3.5. Effects of L. plantarum CCFM8610 on the relative abundance of butyric acid-producing genera and bloating-related genus in IBS-D patients》
3.5. Effects of L. plantarum CCFM8610 on the relative abundance of butyric acid-producing genera and bloating-related genus in IBS-D patients
The bacterial genera Anaerostipes, Anaerotruncus, Bifidobacterium, Butyricimonas, and Odoribacter have notable butyric acidproducing abilities and also significantly changed in relative abundance after the intervention (Figs. 4(a)–(e)). Compared with the placebo group, only the L. plantarum CCFM8610 group significantly increased the relative abundance of Anaerostipes, Anaerotruncus, Bifidobacterium, Butyricimonas, and Odoribacter (p < 0.05). The relative abundance of Methanobrevibacter is related to the production of gas in the intestinal tract. The oral administration of L. plantarum CCFM8610 could significantly reduce the relative abundance of Methanobrevibacter (Fig. 4(f); p < 0.05). Treatment with oligosaccharides could regulate the relative abundance of some genera, but no significant difference was seen with the placebo treatment.
《Fig. 4》

Fig. 4. Relative abundance of butyric acid-producing genera and bloating-related genus. (a) Anaerostipes; (b) Anaerotruncus; (c) Bifidobacterium; (d) Butyricimonas; (e) Odoribacter; (f) Methanobrevibacter. All data were analyzed by the Kruskal–Wallis ANOVA test and expressed as median ± interquartile range. Letters a, b, and c indicate statistically significant differences (p < 0.05).
《4. Discussion》
4. Discussion
This study aimed to investigate the IBS-D symptom-alleviating effects of the bacterial strain L. plantarum CCFM8610, a probiotic strain with multiple health-promoting functions that we screened in a previous study. We designed a randomized, double-blind, placebo-controlled pilot clinical trial and analyzed the ability of L. plantarum CCFM8610 to alleviate the clinical symptoms of IBS-D, improve the quality of life of patients, and alleviate gut microbiota dysbiosis.
In this study, patients were divided into three groups and were given three kinds of study products. In the L. plantarum CCFM8610 product, oligosaccharides were added as an adjuvant, because the oligosaccharides were reported could regulate immune response and promote the proliferation of beneficial microbiome in the intestine [27,28]. However, whether oligosaccharides could alleviate IBS-D has always been controversial [29,30]. Therefore, we set up a separate group to investigate the effects of oligosaccharides on alleviating IBS-D. The results show that oligosaccharides had no IBS-D-alleviating effects. This indicates that the alleviating effect of L. plantarum CCFM8610 products on IBS-D comes from the probiotic strain L. plantarum CCFM8610 alone.
IBS-D is a subtype of IBS, and its severity is greatly affected by the patient’s physiological status, psychological state, dietary habits, and other environmental factors [4]. Compared with other subtypes of IBS, IBS-D usually causes frequent abdominal pain and bloating, bad bowel habits, severe activity impairment, harsh food avoidance, and high treatment costs [31]. Also, patients’ economic productivity is greatly impaired by the clinical symptoms of IBS-D [32]. Due to these factors, the quality of life of IBS-D patients is frequently low [5]. To explore the alleviating effects of L. plantarum CCFM8610 on the quality of life of IBS-D patients, we set up two questionnaire surveys during the study to examine the patients’ feelings.
The IBS-SSS is a questionnaire commonly used in clinical studies to determine the severity of IBS. We found that, of the three intervention strategies in this study, only L. plantarum CCFM8610 reduced the patients’ total IBS-SSS scores (Table 2). These results show that this strain can alleviate the clinical symptoms of IBS-D. Further analysis of the sub-scoring items of the IBS-SSS shows that L. plantarum CCFM8610 significantly alleviated the bloating symptoms and improved bowel habit satisfaction. Bloating is one of the main clinical symptoms of IBS-D and is usually associated with visceral hypersensitivity [33]. For IBS-D patients, visceral hypersensitivity is characterized by an enhanced sense of pain in the intestinal tract, which is essentially a disorder of the peripheral and central nervous systems [34]. This nervous system disorder reduces the threshold of gastrointestinal irritation, so IBS-D patients often suffer from abdominal pain and bloating [33]. In animal models, Lactobacillus species have been proven to inhibit visceral hypersensitivity by increasing the pain threshold, reducing the contractile response of colonic smooth muscle, inhibiting the concentrations of serum corticosterone and spinal neurotransmitters, and regulating the hypothalamus–pituitary– adrenal axis [35,36]. Our results are similar to these conclusions. Therefore, we speculated that the strain L. plantarum CCFM8610 might have similar effects and thereby alleviate the clinical symptoms of IBS-D in humans.
Except for visceral hypersensitivity, IBS-D patients also have typical symptoms of lactose and fructose malabsorption, which prevents the full absorption of dietary carbohydrates in the small intestine. These unabsorbed carbohydrates are fermented by colonic microorganisms to produce a large amount of gas, resulting in severe bloating symptoms [37]. L. plantarum usually plays a probiotic role in the colon. Due to the excellent adhesion and sugar fermentation abilities of L. plantarum, the unfermented sugars in the diet can be thoroughly absorbed in the colon to reduce gas production [38,39]. Therefore, we propose that in addition to inhibiting visceral hypersensitivity, L. plantarum CCFM8610 may also inhibit bloating symptoms by reducing gas production in the colon.
We found that both L. plantarum CCFM8610 and a placebo treatment could improve patients’ bowel habit satisfaction. However, the effect of the placebo appeared only in the first four weeks of the intervention period, whereas the effect of L. plantarum CCFM8610 was consistent throughout the intervention period. In a previous study, L. plantarum CCFM8610 was shown to improve intestinal peristalsis [40], but no placebo (such as maltodextrin) has been reported to exert similar effects. Therefore, it is reasonable to speculate that the improvement of bowel habit satisfaction in the placebo group patients may be due to the placebo effect [41]. L. plantarum CCFM8610 also significantly reduced the number of patients with severe and moderate IBS-D symptoms, but no significant change was seen in the number of patients with various symptoms in the placebo group (Table 2).
We used the IBS-QOL to quantitatively evaluate the patients’ quality of life. After analyzing the results of the IBS-QOL, we found that treatment with L. plantarum CCFM8610 significantly improved the total IBS-QOL score, alleviated concerns about diet and social impression, and improved emotional fluctuations (Table 2). Some foods can cause abdominal pain and abdominal distension, so IBS-D patients often have many restrictions on their diet [4]. Also, the clinical symptoms of IBS-D often lead patients to avoid social activities and group travel, and these patients are usually worried about their social impressions [32,42]. The inconveniences in diet and social activities directly cause significant emotional changes in IBS-D patients. The gut and the brain can communicate in both directions via the gut–brain axis [43]. Intestinal stimulation and discomfort can lead to emotional changes in patients, and these emotional changes can cause intestinal visceral hypersensitivity symptoms and intestinal stress reactions via the vagus nerve [44]. Emotional changes such as depression, anxiety, and compulsion in patients may also aggravate the symptoms of IBS-D and reduce quality of life [44,45]. A meta-analysis of clinical trials of anxiety and depression by Liu et al. [46] found that Lactobacillus has the effect of alleviating anxiety and depression, whereas placebos and oligosaccharides do not. This result suggested that L. plantarum CCFM8610 might regulate the emotions of IBS-D patients. Moreover, due to the excellent carbohydrate utilization character of L. plantarum CCFM8610, the bloating symptoms were alleviated, and concerns about the patients’ diet were improved. Hence, we believe that L. plantarum CCFM8610 can alleviate concerns about diet and improve emotional fluctuations, thus improving the quality of life of IBS-D patients.
Gut microbiota dysbiosis is an important feature of IBS-D [47]. Compared with healthy people, significant changes are seen in the composition of gut microbiota in IBS-D patients, and the diversity of gut microbiota is decreased [48]. Many studies suggest that gut microbiota dysbiosis might be one of the causes of IBS-D [49]. To explore the effects of probiotics on the gut microbiota of IBS-D patients, we sequenced the fecal microbiota and analyzed the diversity and differences in species between each group at the genus level. The results show that the intake of L. plantarum CCFM8610 significantly increased gut microbiota diversity and serve as preliminary evidence that probiotics alleviate gut microbiota dysbiosis (Fig. 2). After further analysis of the various genera between each group, we found that in the placebo group Bacteroides were at a low relative abundance, whereas Prevotella had a high relative abundance. The intervention of oligosaccharides or probiotics can increase the relative abundance of Bacteroides and reduce the relative abundance of Prevotella. This change of relative abundances moves the composition of gut microbiota of IBS-D patients closer to that of healthy people [50,51].
Moreover, except for Bacteroides and Prevotella, we found significant differences among the groups in the relative abundance of Methanobrevibacter, which is a type of anaerobic bacteria that can use hydrogen to reduce carbon dioxide to methane. Due to its gas-producing character, the increase in the relative abundance of Methanobrevibacter often causes severe gastrointestinal flatulence and exacerbates the clinical symptoms of IBS-D [52,53]. In this study, L. plantarum CCFM8610 could reduce the relative abundance of Methanobrevibacter, which is consistent with previous results in which L. plantarum CCFM8610 significantly alleviated bloating symptoms in IBS-D patients. We conclude that L. plantarum CCFM8610 alleviates the IBS-D clinical symptoms by partially alleviating the dysbiosis of gut microbiota.
Compared with other reported probiotics with IBS-D-alleviating effects, we found that L. plantarum CCFM8610 significantly increased the relative abundance of butyric acid-producing bacteria. At the genus level, a series of butyric acid-producing species, such as Anaerostipes, Anaerotruncus, Bifidobacterium, Butyricimonas, and Odoribacter, showed a high relative abundance level in the L. plantarum CCFM8610 group patients. Butyric acid is a short-chain fatty acid with many health-promoting effects. In the gut, butyric acid can reduce the expression of NF-κB, inhibit TNF-α release by macrophages, and activate the transcription factor hypoxiainducible factor (HIF)-1 in intestinal epithelial cells, thus maintaining the integrity of the intestinal barrier, reducing the inflammatory response of the intestine, and alleviating acute neuropathic pain and bloating [54–56]. Butyrate is also an important histone deacetylase inhibitor that can promote histone acetylation in the host organism, thereby affecting brain function and possibly inhibiting depression [57,58]. The relative abundance of butyric acid-producing genera in the gut of IBS-D patients was significantly lower than in healthy people [59]. Therefore, the increase in butyric acid concentrations in the gut may achieve the dual regulation of IBS-D clinical symptoms and emotions. The fermentation of undigested carbohydrates in the gut by gut microbiota is the primary source of butyric acid [60]. Therefore, one possible mechanism could be that the oral administration of L. plantarum CCFM8610 significantly increases the relative abundance of butyric acid-producing genera, which then promote the production of butyric acid in the intestine, thereby alleviating the clinical symptoms and negative emotions of IBS-D patients. This indicates that the alleviating effects of L. plantarum CCFM8610 on IBS-D clinical symptoms and quality of life are related to the increase of the relative abundance of butyric acid-producing genera. Meanwhile, some studies have demonstrated that the low gut microbiota diversity of IBS-D patients is related to the low relative abundance of butyric acid-producing genera [59]. Therefore, the enhancement of the relative abundance of butyric acid-producing genera by L. plantarum CCFM8610 should be one of the mechanisms for the recovery of gut microbiota dysbiosis in IBS-D patients. Similar results have not been reported in previous studies of probiotics that alleviate IBS-D. This indicates that increasing the relative abundance of butyric acid-producing bacterial genera may be a strain-specific effect that is unique to L. plantarum CCFM8610. We believe that this is an advantage of the L. plantarum CCFM8610 strain in alleviating IBS-D.
In addition to the above conclusions, L. plantarum CCFM8610 also has significant advantages in safety over other treatments for IBS-D. Treatment options for IBS are increasing daily but are accompanied by more complicated side effects. A lowfermentable, oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet is only useful for some patients and can cause a massive change in the colon microbiota [61]. Chemical drugs such as alosetron and eluxadoline relieve diarrhea but also can cause severe ischemic colitis, constipation, pancreatitis, and sphincter spasms [62,63]. Some studies support the use of psychotherapy to alleviate IBS, but its efficacy has yet to be explored [4]. These results indicate that most treatments for IBS-D still have severe defects. There also have been reports of adverse health conditions caused by probiotics. This indicates that the probiotics are not an entirely safe dietary ingredient. So, we have investigated the previous literature on adverse health events caused by probiotics. We found that most of the reports of probiotic adverse health events occurred in specific populations, such as intensive care unit patients [64], the elderly with immunodeficiency and malnutrition [65], or structural heart disease patients [66]. For mild patients, probiotics hardly cause any adverse health events. The disease model of this study, IBS-D, does not cause any organic lesions. It is a relatively mild disease. Therefore, the possibility of adverse events is low. Furthermore, most of the adverse health events of probiotics are caused by Saccharomyces. There are very few adverse health events caused by Lactobacillus [67]. Among the reports of Lactobacillus adverse events, Lactobacillus rhamnosus adverse events are the most common, which may be related to fimbriae structure and extracellular polysaccharide composition [68]. Currently, there have been no reports of adverse events caused by L. plantarum. Moreover, before this study was conducted, our strain L. plantarum CCFM8610 had conducted several animal experiments [22,23,69] and one clinical study [24], and no adverse health events have occurred. Therefore, combined with the conclusion that no adverse events occurred in this study, we preliminarily concluded that the intervention of IBS-D with L. plantarum CCFM8610 is a healthy strategy.
《5. Conclusions》
5. Conclusions
The purpose of this study was to investigate the clinical effects of L. plantarum CCFM8610 on alleviating IBS-D symptoms. For this purpose, we designed a randomized, double-blind, placebocontrolled pilot clinical trial. The results show that L. plantarum CCFM8610 significantly restores the gut microbiota composition and diversity and alleviates clinical symptoms and negative emotions in IBS-D patients. We also found that the beneficial effects of L. plantarum CCFM8610 on gut microbiota, IBS-D clinical symptoms, and quality of life may be related to the increase in the relative abundance of butyric acid-producing bacterial genera. Our results verify the IBS-D-alleviating effect of probiotics and lay a foundation for the exploration of the mechanisms of alleviating IBS-D symptoms.
《Acknowledgements》
Acknowledgements
This work was supported by the National Natural Science Foundation of China Program (31871773 and 31820103010); National Key Research and Development Project (2018YFC1604206); Projects of Innovation and Development Pillar Program for Key Industries in Southern Xinjiang of Xinjiang Production and Construction Corps (2018DB002); National First-Class Discipline Program of Food Science and Technology (JUFSTR20180102); the Biotechnology and Biological Sciences Research Council (BBSRC) Newton Fund Joint Centre Award; and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Yang Liu, Xinjie Yu, Leilei Yu, Fengwei Tian, Jianxin Zhao, Hao Zhang, Long Qian, Qun Wang, Zhengqing Xue, Qixiao Zhai, and Wei Chen declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




