《1. Introduction》
1. Introduction
Pulmonary diseases present one of the most severe threats to human society. Since late 2019, the coronavirus disease 2019 (COVID-19) pandemic has significantly impacted the lifestyle, culture, and politics of almost everyone in the world. COVID-19 causes severe pulmonary dysfunction, which is a major cause of mortality for those affected [1]. This pulmonary disease also causes significant cardiovascular damage [2] and neurological problems [3], which could lead to chronic health issues such as an increased risk of stroke and heart failure. Before COVID-19, infectious airway diseases such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and influenza had already caused millions of mortalities. Likewise, chronic airway diseases such as asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, idiopathic pulmonary fibrosis (IPF), and lung cancer continue to impact millions of people around the world.
Diagnosis of lung disease is usually achieved through DNA amplification and sequencing, pathogen culturing, immunostaining, and medical image analysis in centralized labs. A wellrecognized problem that has persisted throughout the COVID-19 pandemic has been the poor global availability of rapid tests that can deliver results on-site. The shortcomings in COVID-19 diagnostics highlight some of the fundamental limitations of existing tests and motivate a significant need for innovations in diagnostic technologies for all pulmonary diseases. Even for patients that have been diagnosed, major obstacles in therapeutics make many pulmonary diseases particularly deadly. Current treatments for pulmonary diseases largely rely on medication that is taken orally or through intravenous injections and delivered to the airway through the circulation. Besides their poor efficiency of delivery to the target organ, these methods of delivery are often highly nonspecific and can be toxic to multiple unintended end organs. It would be ideal to use a customized delivery approach that can efficiently deliver drugs to the target organ (i.e., the lungs) via the airways.
In addition to diagnostics and therapeutics, breakthroughs in disease modeling are required in order to gain insight into poorly understood pulmonary diseases. Human studies reveal the consequences of a disease, but often fail to reveal the underlying pathophysiology. While animal models have been used extensively to study disease, the airway structures of animals can be significantly different from those of humans; thus, these models commonly fail to accurately reflect the pulmonary disease processes observed in humans, affecting both our understanding of symptom progression and treatment efficacy. While in vitro cell studies can provide valuable insight into the fundamental mechanisms of disease, they fail to replicate the complexity of cell types, cell–cell interactions, physiological environments, or the structure of the tissue microenvironment observed in airways. To gain a deeper insight into disease progression and develop more effective treatments, all of these barriers need to be addressed with better models for pulmonary disease study.
《2. The role of microfluidic technologies in addressing pulmonary disease》
2. The role of microfluidic technologies in addressing pulmonary disease
Microfluidics is a broad field of technologies capable of controlling the motion of fluids and particles at a micrometer and nanometer scale, often via driving forces generated from various energy sources, such as acoustic waves, capillary action, light, or electromagnetic fields (Table 1 [4–9]). These technologies can actuate fluids and move particles against the laminar flow. Microfluidic technologies have several properties that make them particularly suitable for biomedical applications (Fig. 1) [10]. First, microfluidic technologies are versatile, being capable of manipulating objects ranging from several nanometers to several millimeters in size. They can also control a diverse range of objects such as liquids, molecules, particulates, cells, and small-model organisms such as Danio rerio and Caenorhabditis elegans, all of which have significant implications in pulmonary disease research and therapeutics. The temporal scale of microfluidic manipulation can range from microseconds (e.g., particle deflection in fluorescentactivated cell sorting [11]) to days (e.g., patterned cell culture). These technologies enable a wide spectrum of functionalities such as liquid transfer, mixing, extraction, particle translation, sorting, pairing, and aggregation. Second, microfluidic technologies are biocompatible. The forces driving the manipulation can be used at amplitudes low enough to prevent damage to biological objects. These properties help microfluidic manipulation technologies to minimize—and often prevent—damage or altered function of cells and biomolecules. Third, microfluidic technologies are highly adaptable. Because their form factors (e.g., channel design, device material, and power supply) can be easily modified, they can be integrated into many instruments or devices. Fourth, compared with conventional methods, microfluidic devices are more compact and thus consume less reagent. The large surface-to-volume ratio of microfluidic devices enables quick heat dissipation, allowing the suspended molecules and cells in the fluid to be processed at physiologically relevant temperatures.
《Table 1》
Table 1 Mechanism and applications of microfluidic manipulation in pulmonary diseases.

SARS-CoV-2 CRISPR: clustered regularly interspaced short palindromic repeats (CRISPR) assay in to detecting SARS-coronavirus 2 (CoV-2).
《Fig. 1》
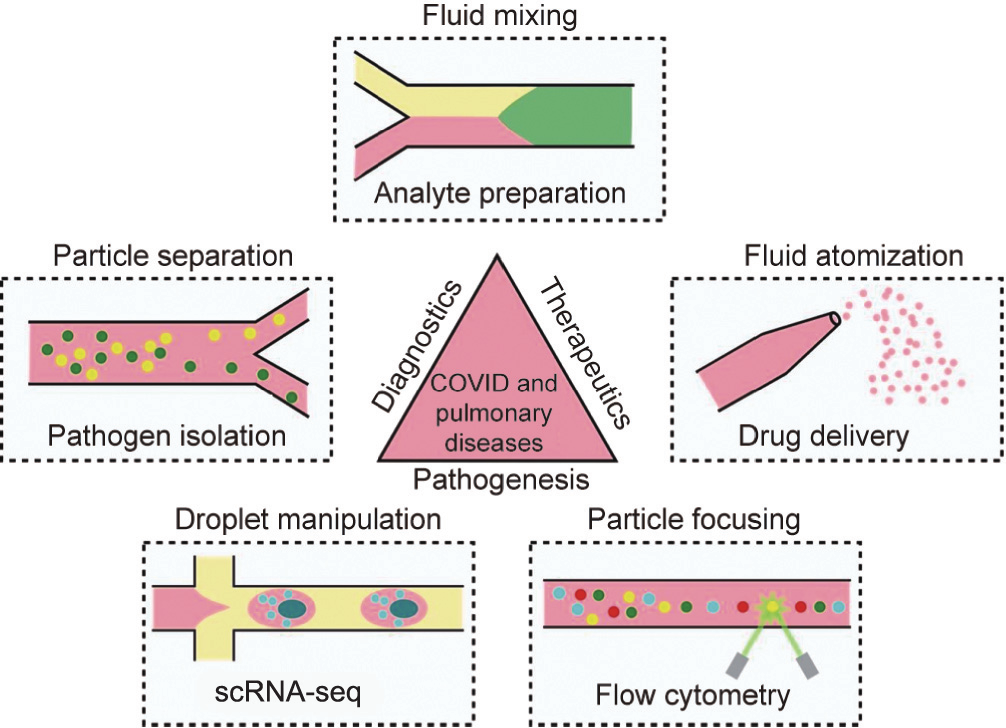
Fig. 1. Applications of the microfluidic manipulation of fluids, particles, and cells in fundamental biological studies, diagnostics, and development of therapeutics for COVID-19 and other pulmonary diseases. scRNA-seq: single-cell RNA sequencing.
Despite the many advantages of microfluidics, more traditional methods for fluid/particle manipulation still dominate pulmonary research and clinical labs, including sample centrifugation, shaking, mixing, filtration, extraction, and two-dimensional (2D) cell culture. To further expand the use of microfluidics for pulmonary disease, it is necessary to understand the context of pulmonary research. Most research is conducted at centralized labs; there is no shortage of financial support in these environments, but there is a significant need for highly standardized protocols to obtain repeatable results. Because there is a lack of standardization for many microfluidic technologies, researchers tend to forego using these devices in favor of traditional procedures, even when there are significant limitations to these traditional procedures. In addition, diagnosis—especially point-of-care diagnostics—typically takes place in a resource-limited environment. In order for microfluidic devices to gain more widespread use, it is necessary to reduce their use of bulky peripheral equipment. Recent microfluidic technologies can be integrated with a low-power driver (e.g., the universal serial bus (USB) port from a cell phone) and utilize microscope-free detection (e.g., the camera from a cell phone), greatly simplifying their instrumentation and allowing for integrated rapid diagnostic devices to be fully realized as a true point-of-care technology [12].
《3. Microfluidic technologies in pulmonary disease research》
3. Microfluidic technologies in pulmonary disease research
Commercial devices utilizing microfluidics have been used to elucidate the mechanisms of pulmonary diseases at the single-cell level. In single-cell analysis, genetic, epigenetic, and/or proteomic information is acquired from individual cells. This information is then further integrated to provide an unprecedented means of understanding cell statuses and cell-environment interactions [13]. For example, high-throughput single-cell RNA sequencing technology (scRNA-seq) [14,15] has been achieved by designing a microfluidic system for droplet creation and manipulation (Fig. 1). scRNA-seq has been successfully applied in research to reveal the transcriptome of cystic fibrosis by identifying ionocyte cells [16,17], which predominantly determine the cross-membrane transport of ions. In another example, commercial flow cytometry, which takes advantage of hydrodynamic focusing and optical detection, was used to study cell phenotypes in pulmonary diseases at the single-cell level (Fig. 1). Woodruff et al. [18] characterized B-cell responses through high-dimensional flow cytometry to reveal substantial heterogeneity in both effector and immature populations in patients with COVID-19.
Microfluidic technologies can also be used to establish disease models at the cellular level. Researchers have used these devices to aggregate cells with bacteria and parasites in order to study the pathogenesis of non-pulmonary diseases such as malaria [19]. When used to study pulmonary disease, this type of method could shed light on the pathogenesis of many bacterial diseases. Another potential strength of these technologies is the ability to coculture multiple cell types, which more accurately imitates the complex airway of epithelial systems [20] in comparison with traditional in vitro studies, which only investigate one type of cell at a time.
Beyond cell studies, microfluidics has significant potential as a tool for the development of disease models at the cell, tissue, and organ levels. Recent approaches have focused on the development and study of organoids [21], which have been extensively used in the investigation of disease mechanisms for COVID-19 [22] and other airway diseases including cystic fibrosis, asthma, and lung cancer [23]. Microfluidic approaches have the ability to aggregate suspended cells to form organoids in a controlled and repeatable manner [24]. In addition, these technologies can be used to apply controlled mechanical stimulation, chemical gradients, and shear stresses to organoids in order to study how such variables affect organoid physiology [21].
Moreover, physiological processes in pulmonary diseases can be modeled using non-biological systems that are formed via microfluidics. Microfluidic atomization [4] can be used to model the spread of droplets and pathogens ejected from the nose and mouth during coughing or sneezing. These models can help scientists investigate the mechanisms for the airborne transmission of diseases with high precision. Microfluidics has also been used to develop a model for the process of mucus secretion in the submucosal glands [6], a major site of mucus production in the trachea and bronchi. Actuated by acoustic streaming, mucus was released from mucin-containing vesicles to form mucus strands under different pH conditions. A flow was then introduced to clear the mucus strands, mimicking airway cilia beating. This study found that decreases in the pH of submucosal glands, due to loss of cystic fibrosis transmembrane conductance regulator (CFTR) function, impair the clearance of mucus from airway surfaces; thus, it identified submucosal glands as a key site for early pathogenesis in cystic fibrosis.
《4. Microfluidic technologies in pulmonary disease diagnostics》
4. Microfluidic technologies in pulmonary disease diagnostics
Microfluidic technologies are well-suited for analyte preparation (e.g., mixing, lysis, and focusing) (Fig. 1). Recently, researchers discovered that electric field gradients can be used to co-focus Cas12-guide RNA (gRNA), reporters, and targets within a microfluidic chip to accelerate reagent mixing for a clustered regularly interspaced short palindromic repeats (CRISPR) assay to detect SARS-coronavirus 2 (CoV-2) [9]. Using this approach, the group achieved rapid detection (35 min from raw nasopharyngeal swab samples to the result) of SARS-CoV-2 RNA on a microfluidic chip with small sample volumes (~100 pL). Wang et al. [8] developed a microfluidic system based on real-time colorimetry for diagnosing multiple respiratory viruses. Magnetic beads were utilized for nucleic acid extraction in conjunction with a multi-channel array chip with integrated isothermal amplification to achieve the high-specificity (100%) and high-sensitivity (96%) detection of multiple influenzas and adenoviruses. In another study, Deng et al. [25] utilized the thermophoretic effect in a microfluidic device to develop a rapid diagnostic platform for COVID-19. In this method, aptamers were bonded to the SARS-CoV-2 spike protein and were then separated by temperature and polyethylene glycol (PEG) concentration gradients for detection. The researchers achieved an approximately 170 particles per microliter (26 fmol·L–1 of the spike protein) detection limit within a 15 min processing time.
Microfluidic technologies can also aid in pulmonary disease diagnosis through the isolation and analysis of pathogens, viruses, extracellular vesicles, and DNA (Fig. 1). For example, microfluidic nanoparticle manipulation could be used to help isolate cell-free DNA, which has gained significant interest as a potential biomarker for liquid biopsies. A recent study [26] found elevated levels and divergent tissue sources of cell-free DNA in COVID-19 patients compared with patients who had influenza and/or respiratory syncytial virus, and with healthy controls. In another study [27], researchers found significantly higher plasma cell-free DNA levels in non-small-cell lung cancer patients than in subjects with chronic respiratory inflammation and healthy individuals.
In addition to cell-free DNA isolation, microfluidic nanoparticle manipulation can be used for the isolation and detection of extracellular vesicles from biofluids. Rosell et al. [28] found that COVID-19 infection induces tissue factor expression and increased levels of circulating tissue-factor-positive extracellular vesicles. Extracellular vesicles have also been found to hold significance in the pathology of COPD, pulmonary hypertension, lung fibrosis, and asthma [29]. Wu et al. [30] demonstrated the use of acoustofluidics to rapidly isolate exosomes, a specific type of extracellular vesicle, from whole blood samples with high purity (98%) and yield (82%). Their study established acoustofluidics as an effective microfluidic manipulation platform for exosomal isolation—a result with enormous potential in biology and medicine, as the burgeoning field of exosome-based diagnostics and therapeutics shows significant promise for pulmonary diseases that are difficult to diagnose or treat. It should be noted that throughput can be a potential limitation of microfluidic separation and is expected to be addressed in rare cell isolation practice [31].
Microfluidic technologies could further contribute to the pointof-care diagnostics of pulmonary diseases. Point-of-care diagnostic methods have rapid processing times compared with lab tests, which enables physicians to make faster, better-informed decisions. Microfluidics can significantly increase the efficiency of diagnostic testing for many pulmonary diseases and thereby play a critical role in administering life-saving treatments and mitigating the spread of disease. Paper-based microfluidic devices [32] have also been used for pulmonary disease diagnostics; they have the unique advantage of further reducing manufacturing costs and minimizing the instrumentation required for analysis [33]. Microfluidics has been revolutionizing point-of-care technologies through its integration into commercial products (e.g., centrifugation-based microfluidics [34]), combination with existing procedures (e.g., electrophoresis-based microfluidic manipulation [35]), and iterative improvements in device design and user experience.
《5. Microfluidic technologies in pulmonary disease therapeutics》
5. Microfluidic technologies in pulmonary disease therapeutics
Current therapeutics for pulmonary diseases are based on the oral delivery and whole-body circulation of medications. Although this makes medication administration simple, the systemic side effects and low efficiency in delivery are driving a critical need for innovations in drug delivery approaches. Compared with conventional drug delivery methods, a localized treatment would enhance the local concentration of medication, thus reducing the necessary dose and mitigating many side effects of the drug. Microfluidic technologies can provide methodologies for disease therapeutics and are particularly well-suited for localized therapeutics. Devices employing the microfluidic atomization and aerosolization of liquid samples could be used to replace traditional delivery methods of medication to airway surfaces (Fig. 1). Compared with a conventional nebulizer or inhaler, microfluidic atomization allows the application of a smaller amount of reagent by targeting the delivery tissues through precise control over the droplet size. With this aim, Qi et al. [4] developed an acousticbased atomization method to generate droplet sizes of (2.84 ± 0.14) μm. Microfluidics could also enable the deep penetration of drug delivery into the airways, which is not possible with conventional methods. Ramesan et al. [5] developed an acousticbased method to deliver nanoparticles up to 700 μm deep into the epithelial cells of oral tissue, compared with a depth of about 100 μm without acoustic actuation. This method could enable drug delivery to submucosal tissues such as cartilages, connective tissues, and neural cells in the airway system. Although they hold promise, microfluidic-based drug delivery methods are still at their proof-of-concept stage. To push toward clinical applications, more efforts are needed in system integration, animal experiments, and clinical trials of their performance.
《6. Conclusions and outlook》
6. Conclusions and outlook
In the past few decades, microfluidic technologies have enabled a wide spectrum of functionalities and applications in pulmonary medicine (Fig. 1). These applications include single-cell studies; disease models; sample preparation; detection of pathogens, DNA, and vesicles; and novel drug delivery methods. Although significant challenges exist in device fabrication, system integration, throughput, and standardized operation protocols, we expect that microfluidics will continue to provide a significant contribution to the research, diagnostics, and therapeutics of pulmonary diseases.
《Acknowledgments》
Acknowledgments
We acknowledge support from the National Institutes of Health (U18TR003778, R01GM141055, R01GM132603, and R01GM135486), National Science Foundation (ECCS-1807601) to Tony Jun Huang, and Roy J. Carver faculty start-up fund and University of Iowa to Yuliang Xie.














 京公网安备 11010502051620号
京公网安备 11010502051620号




