《1. Introduction》
1. Introduction
Antimicrobial resistance (AMR) is now recognized as one of the most serious global threats to health and the economy [1]. Global concern has been heightened by the rapid increase in carbapenemresistant Enterobacterales (CRE) expressing Klebsiella pneumoniae carbapenemase (KPC-1) or New Delhi metallo-b-lactamase (NDM) [2,3], which has threatened the clinical utility of carbapenems. Thus far, colistin (polymyxin E), which was recently reintroduced into clinical medicine, has been recognized as one of the antimicrobial agents of last resort for the treatment of lifethreatening human infections caused by multidrug-resistant (MDR) Gram-negative pathogens, such as CRE [4].
Since the discovery of the mobile colistin resistance (mcr) mechanism, mcr-1, in Enterobacterales of both food-producing animal and human origins in China in November 2015, there have been increasing reports of MCR-producing Enterobacterales being isolated from food animals, animal products, humans, and the environment from 45 countries across six continents, including Southeast Asia (SEA), Europe, Africa, North America, South America, and Oceania [5,6]. Multiple mcr genes (mcr-1 to mcr-10) have been described from human, animal, and environmental sources. Plasmids are considered to be key drivers in the global dissemination of mcr genes. IncI2, IncH12, and IncX4 are the major incompatibility groups that have been reported in association with mcr [6–8].
Within SEA, an increasing prevalence of mcr genes in Enterobacterales has been observed in Vietnam and Thailand, and the usage of colistin is considered to be the driving force for this growing resistance [6,9–12]. A recent report from the Lao People’s Democratic Republic (Laos) revealed the widespread prevalence of mcr within the country but did not analyze the drivers for mcr dissemination, such as the role of the environment or the potential of plasmid vectors to spread mcr [13]. The present study was designed to investigate the molecular epidemiology of mcr in Laos using a ‘‘One-Health” approach and to predict whether any dominant plasmid and/or strain type might influence the dissemination of mcr by evaluating both genetic and functional properties.
《2. Materials and methods》
2. Materials and methods
The study outline is described in Fig. 1.
《Fig. 1》
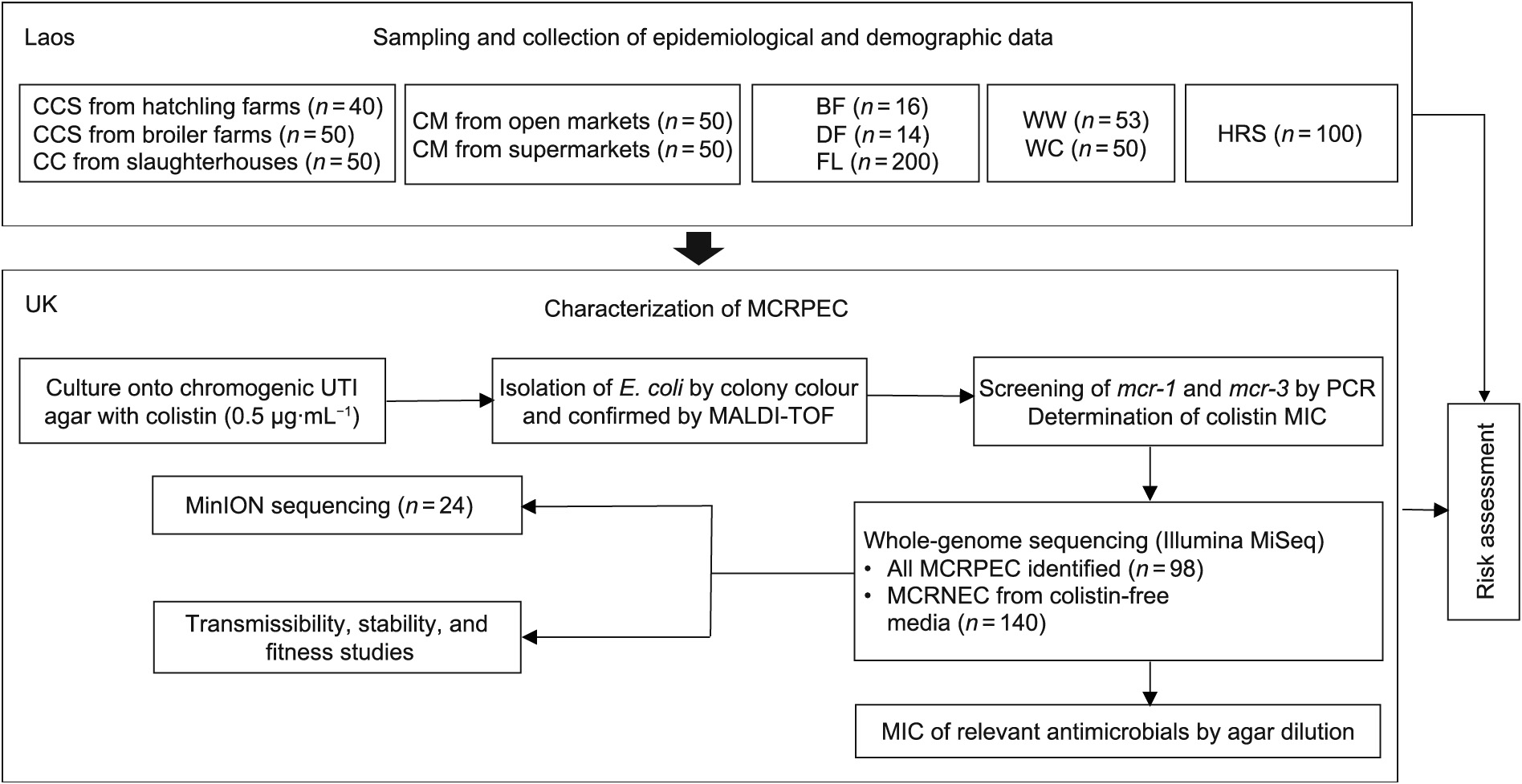
Fig. 1. Schematic workflow of the study. (CCS: chicken cloacal swabs; CC: chicken caeca; CM: chicken meat; BF: bird feces; DF: dog feces; FL: flies; WW: wastewater; WC: water from canals, rivers, and reservoirs; HRS: human rectal swabs; E. coli: Escherichia coli; MCRPEC: MCR-positive E. coli; PCR: polymerase chain reaction; MALDI-TOF:matrix-assisted laser desorption/ionization time-of-flight; UTI: urinary tract infection; MIC: minimum inhibitory concentration.
《2.1. Sampling strategies》
2.1. Sampling strategies
A comprehensive sampling strategy based on a ‘‘One-Health” approach was conducted in Vientiane, Laos, between May and September 2018. The 673 samples collected comprised human rectal swabs (HRS) from healthy volunteers (n = 100); chicken cloacal swabs (CCS) from commercial farms (n = 90) (including both hatchlings and broilers); chicken caeca (CC) from slaughterhouses (n = 50); chicken meat (CM) from both open markets and supermarkets (n = 100); bird feces (BF) (n = 16); dog feces (DF)(n = 14); flies (FL) from open markets and slaughterhouses (n = 200); wastewater from farms, markets, and slaughterhouses(WW) (n = 53); and water from canals, rivers, and reservoirs (WC) (n = 50). Sampling sites were selected within a 10 km radius of poultry farms (Fig. S1 (a) in Appendix A). Flies were captured using fly glue boards (PEST-STOP, UK) and then individually transferred to Eppendorf tubes aseptically [14]. Other samples were collected using Amies transport swabs with charcoal (Deltalab, Spain). Sampling details (i.e., type of sample, location, and date of sampling) and demography of human subjects (e.g., sex, locality, occupations based on farming products, dietary habits, drinking habits, sanitation status, and whether or not they owned a farm) were collected (Table S1 in Appendix A). This project was approved by the Oxford Tropical Research Ethics Committee (524-18) and the National Ethics Committee for Health Research of Laos (2018.62. MC). All samples were transferred from Laos to the UK in UN3373 containers (UN3373, Netherlands) with appropriate documentation.
《2.2. Phenotypic characterization of MCR-positive Escherichia coli (E. coli)(MCRPEC)》
2.2. Phenotypic characterization of MCR-positive Escherichia coli (E. coli)(MCRPEC)
Screening included the culture of specimens on chromogenic urinary tract infection (UTI) medium (Merck Life Science, Germany) with colistin (0.5 μg∙mL–1 ), the isolation of pink colonies, and the confirmation of E. coli by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS; Bruker Daltonics, Germany). Flies were mashed and pre-treated with Luria broth (LB; Sigma–Aldrich, Germany) at 37 °C for 4 h, followed by plating with colistin selection (0.5 μg∙mL–1 ) on chromogenic UTI media. Initially, E. coli were screened for mcr-1 and mcr-3 by polymerase chain reaction (PCR) using primers described previously [15] and determination of the minimum inhibitory concentration (MIC) of colistin [16]. Samples negative for colistin-resistance predictors (i.e., if the isolated E. coli were sensitive to colistin or negative for mcr-1 or mcr-3) were plated on colistin-free chromogenic UTI to recover colistinsensitive E. coli for risk analysis. Out of 673 samples, a total of 238 E. coli isolated from non-duplicative samples were analyzed in this study, which included 98 MCR-positive E. coli (MCRPEC) and 140 MCR-negative E. coli (MCRNEC). The agar dilution method was used to determine the MICs of clinically relevant antimicrobials (co-amoxiclav, piperacillin-tazobactam, ceftriaxone, ceftazidime, cefotaxime, cefepime, imipenem, meropenem, ciprofloxacin, levofloxacin, amikacin, gentamicin, tigecycline, fosfomycin, and sulfamethoxazole-trimethoprim), and the results were interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [16].
《2.3. Whole-genome sequencing》
2.3. Whole-genome sequencing
E. coli isolated from primary screening (n = 238) were sequenced using the Illumina MiSeq platform (Illumina Inc., USA). Plasmid backgrounds in association with mcr were predicted based on MiSeq assembly, and 24 E. coli harboring mcr on diverse plasmid backgrounds were selected accordingly for MinION sequencing (Oxford Nanopore Technologies, UK). In brief, genomic DNA (gDNA) was extracted from overnight culture using QIAcube (Qiagen, Germany). DNA libraries were prepared for paired end sequencing (2 × 301 cycles) using Nextera XT. Quality control of raw reads included fastqc (v0.11.2), and adaptor trimming was performed using Trimgalore (v0.4.3). Reads were assembled in contigs using the de novo assembler SPAdes (v3.9.0) (.fasta) and were aligned to the original fastq reads using Burrows-Wheeler Aligner (BWA; 0.7.15). Any error was corrected using Pilon (v1.22). Assembly metrics were evaluated using Quast (v2.1). The de novo assembly was then annotated with Prokka (v1.12), and the outputs (.gff) were subjected to core-genome alignment using Roary (v3.12.0). We constructed maximum likelihood (ML) trees with core alignment using RA ×ML-ng (v0.9.0.git-mpi) with a general time-reversible (GTR) evolutionary model and gamma correction with iterations until bootstrapping converged with a cut-off value of 3% (by default), followed by visualization using Interactive Tree of Life (iTOL; v5). Demultiplexing of the raw reads obtained from MinION sequencing was performed using Porechop (v0.2.3). Unicycler (0.4.4) was used to yield hybrid assembly using both Illumina short reads and MinION long reads. The databases used in this study were the multi-locus sequence typing (MLST) databases (v2.0.0) from the Center for Genomic Epidemiology (CGE), the Clermont database (v1.4.0), and Resfinder and PlasmidFinder in ABRicate (v0.9.7). All whole-genome sequencing data from this study have been deposited in the GenBank under BioProject accession No. PRJNA763111.
《2.4. Conjugation, plasmid stability, and growth kinetics》
2.4. Conjugation, plasmid stability, and growth kinetics
Ten MCRPEC carrying five different mcr-bearing Inc-type plasmids (IncX4 (n = 2), IncI2 (n = 2), IncHI2 (n = 2), IncP1 (n = 2), and IncFII (n = 2)) were selected as donors, and four recipients from different geographical regions (Recipient 1 (R1): sequence type 10 (ST10) from this study; R2: ST131 from Brazil; R3: ST1193 from China; and R4: ST167 from Bangladesh) and E. coli J53 were chosen for the conjugation assays. The following criteria were used for donor and recipient selection: ① Donors and recipients did not belong to the same STs; ② recipients were mcr-negative; and③ recipients belonged to epidemiologically important E. coli STs (e.g., ST10 is regarded as the largest reservoir of mcr-1 [17], ST131 is an epidemic clone for blaCTX-M-15 [18], ST1193 is a highly virulent clone [19], and ST167 is a high-risk clone for blaNDM [3]). The recipients did not contain the same Inc group plasmids as the donors except that the isolates from Brazil and Bangladesh had IncFII plasmids (Table S2 in Appendix A). Mating experiments were deployed with each recipient against each donor. Donors and recipients were grown in LB media at 37 °C with shaking at 170 r∙min–1 until they reached the exponential growth phase (an optical density at 620 nm (OD620) of 0.6). Broth mating was undertaken with 1:3 donor–recipient mixtures at 37 °C overnight. Serial dilutions of the overnight mating cultures were then plated on chromogenic media with colistin (2 mg∙L–1 ) and with colistin plus a selective antibiotic (based on the susceptibility pattern of pertinent recipients). Successful conjugation was confirmed by the PCR of mcr followed by Clermont typing, repetitive element sequence-based PCR (rep-PCR), or MLST (Table S3 in Appendix A), where appropriate. Transfer frequencies were calculated by colony-forming unit (CFU) counts of transconjugants against those of donors.
The stability of all the transconjugants obtained (n = 41) was investigated by 15 days’ serial passaging in an antibiotic-free environment according to a previously described protocol [20]. Overnight cultures were diluted as 1:1000 in fresh LB medium without colistin and incubated with vigorous shaking (220 r∙min–1 ) at 37 °C for 24 h. Biological triplicates were performed for each strain. Total gDNA was extracted on days 0, 3, 6, 9, 12, and 15 using the boiling lysis method [21]. The changes in the abundance of mcr-carrying plasmids over 15 days’ passaging were measured by quantitative PCR (qPCR) using a StepOnePlus qPCR machine (Applied Biosystems, UK) with specific primers and probes for mcr variants and a housekeeping gene (HKG), rpoB (Table S3). The relative abundance of mcr compared with the HKG was calculated by the delta–delta Ct method  . The experiment was performed in three replicates.
. The experiment was performed in three replicates.
The growth kinetics of all transconjugants (n = 41) and recipients (n = 5) were investigated [20]. Overnight bacterial cultures (37 °C in fresh LB broth) were diluted as 1:1000 in fresh LB medium. Bacterial growth was recorded by monitoring the OD620 at half-hour intervals for 24 h with shaking at 100 r∙min–1 , using a FLUOstar Omega microplate reader (BMG LABTECH Ltd., UK). Three biological repeats and two technical repeats were conducted for each strain.
《2.5. Questionnaire and statistical analysis》
2.5. Questionnaire and statistical analysis
Univariable logistic regression using SPSS (v26) was performed to assess the potential risks for human carriage of MCRPEC with socio-demographic indices (i.e., sex, locality, occupation, dietary habit, source of daily drinking water, type of toilet, and previous use of antibiotics in the last three months) and to examine the associations between MCRPEC and other variables of interest (i.e., prevalence of MCRPEC in different sources and locations, distribution among different STs, and resistance and virulence profiles). One-way analysis of variance (ANOVA) was employed in GraphPad Prism (v7.04) to investigate the effects of the particulars of donors, recipients, and plasmids on the conjugation frequency. Statistical significance was set at p < 0.05.
《3. Results》
3. Results
《3.1. Prevalence of MCRPEC》
3.1. Prevalence of MCRPEC
The overall prevalence of MCRPEC was found to be 14.6% (98/673), with the highest prevalence in HRS (45.9% (45/98), p < 0.0001, odds ratio (OR): 0.125, 95% confidence interval (CI): 0.077–0.202). The percentages of MCRPEC from other samples were 14.3% (2/14) in dog feces, 12.0% (24/200) in flies, 11.0% (11/100) in chicken meat, 8.9% (8/90) in chicken cloacal, 8.0% (4/50) in chicken caeca, and 7.5% (4/53) in wastewater. No MCRPEC were found in bird feces or water from canals, rivers, and reservoirs (Fig. S1 and Table S4 in Appendix A). The comparative prevalence of MCRPEC from different locations of Vientiane is shown in Table 1, Fig. S1(b), and Table S5 in Appendix A, which show that the prevalence of MCRPEC was significantly higher in Xaythany and Xaysetha than in the other locations. The most common variant of mcr was mcr-1 (14.3%, 96/673), followed by mcr-3 (2.4%, 16/673). The majority (87.5%, 14/16) of mcr-3 was found in association with mcr-1 (Table 2).
《Table 1》
Table 1 Univariable logistic regression analysis for the prevalence of MCRPEC in different locations of Vientiane isolated from different sampling sectors.
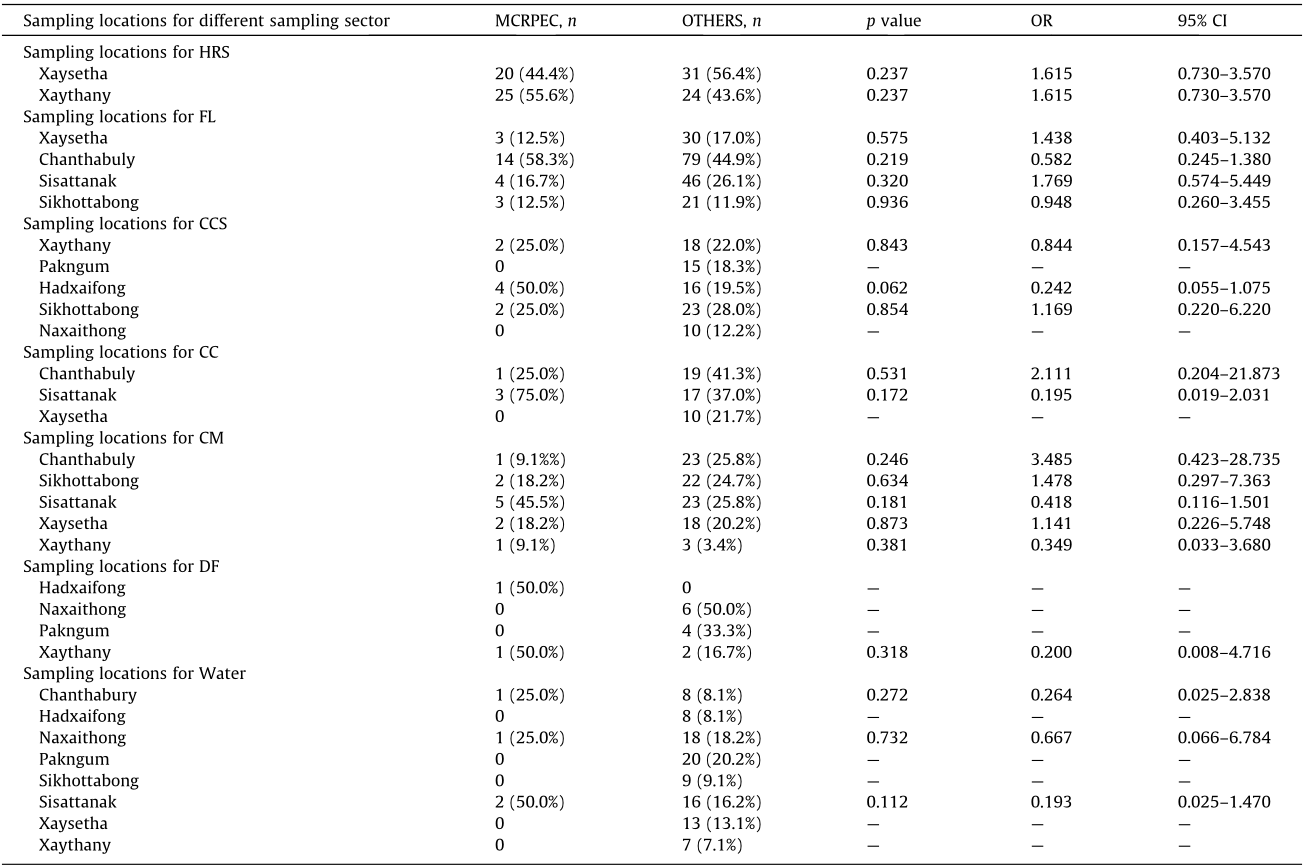
OTHERS refers to samples from which MCRPEC were not isolated. Values in parentheses indicate column percentage. Data for bird feces was not shown in this table as no MCRPEC was isolated from this sampling sector. Statistical significance was set at p < 0.05.
《Table 2》
Table 2 Prevalence of mcr variants in samples from different sectors.
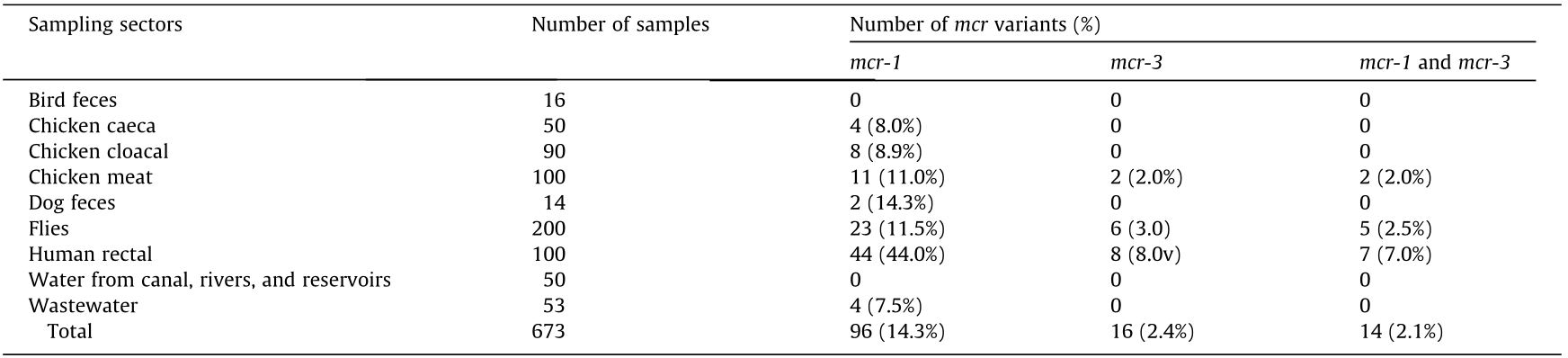
Values in parentheses indicate column percentage.
《3.2. Risk factors associated with human fecal carriage of MCRPEC》
3.2. Risk factors associated with human fecal carriage of MCRPEC
Univariate logistic regression did not infer significant association by gender, locality, households’ drinking and sanitation facilities, and antibiotic intake among the participants carrying MCRPEC compared with participants with non-MCRPEC. Statistical significance in relation to harboring MCRPEC was only observed for participants with domestic animals, in comparison with their counterparts without domestic animals (p < 0.05) (Table S6 in Appendix A).
《3.3. Antimicrobial resistance profiles of MCRPEC》
3.3. Antimicrobial resistance profiles of MCRPEC
The resistance rates of MCRPEC were significantly higher for co-amoxiclav, sulfamethoxazole-trimethoprim, levofloxacin, ciprofloxacin, and gentamicin compared with those of MCRNEC (p < 0.05), and the percentages of resistance were 94.9%, 85.7%, 56.1%, 54.1%, and 26.5%, respectively. However, only 6.1%, 5.1%, and 1.0% of MCRPEC showed resistance to tigecycline, cephalosporins, and fosfomycin, and all MCRPEC were susceptible to amikacin, piperacillin-tazobactam, imipenem, and meropenem (Table 3).
《Table 3》
Table 3 Univariable logistic regression analysis for resistance profiles to antibiotics tested for MCRPEC and MCRNEC.
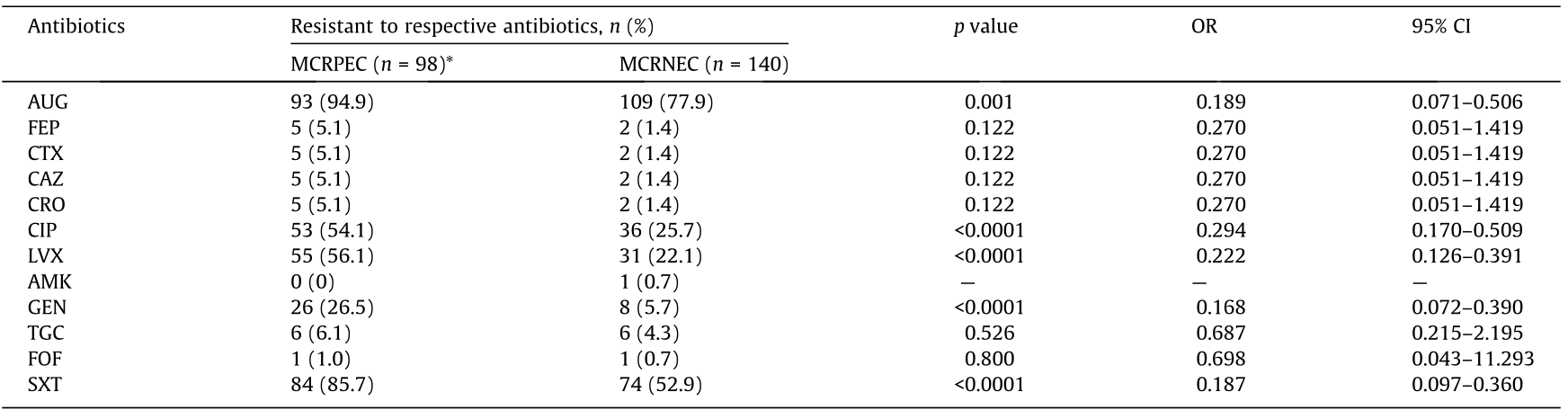
*Only E. coli confirmed for mcr by sequencing were included in this analysis. AUG: co-amoxiclav; FEP: cefepime; CTX: cefotaxime; CAZ: ceftazidime; CRO: ceftriaxone; CIP: ciprofloxacin; LEV: levofloxacin; AMK: amikacin; GEN: gentamicin; TGC: tigecycline; FOF: fosfomycin; SXT: sulfamethoxazole-trimethoprim. All MCRPEC and MCRNEC tested in this study were susceptible to piperacillin-tazobactam, imipenem, and meropenem. Statistical significance was set at p < 0.05.
Values in parentheses indicate column percentage.
Aminoglycoside-resistance genes (aac(3)-IId, aadA2, aph(3',)-Ib, aph(3')-Ia, and aph(6)-Id), β-lactamase gene blaTEM-1B, phenicolresistance genes (floR, cmlA1, and dfrA12), macrolide-resistance genes (mef(B) and mph(A)), fluoroquinolone-resistance gene qnrS1, sulfonamide- and trimethoprim-resistance genes (sul2 and sul3), and tetracycline-resistance genes (tet(A) and tet(M)) were significantly correlated to MCRPEC (p < 0.05), and oqxA and oqxB were only found in MCRPEC (Table S7 in Appendix A).
《3.4. Clonal distribution of the population of E. coli》
3.4. Clonal distribution of the population of E. coli
The E. coli (n = 238) sequenced in this study were distributed among 134 diverse STs. The most prevalent STs were ST48 (n = 17), ST206 (n = 10), ST10 (n = 9), and ST58 (n = 9) (Fig. 2). There was no significant association between particular STs and the presence of mcr; however, all isolates of ST5229 carried mcr (Table S8 in Appendix A). E. coli ST58 were recovered from flies only, and E. coli belonging to ST48 and ST206 were significantly associated with chicken caeca and chicken meat, respectively (p < 0.05) (Table S9 in Appendix A). Out of 98 MCRPEC, the majority belonged to phylogroup A (59/98, 60.2%) and B1 (30/98, 30.6%) (Fig. 2 and Table S10 in Appendix A).
《Fig. 2》
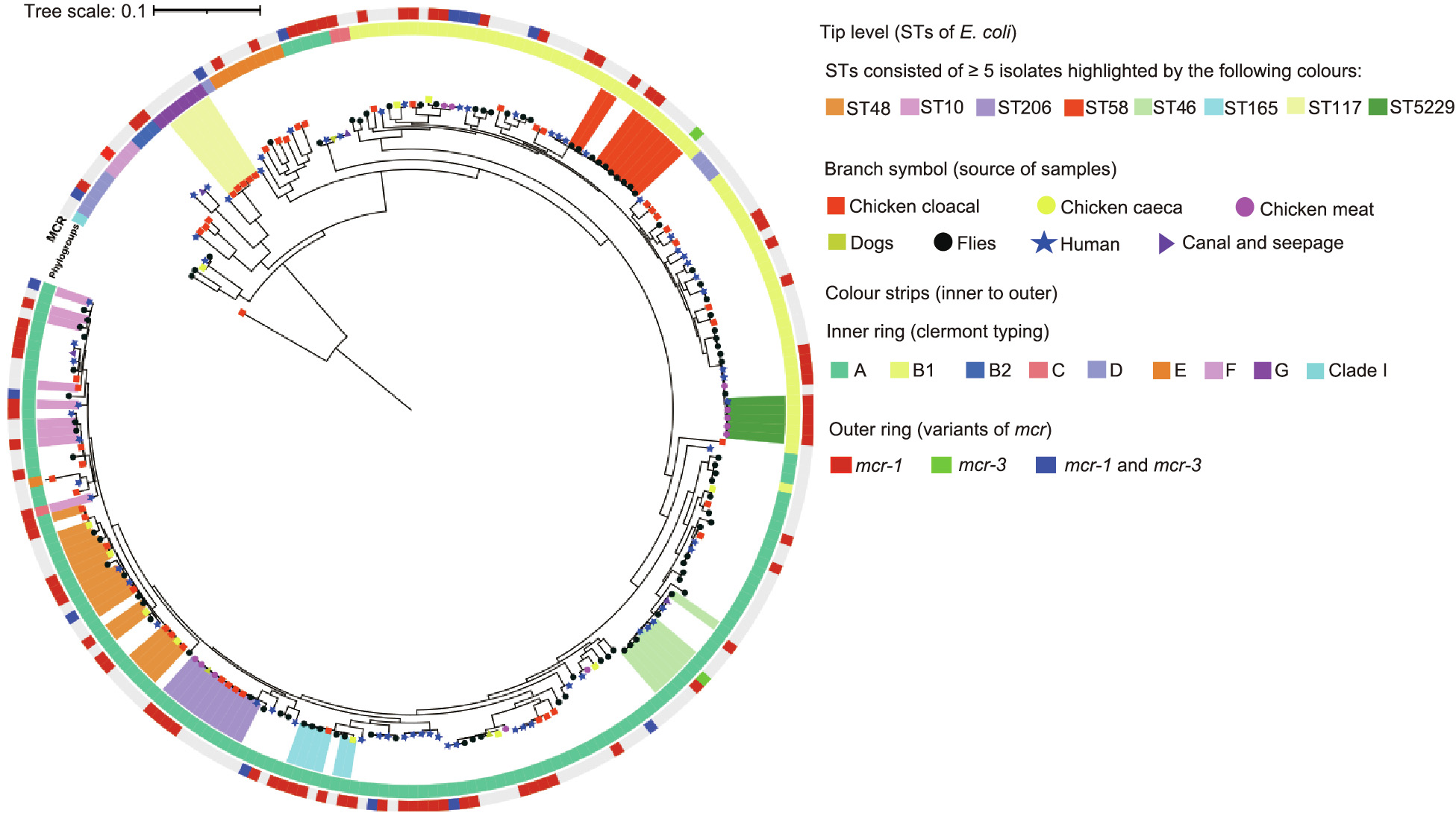
Fig. 2. ML tree generated from a core-genome analysis of E. coli (n = 238) in this study. Core-genome alignment was performed using Roary (v3.12.0). The ML tree from the core genome was built with RA ×ML-ng (v0.9.0.git-mpi) using a GTR model and gamma correction with bootstrapping.
《3.5. Associations between virulence genes and MCRPEC》
3.5. Associations between virulence genes and MCRPEC
Virulence-associated genes encoding enterobactins (entC (238/238, 100.0%), entE (238/238,100%), entB (237/238, 99.6%), and entS (234/238, 98.3%)) and ferrienterobactins (fepD (238/238, 100%), fepB (237/238, 99.6%), fepC (237/238, 99.6%), fepA (233/238, 97.9%), and fes (237/238, 99.6%)) were highly prevalent among the E. coli sequenced in this study. Furthermore, virulence genes related to the E. coli hemin-uptake system, such as chuU, chuV, chuW, shuA, and shuX, were significantly associated with MCRNEC (p < 0.05), while only fimF and fimG (type 1 fimbriae) were associated with MCRPEC (p < 0.05) (Table S11 in Appendix A).
《3.6. Characterization of plasmids harboring mcr-1》
3.6. Characterization of plasmids harboring mcr-1
Complete circular plasmids carrying mcr-1.1 belonging to IncX4 of 33–34 kb (n = 8), IncHI2 of 195–280 kb (n = 4), IncP1 of 47–56 kb (n = 4), IncI2 of 59–87 kb (n = 4), and IncFIA of 209 kb (n = 1) were obtained in this study. Genomic comparisons at the nucleotide level showed that IncX4 plasmids were ≥ 99% similar at ≥ 99% coverage, IncHI2 were ≥ 99% similar at ≥ 85% coverage, IncP1 were ≥ 99% similar at ≥ 98% coverage, and IncI2 were ≥ 99% similar at ≥ 91% coverage. The host origins of IncX4, IncHI2, IncP1, and IncI2 plasmids are shown in Fig. S2 in Appendix A. An analysis of the genetic environment adjacent to mcr-1 revealed that all plasmids had a conserved segment with 2300–2400 bp containing mcr-1.1 and pap2 (upstream of mcr-1.1). The conserved region was bracketed by two complete ISApl1 (IS: insertion sequence) in only two plasmids of IncFIA (n = 1) and IncP1 (n = 1). Plasmids of IncI2 (n = 2) and IncFIA (n = 1) had one complete ISApl1 downstream of mcr-1.1. Complete loss of ISApl1 around mcr-1.1 was found in the remaining plasmid sequences characterized in this study (Fig. 3(a) and Fig. S2). All plasmids belonging to IncX4, three belonging to IncP1, and two belonging to IncI2 carried only mcr as a resistance gene; however, all IncHI2 plasmids were shown to be MDR (Fig. S3 in Appendix A).
《Fig. 3》

Fig. 3. Linear comparison of the genetic context of mcr on various Inc-type plasmids. Arrows represent the position and transcriptional direction of the open reading frames. Grey cross-links between sequences demonstrate regions of sequence homology (> 77% identity). The genomic comparison was performed by Easyfig (v2.2.5). (a) Genetic environment of mcr-1 on IncFIA, IncHI2, IncI2, IncP1, and IncX4 plasmids. (b) Genetic environment of mcr-3 on IncFIB, IncFII, IncR, IncP1, and IncFIA plasmids; NADPH: reduced nicotinamide adenine dinucleotide phosphate; DEAD: Asp–Glu–Ala–Asp amino acid sequences; DEAH: Asp–Glu–Ala–His amino acid sequences; SMC: structural maintenance of chromosomes family protein; NTPase: nucleoside-triphosphatase.
《3.7. Characterization of plasmids harboring mcr-3》
3.7. Characterization of plasmids harboring mcr-3
Plasmid sizes ranging from 71 to 87 kb for IncFII (n = 3), 53 kb for IncP1 (n = 1), 64 kb for IncR (n = 1), 112 kb for IncFIA (n = 1), and 103 kb for IncFIB (n = 1) could be closed by the hybrid assembly of short- and long-read sequence data. The variants of mcr-3 characterized were mcr-3.5 on IncFII (n = 2), IncFIA, and IncP1; mcr-3.1 on IncR; mcr-3.19 on IncFIB; mcr-3.21 on IncFII (n = 1); and mcr-3.1 and mcr-3.4 on undetermined plasmids. Genomic comparisons at the nucleotide level of IncFII carrying mcr-3 demonstrated 97% identities at 69%–86% coverage. The host origins of IncFII plasmids are shown in Fig. S4 in Appendix A. A core segment 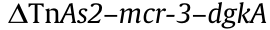 was found in all plasmids except IncFIA, where TnAs2 was lost downstream of mcr-3. ISKpn40 and IS26 in association with the conserved region of mcr-3 were found to be distributed among plasmids of diverse Inc types. Upstream of dgkA was flanked by ISKpn40 in IncFIB, IncFII (n = 1), and IncR plasmids, and by IS26 in IncP1. Interestingly, IS26-flanked (same direction at each end) mcr-3–dgkA was found in IncFIA plasmid. IS15DI was also associated with the conserved segment of mcr-3, but the distribution of IS15DI was restricted to IncFII (n = 2) and IncFIB plasmids (Fig. 3(b)). The plasmids harboring mcr-3 were shown to be MDR except for one IncFII and one IncP1 (only encoded mcr-3), and the resistance profiles were variable (Fig. S3).
was found in all plasmids except IncFIA, where TnAs2 was lost downstream of mcr-3. ISKpn40 and IS26 in association with the conserved region of mcr-3 were found to be distributed among plasmids of diverse Inc types. Upstream of dgkA was flanked by ISKpn40 in IncFIB, IncFII (n = 1), and IncR plasmids, and by IS26 in IncP1. Interestingly, IS26-flanked (same direction at each end) mcr-3–dgkA was found in IncFIA plasmid. IS15DI was also associated with the conserved segment of mcr-3, but the distribution of IS15DI was restricted to IncFII (n = 2) and IncFIB plasmids (Fig. 3(b)). The plasmids harboring mcr-3 were shown to be MDR except for one IncFII and one IncP1 (only encoded mcr-3), and the resistance profiles were variable (Fig. S3).
《3.8. Plasmid transferability, stability, and fitness cost》
3.8. Plasmid transferability, stability, and fitness cost
All liquid broth matings gave conjugation frequencies ranging from low (1 ×10–8) to high (1 ×10–1) (Table S12 in Appendix A); however, conjugation assays were unsuccessful in some instances (IncHI2 to ST10, ST131, and ST167; IncI2 to ST131; IncP1 to ST167; and IncX4 to ST131) (Fig. 4 and Table S12). The mean conjugation frequency into the recipient of ST1193 was significantly higher than that for recipients of other STs (p < 0.05). Significant differences in the rates of transfer were not observed in respect to donors’ STs, donors’ origins, and plasmid Inc types (Table S13 in Appendix A).
《Fig. 4》
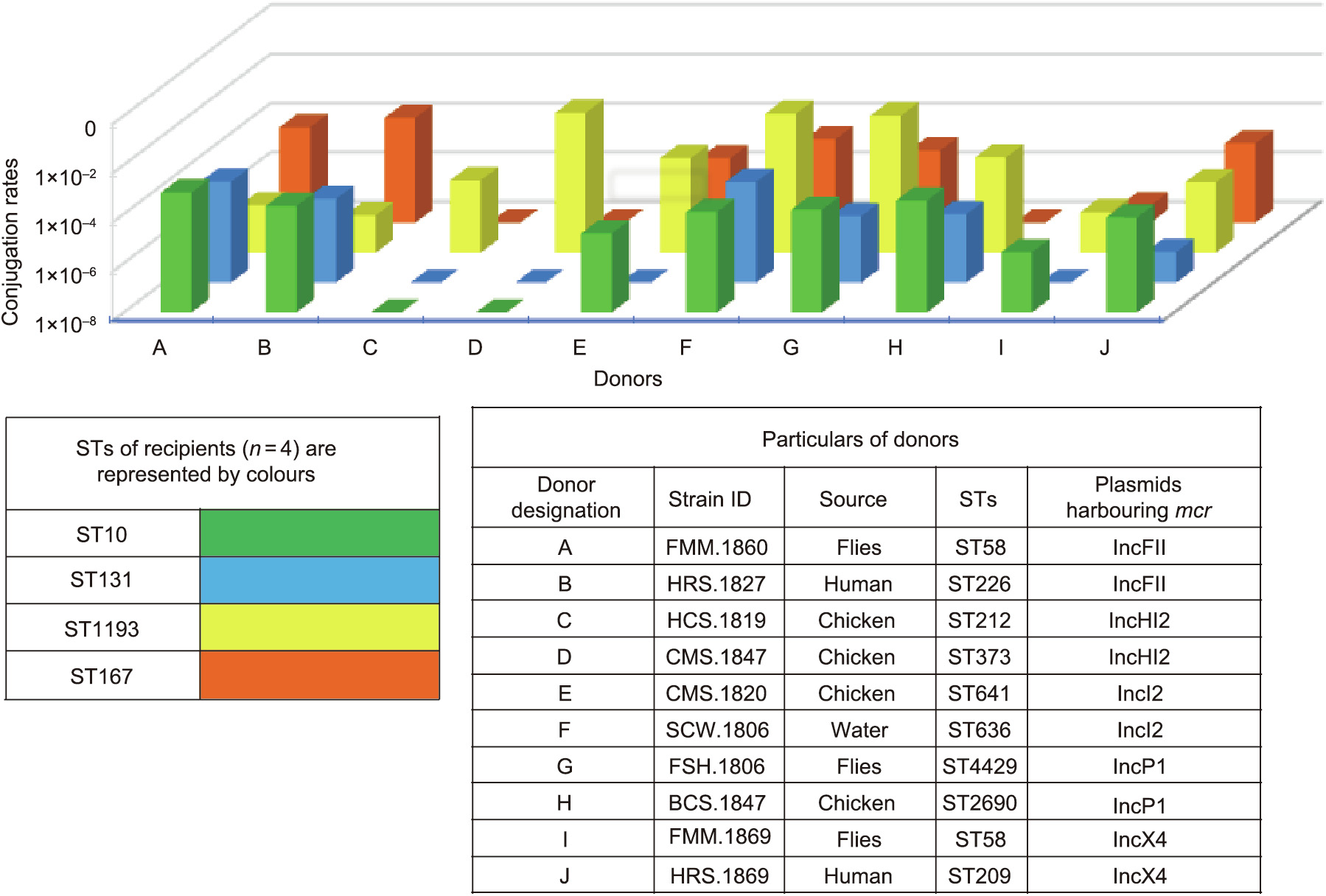
Fig. 4. Graph showing variable transfer rates of plasmids carrying mcr among ten donors and recipients of four STs. E. coli J53 was used as the control for each donor, and the conjugation rates for J53 are not shown in this figure.
Serial passaging of transconjugants in antibiotic-free media revealed that the copy numbers of mcr up to day 15 were static compared with those of day 0. A decline of the relative abundance of mcr-1 after day 9 was observed only with the transconjugants of CX-17 (ST1193) carrying IncP1. The copy number of mcr-1 among the transconjugants with IncHI2 was considerably lower across 15 days’ passaging than that of the transconjugants with other Inc types (Fig. 5).
《Fig. 5》
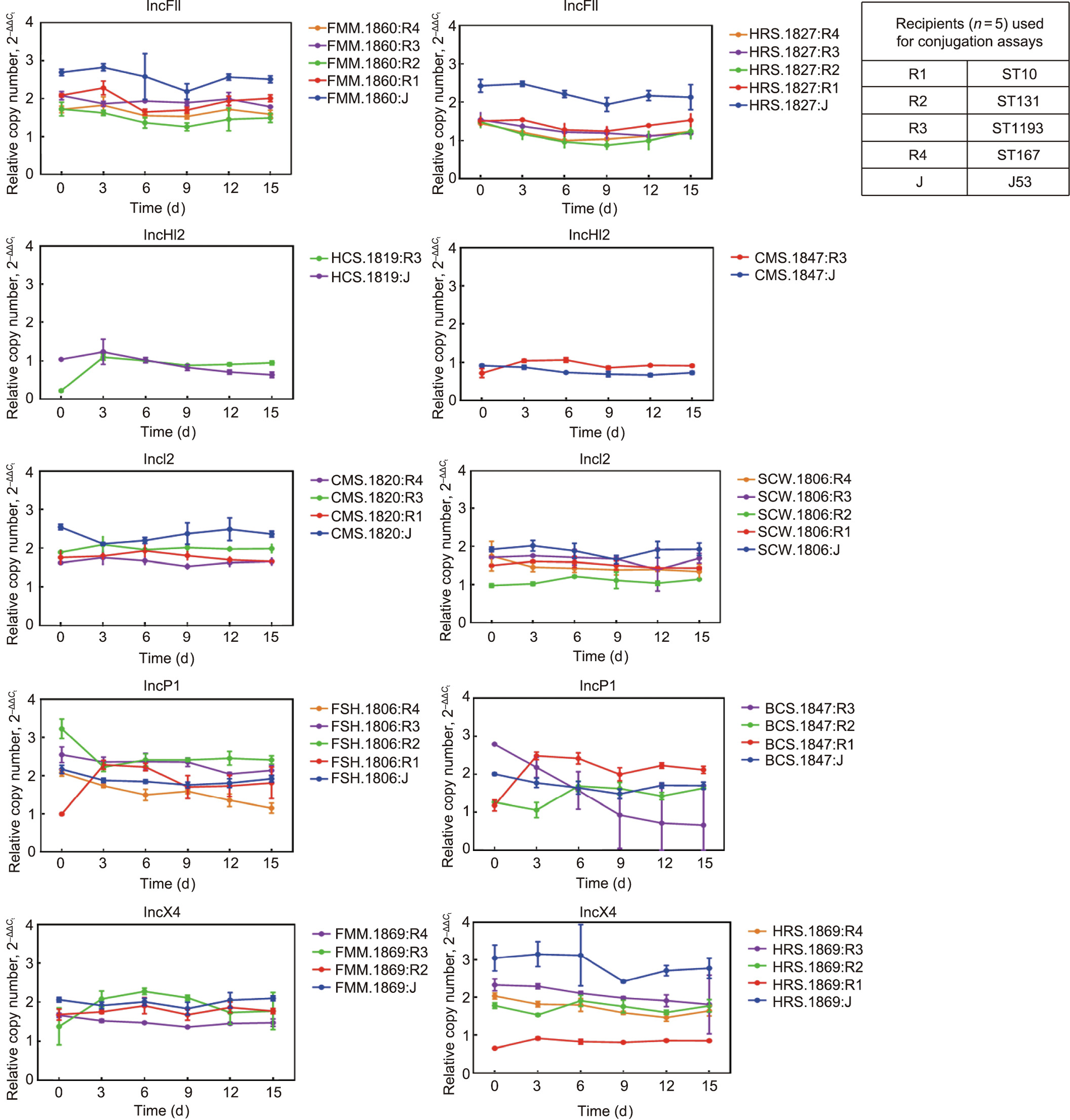
Fig. 5. Abundance of mcr plasmids under antibiotic-free conditions. The dynamic change of the mcr plasmids’ copy number in all transconjugants (n = 41) was investigated by means of 15 days’ passaging. The transconjugants of five recipients (R1, R2, R3, R4, E. coli J53) carrying plasmids of five Inc types (IncFII (containing mcr-3), IncHI2 (containing mcr-1), IncI2 (containing mcr-1), IncP1 (containing mcr-1), and IncX4 (containing mcr-1)) were examined. The delta–delta Ct method (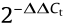 method) was used to model the change of mcr copy number over time.
method) was used to model the change of mcr copy number over time. 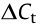 refers to the difference in threshold cycle between mcr and the chromosomally encoded gene. Each strain included three independent replicates, and the values represent the mean of the three independent assays. Error bars represent standard deviations (n = 3).
refers to the difference in threshold cycle between mcr and the chromosomally encoded gene. Each strain included three independent replicates, and the values represent the mean of the three independent assays. Error bars represent standard deviations (n = 3).
Growth kinetics assays showed a variable degree of fitness cost among the transconjugants with mcr-positive plasmids of different Inc types. Compared with the recipients, significant reduction of growth rate was observed in the transconjugants that acquired mcr-positive plasmids belonging to IncFII (FMM.1860:J, FMM.1860:R1, FMM.1860:R2, HRS.1827:R1, HRS.1827:R2, and HRS.1827:R3), IncHI2 (CMS.1847:J, CMS.1847:R3, HCS.1819:J, and CMS.1847:R3), IncI2 (SCW.1806:R1 and SCW.1806:R2), InP1 (BCS.1847:R1), and IncX4 (FMM.1869:R3 and HRS.1869:R1) (p < 0.05). However, 26 of 41 transconjugants (63.4%) exhibited low fitness burden (Fig. 6).
《Fig. 6》

Fig. 6. Growth kinetics of strains with and without mcr plasmids. Five recipients (R1, R2, R3, R4, E. coli J53) and their corresponding transconjugants (n = 41) were examined. Growth fitness was tested for plasmids of five Inc types: IncFII (containing mcr-3), IncHI2 (containing mcr-1), IncI2 (containing mcr-1), IncP1 (containing mcr-1), and IncX4 (containing mcr-1). Three biological repeats and two technical repeats were conducted for each strain. OD620nm indicates the optical density of a sample measured at a wavelength of 620 nm. Data points represent the mean of the independent assays. Error bars represent standard deviations (n = 6). The differences in fitness were tested by unpaired t test (GraphPad v7.04). *: p < 0.05; **: p < 0.01; ***: p < 0.001.
《4. Discussion》
4. Discussion
This study has confirmed the widespread occurrence of mcr genes in human, animal, and environmental reservoirs in Laos, representing a potential risk to human health. mcr-1 was the most common variant, followed by mcr-3, as found in previous studies [13,22–26]. Very little data is available regarding the usage of colistin in Laos, and a review of the scientific literature provided no evidence of its use in humans in Laos [27,28]. However, colistin has been widely utilized metaphylactically in livestock in neighboring countries such as Thailand and Vietnam [9,12]. Livestock has been shown to be the most frequent source of mcr, as described recently in China, as well as in Laos [6,13]; however, in our study, the prevalence of mcr was highest in humans, and human sampling from Xaythany and Xaysetha consistently yielded the highest rate of mcr from these two areas of Vientiane (Table 1, Fig. S1, and Table S5. This result is unlikely to have been driven by the clinical use of colistin in humans, which is very uncommon in Laos, but may relate to contamination of the food chain and the environment, with flies potentially being involved in the dissemination of mcr genes (Table 2 and Fig. S1(b)). Interestingly, we found a significant association between the presence of MCRPEC and the participants having domestic animals (p < 0.05) (Table S6), suggesting a transmission link between animal and human [40].
E. coli is an opportunistic pathogen that contributes to the intestinal flora in a variety of animals, including humans, and can also persist in soil and aquatic environments [29]. The phenotypic and genomic screening of E. coli for resistance in this study provides an insight into the ubiquity of MDR bacteria in Laos. It is particularly worrying that 14.6% of the E. coli tested (MCRPEC, n = 98) showed a high rate of resistance to clinically important antimicrobial classes (β-lactams, aminoglycosides, and quinolones), in addition to the World Health Organization (WHO)-listed ‘‘reserve” antibiotic, colistin (Table 3, Fig. 1, and Table S7) [30].
The horizontal transmission of mcr has played a pivotal role in the dissemination across a variety of niches, as described elsewhere, including in Laos [6,13]. We also found mcr to be present in a wide range of E. coli STs (Fig. 2). In addition, 24 mcr-bearing plasmids were characterized and completed by the hybrid assembly of short-read and long-read data, which demonstrated a complex horizontal dissemination of mcr in Laos in terms of the varied genetic context immediately adjacent to mcr and diverse plasmid backgrounds (Fig. 3, Figs. S1 and S3). The ancestral vehicle for mcr-1 mobilization has been thought to be Tn6330 (ISApl1-mcr-1-pap2-ISApl1) [31,39]. Two plasmids (IncFIA and IncP1) characterized in this study had the classical features (Tn6330) of mcr-1. Nonetheless, the presence of ISApl1 down-stream of mcr-1.1 on IncI2 also predicted the IS-derived mobility of mcr-1, while the absence of ISApl1 adjacent to mcr-1 on IncX4, IncHI2, one IncI2, and some IncP1 indicated stabilization of mcr-1 in a diverse range of plasmid backgrounds (Fig. 3(a) and Fig. S2) [32]. We built a hypothesis for mcr-3 mobilization in Laos based on nucleotide position and the distribution of various IS elements in different plasmid backbones. We hypothesized that mcr-3 was captured by ISKpn40, TnAs2, or IS26 and subsequently dispersed into plasmids of varied backgrounds (Fig. 3 (b) and Fig. S4). Although mobile elements, such as TnAs2 and ISKpn40, have commonly been found in association with mcr-3, others have also described the IS26-mediated mobility of mcr-3 [7,33]. In particular, ISKpn40 in the form of a composite transposon (ISKpn40-mcr-3-dgkA-ISKpn40) was recovered in isolates from Laos [25]. The distribution of highly similar mcr-bearing plasmids in different sampling sectors (e.g., IncHI2 found in isolates from humans and chickens (Fig. S2(a)); IncI2 in isolates from humans, water, flies, and chickens (Fig. S2(b)); IncP1 in isolates from humans, chickens, and flies (Fig. S2(c)); IncX4 in isolates of humans, dogs, chickens, and flies (Fig. S2(d)); and IncFII in isolates from humans and flies (Fig. S4)), suggests possible plasmid-mediated inter-host transmission and a potential role for flies in the dissemination of mcr in Laos. Notably, the mcr-1.1 carrying IncX4 (accession No. CP063335) and the mcr-3.5 carrying IncFII (accession No. CP063484) that were characterized in a recent study in Laos [13] were very similar to the mcr-positive IncX4 and IncFII plasmids found in this study(at ≥ 97% coverage, ≥ 99% identity), highlighting a dominant role of plasmids in the spread of mcr in Laos. Conjugation assays confirmed the transferability of all the mcr-positive plasmids examined in this study; however, variations in transferability were observed (Fig. 4). Unsuccessful conjugation results may be explained by the variations in recipient genotypes and plasmid backgrounds [34]. The recipients we chose did not possess plasmids of similar Inc types to the donors, which potentially excluded conjugation failure by replication-control mechanisms (Tables S2 and S13) [41]. Our findings suggested a predilection for the acquisition of mcr into ST1193 rather than E. coli ST10, ST131, or ST167 (Fig. 4 and Table S12). It is also possible that IncHI2 harboring mcr could not be transferred into E. coli ST10, ST131, or ST167 due to its high molecular weight of >250 kb (Tables S2 and S12) [37]. Further more, a high rate of conjugation into ST1193 should act as a warning for the emergence of mcr in a hypervirulent clone (Table S13) [19].
Post-segregational killing systems are ubiquitous in conjugative plasmids, which facilitates the maintenance of plasmids in subsequent generations during cell division and may be influenced by the fitness costs due to the plasmids or adaptive evolution [35,36]. The plasmids of IncFII, IncHI2, IncI2, InP1, and IncX4 that were recovered in this study appeared to exert a fitness burden; however, these effects were not observed in all transconjugants and were not confined to particular STs of the transconjugants (Fig. 6). Consistent with earlier findings, mcr-positive IncHI2 had a high molecular weight and carried more ARGs compared with other Inc-type plasmids, which is likely to have imposed a fitness burden (Fig. 6, Figs. S1(a) and S2) [37]. Despite the variations in fitness costs, the mcr-positive plasmids were stable in all transconjugants except BCS.1847:R3, irrespective of Inc types and without any antibiotic selection (Fig. 5). Some transconjugants can maintain plasmids for 50 generations without selection [38]. It is also worth noting that the plasmids of IncHI2 were maintained in the transconjugants at low copy numbers following 15 days’ passaging, despite the significant fitness effects (Figs. 5 and 6).
《5. Conclusions》
5. Conclusions
We have demonstrated a high prevalence of mcr-related colistin resistance, which appears to have been disseminated horizontally in a wide range of hosts and environments in Laos. This finding implies a need for the urgent implementation of both theoretical and practical interventions across individual professional boundaries, including antimicrobial stewardship programs in both the healthcare and agricultural sectors in this country, to impede the spread of AMR.
《Acknowledgments》
Acknowledgments
We thank the staff of the Lao-Oxford-Mahosot HospitalWellcome Trust Research Unit (LOMWRU) and the Mahosot Hospital Microbiology Laboratory for their advice and support in undertaking this investigation, particularly Anisone Chanthongthip and Sengkham Simanivong for logistical support. Dr. Vilada Chansamouth kindly shared pre-publication data from a review of AMR in Laos. We are grateful to the staff at the Ministry of Health, the University of Health Sciences, the directors of Mahosot Hospital and the late Dr. Rattanaphone Phetsouvanh for their support of this study and the work of LOMWRU. This project was funded partly by the Wellcome Trust (214207/Z/18/Z). For the purpose of open access, the author has applied a CCBY public copyright license to any Author Accepted Manuscript version arising from this submission.
《Authors’ contribution》
Authors’ contribution
Yuqing Zhou: conceptualization, methodology, validation, investigation, formal analysis, writing – review & editing, visualization. Refath Farzana: conceptualization, methodology, validation, investigation, formal analysis, writing – review & editing. Somsavanh Sihalath: investigation, writing – review & editing. Sayaphet Rattanavong: investigation, project administration, writing – review & editing. Manivanh Vongsouvath: project administration, writing – review & editing. Mayfong Mayxay: methodology, writing – review & editing. Kirsty Sands: investigation, formal analysis, writing – review & editing. Paul N. Newton: writing – review & editing. David A.B. Dance: conceptualization, validation, writing – review & editing. Brekhna Hassan: conceptualization, methodology, investigation, writing – review & editing. Timothy R. Walsh: conceptualization, funding acquisition, methodology, validation, writing – review & editing, and a supervision.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Yuqing Zhou, Refath Farzana, Somsavanh Sihalath, Sayaphet Rattanavong, Manivanh Vongsouvath, Mayfong Mayxay, Kirsty Sands, Paul N. Newton, David A. B. Dance, Brekhna Hassan, and Timothy R. Walsh declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2022.01.013.














 京公网安备 11010502051620号
京公网安备 11010502051620号




