《1. Introduction》
1. Introduction
Microalgae are usually defined as a group of unicellular photosynthetic microorganisms that live in aquatic (marine and fresh water) and terrestrial environments [1]. To date, more than 3000 species of microalgae have been reported. Using chlorophyll a, microalgae can transform carbon dioxide (CO2) into diverse bioactive compounds via photosynthesis and thereby achieve about tenfold higher efficiency in fixing CO2 than terrestrial plants. Their natural bioactive compounds include amino acids, proteins, lipids, polysaccharides, and carotenoids [2,3]. In the ocean, microalgae make the greatest contribution to flora biomass feedstock due to their fast growth and reproduction with low requirements [4]. Microalgae-based bioproducts have been widely used for assisting various human activities [5–10]. Their metabolism activities and products are useful in various fields, including food production, biofuel, sewage treatment, the biopharmacy industry, and biomedical therapy [1,11–19]. Nevertheless, their usage as living organisms in the biomedical field has not drawn significant attention until recent years.
Along with the rapid development of biomaterials in which the nano-bio interface plays a critical role in determining performance, research interest in biohybrid materials containing biological components is increasing. Biohybrid materials with live organisms exhibit apparent advantages compared with conventional materials in many aspects, especially for biomedical applications. These advantages include: ① significantly improved biocompatibility and biodegradability [20–23]; and ② the functional reproduction of naturally efficient systems in the external environment, which are thus far inimitable using basic artificial elements. Furthermore, these live-organism-based materials will facilitate the application of biological functions by manipulating the spatial displacement and structural fabrication of organisms. Live cells including cardiomyocytes, skeletal muscles, and microorganisms (e.g., bacteria) have been reported to be promising elements for integration into biohybrid materials [20,22,24]. Moreover, the ingenious integration of microalgae into natural or artificial biomaterials for various biomedical applications is gradually being reported. Over the past decade, microalgae-based biohybrid materials (MBBMs) composed of living microalgae and biomaterials have presented tremendous potential for solving a series of medical challenges, such as oncotherapy, tissue reconstruction, drug delivery, and more.
Microalgae are multifunctional living factories that can produce oxygen, diverse metabolites, and autofluorescence due to the physiological abilities of unicellular organisms. These natural activities can be incorporated into MBBM design for various biomedical applications. In addition, the autokinetic movement of dinoflagellates endows well-designed MBBMs with the specific ability to act as transport vehicles for delivering drug molecules, such as for cancer therapy [25,26]. Herein, we review a comprehensive background of MBBMs and summarize their fabrication, biological physiology, and impetus, along with several practical applications in the biomedical field (Fig. 1). Finally, we raise a critical discussion on the challenges and perspectives in this area, depicting rational considerations about the development of MBBM in the future.
《Fig. 1》
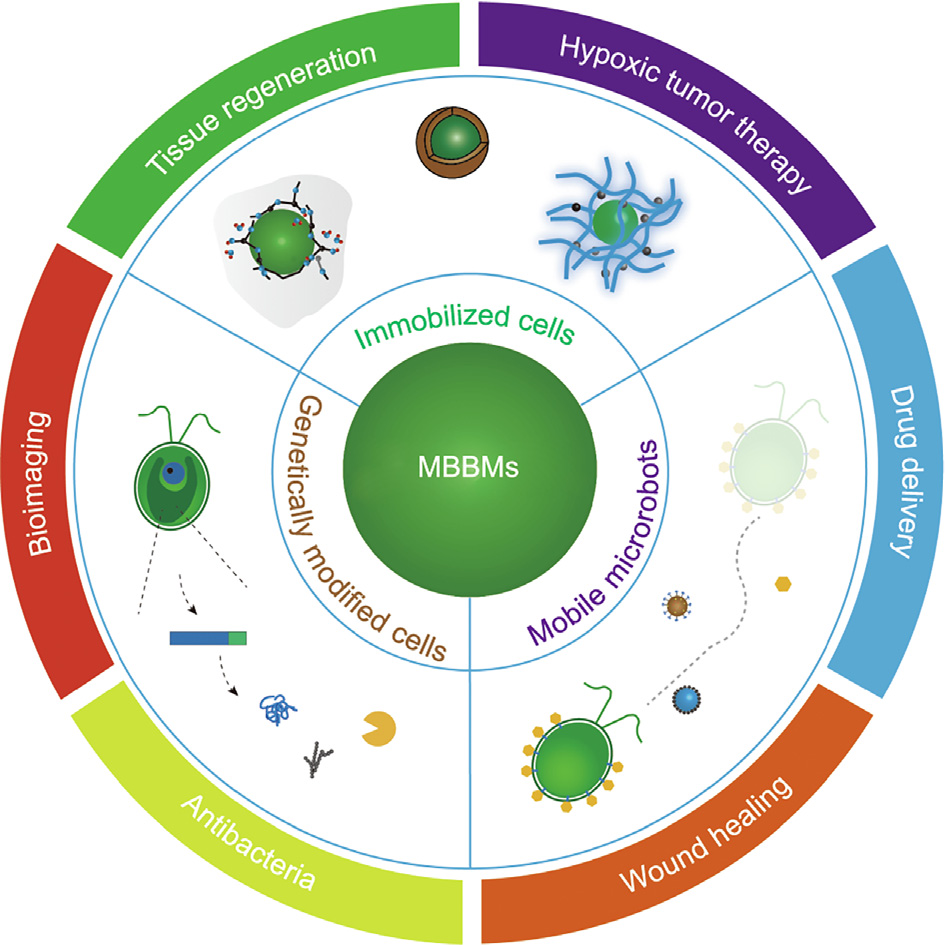
Fig. 1. Design of MBBMs and their diverse biomedical applications.
《2. Conventional usage of microalgae biomass in biomedicine》
2. Conventional usage of microalgae biomass in biomedicine
Biomass is organic material derived from plants (including algae) and animals [27]. Microalgae biomass has been applied in diverse human productive activities, due to its wide variety, rapid growth, and low cost of industrial production. Despite this, only a few clues on the integration of microalgae biomass within biohybrid materials for biomedical applications have been published thus far. For example, Chlorella vulgaris (C. vulgaris) extract (CVE)- A has been defined as a group of water-soluble, high-molecularweight fractions including proteins, carbohydrates, and nucleic acids extracted from dialyzed and lyophilized C. vulgaris, a kind of unicellular green algae. It has been reported that, after being pretreated with CVE-A via intraperitoneal, intravenous, or subcutaneous administration, mice exhibited a significantly higher survival rate during intraperitoneal infection by Escherichia coli, as evidenced by the increased elimination of bacteria from their spleens [28]. Pre-administrated CVE-A may enhance the activity of polymorphonuclear leucocytes, promoting superoxide generation and thus killing bacteria more efficiently at infected sites. Lesion of the intestinal barrier is a significant mechanism of endotoxemia in obstructive jaundice. Chlorella sp. extracts were found to reduce the bacterial translocation and malondialdehyde activities of oxidative stress responses in rat intestine after bile duct ligation, thereby revealing the positive regulation of Chlorella sp. on intestinal mucosal lipid peroxidation and reduction in villous atrophy [29].
In recent years, microalgae biomass has been used as a bioactive filler in bio-scaffolds for regenerative medicine. Nanofibrous scaffolds prepared via electrospinning have mechanical and topographical characteristics similar to those of the extracellular matrix (ECM) and can thus mimic its natural functions; as a result, they have been widely investigated for various biomedical applications [30–34]. Spirulina, a kind of blue-green microalgae, has been developed as a source of potential pharmaceuticals, due to the therapeutic (e.g., antioxidant, anti-inflammatory) functions of its bioactive compounds [35,36]. The incorporation of Spirulina biomass into biocompatible nanofibers with polymers by means of electrospinning results in the construction of versatile scaffolds with high porosity, hydrophilicity, large surface area, and improved capability to support cell adhesion and proliferation. de Morais et al. [37] developed a highly porous fibrous nonwoven scaffold via electrospinning, in which Spirulina sp. LEB 18 biomass was used as a bio-filler within polyethylene oxide. By optimizing the viscosity and conductivity of the biohybrid solution, beadfree nanofibrous scaffolds (~110 nm diameters) were prepared with the biomass at a concentration of up to 67 wt%. These biohybrid fibrous scaffolds possessed an ECM-mimicking structure with a high content ratio of biomass and showed promise for tissue engineering.
When Spirulina extracts were added into the fabrication of silk fibroin-based nanofibers via electrospinning, the nanofibers’ morphology was found to be greatly dependent on the concentration of Spirulina extracts. The composite fibers exhibited low cytotoxicity, supported the adhesion and growth of both fibroblasts and human umbilical vein endothelial cells, and remarkably inhibited blood clotting or antithrombogenicity, suggesting that these scaffolds could find potential application in blood vessel regenerative treatment [38]. Similarly, biohybrid nanofibers composed of poly-D,L-lactic acid (PDLLA) and Spirulina (Arthrospira) extracts were found to be atoxic to stem cells and even promoted cell viability and adhesion in comparison with PDLLA on its own, which might be attributed to several bioactive components in the biomass [39]. Spirulina extracts added to culture media were found to enhance the cell growth and metabolism of rat primary astrocytes; however, when they were combined within electrospun polycaprolactone (PCL) nanofibers, the bioactivities of the astrocytes were alleviated, although no significant toxicity was induced [40]. It is reasonable to speculate that the embedded extracts were not efficiently released from the PCL nanofibers during culture.
To summarize, although the implementation strategies in some specific applications require further investigation, the therapeutic effects of several applications of microalgae biomass have been demonstrated and shown to hold promise for biomedicine. Readers interested in this aspect are recommended to view previously published reviews of the biomedical applications of microalgal bioactive compounds [15,41], while this review will move on to the integration of living microalgae cells in MBBMs.
《3. Integration of living microalgae in MBBMs》
3. Integration of living microalgae in MBBMs
Over the past decade, an increasing number of publications have highlighted the superiority of biohybrid materials containing living microalgae for diverse applications in the biomedical field. Compared with conventional biomaterials, MBBMs can provide benefits based on the physiologies of live microalgae, such as photosynthetic activity, autofluorescence, and autokinetic activities. For example, photosynthetic tissue constructs can be obtained, in which algae cells provide local oxygen to support the growth of mammalian cells. In this sense, the oxygen supplied by microalgae photosynthesis could alter the hypoxia condition caused by poor vascularization in tumors. Most microalgae can exhibit green autofluorescence through chloroplasts or by the accumulation of vegetative cells, cysts, and cell spines; examples of such microalgae include dinoflagellates, diatoms, cyanobacteria, and green algae [42]. Besides, the chlorophyll in microalgae also exhibits red autofluorescence [43,44]. These forms of autofluorescence can function as indicators of cell viability and biocompatibility or can provide sufficient signals for the tracing of most microalgaebased biohybrid systems through the spectroscopic monitoring of changes in photosynthetic pigments. Moreover, the autokinetic movement of dinoflagellates can endow MBBMs with elaborate mobility for various applications, such as drug delivery. Herein, classified by their motility (i.e., immobilized cells or mobile microrobots), we summarize how live microalgae have been incorporated into MBBMs and review the biomedical applications of both categories.
《3.1. Immobilized cells》
3.1. Immobilized cells
Since most microalgae are suspension-type microorganisms, the appropriate immobilization of microalgae cells on a solid surface can reduce the occupied growth space and medium, and enable the reuse of cells for sustainable product collection. Moreover, compared with free cells, irreversible malformation can be avoided in immobilized microalgae. The encapsulation of live microalgae in a three-dimensional (3D) matrix (e.g., a polymeric material or inorganic sphere) is a key method for cell immobilization [45]. It is worth mentioning that, in order to ensure the photosynthetic activities of immobilized microalgae, the matrix should generally possess a certain degree of transparency for creating an illumination environment. In this way, immobilized microalgae cells can maintain their natural bioactivities. Various encapsulation approaches for carrying out MBBM hybridization are reviewed in this section, along with their potential use in the biomedical field.
3.1.1. The sol–gel approach for microalgae cell encapsulation
The sol–gel approach—one of the most common techniques for preparing silica matrices—has also been used for encapsulating microalgae cells in a matrix (Fig. 2) [46–48]. The immobilization of microalgae cells in porous silica matrices is helpful in maintaining their structural stabilization; however, it is still essential to optimize the sol–gel process to improve cell viability in the MBBM system. Several feasible methods have been developed for optimizing the encapsulation process, including: ① maintaining a partially hydrated environment; ② selecting specific precursors (e.g., aqueous sodium silicate/colloidal silica) to promote the matrix biocompatibility [49–53]; and ③ adding glycerol to reduce the osmotic stress imposed on the embedded cells by adjusting the porosity and pore connectivity of the MBBMs [46,54]. Thus far, porous and light-admitting silica matrices have been successfully used for encapsulating C. vulgaris, Synechococcus, Cyanidium caldarium, Synechocystis, Porphyridium purpureum, and Chlamydomonas reinhardtii (C. reinhardtii) cells and have favorably preserved the cells’ viability over periods of days to weeks. In general, microalgae are harvested and mixed with an as-prepared silicate solution, followed by a poly-condensation process. This technique has been further applied for developing a photo-oxygen bioreactor for CO2 removal, O2/H2O2 release, or fluorescence imaging [46,49–51, 53–58].
Alternatively, a core–shell structure composed of a SiO2-based hybrid shell with a microalgae cell as the core provides another path to achieve encapsulation. For example, the cell surface of cyanobacteria Synechocystis sp. strain PCC 6803 cells was coated with multiple layers composed of a biocompatible polyamine, poly(diallyldimethylammonium chloride) (PDADMAC), and poly(styrene sulfonate) via a modified layer-by-layer (LbL) method. This in situ silicification appeared to promote the photosynthesis and biomass production of cyanobacteria under high light conditions [59]. In another study, a mixture of alginate and mineralized porous silica was used to envelop Dunaliella tertiolecta, a cyanobacteria strain, through the condensation of polycations on the charged silica surface. This process produced a shell with semipermeability, in which abundant PDADMAC acted as catalyst and flocculating agents for the condensation of silica sol. The extremely long-term viability (over 13 months) and high bioactivity of the cyanobacteria wrapped within the alginate–silica hybrid matrix suggested the generation of fixed biomass photobioreactors in the future [47].
Recently, a microalgae–gel patch was developed to address the chronic healing of skin wounds in diabetes. In this patch, Synechococcus elongatus PCC 7942 cells were encapsulated in millimeter alginate hydrogels and used to provide O2 and CO2 in the wound area through photosynthesis and respiration (Fig. 2(f)). Compared with topical gaseous oxygen, the microalgae–gel patch provided an oxygen supply over 100-fold higher due to the more efficient penetration capability of the microalgae’s dissolved oxygen. Consequently, the patch more efficiently promoted fibroblast proliferation and angiogenesis, leading to faster wound healing and skin graft survival in a diabetic mouse model in comparison with the untreated control (Fig. 2(g)) [48]. These results demonstrate the possibility of encapsulating living microalgae cells in a gel matrix, which holds promise for the construction of photosynthetic biohybrid materials for biomedical applications.
《Fig. 2》
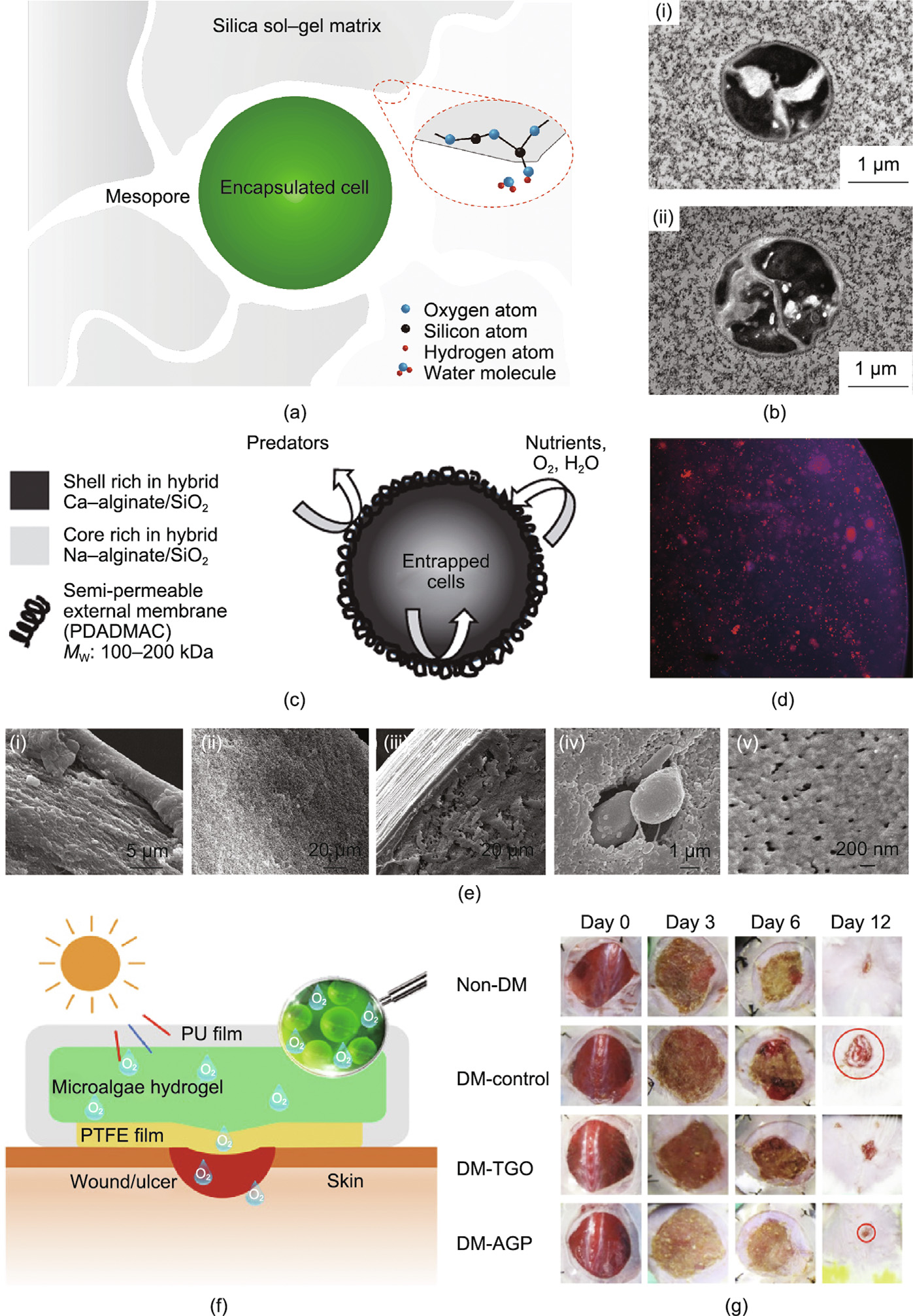
Fig. 2. Entrapment of microalgae cells in sol–gel-based materials. (a) Schematic of microalgae cell entrapment in silica gel. (b) Transmission electron microscopy images of microalgae cells immobilized in silica gel. (c) Structural schematic of an alginate–silica composite bead with enveloped microalgae. (d) Fluorescent image of biohybrid beads; red fluorescence represents the autofluorescence in the chlorophyll of live microalgae, and silica is marked by the violet fluorescent probe, 2-(4-pyridyl)-5-[(4-(2- dimethylaminoethylaminocarbamoyl)methoxy)-phenyl]oxazole (PDMPO). (e) Scanning electron microscopy (SEM) images of hybrid alginate–silica beads (i) with and (ii) without poly(diallyldimethylammonium chloride) (PDADMAC), and the (iii, v) distal layer and (iv) core of a composite bead containing microalgae cells. (f) Schematic illustration of the structure of a microalgae–gel patch and the release of dissolved oxygen for healing chronic wounds. (g) Representative photos of the wound area under various treatment at different timepoints after the operation. PU: polyurethane; PTFE: polytetrafluoroethylene; DM: diabetic mouse; TGO: topical gaseous oxygen; AGP: alga–gel patch. (b) Reproduced from Ref. [46] with permission; (c–e) reproduced from Ref. [47] with permission; (f, g) reproduced from Ref. [48] with permission.
3.1.2. Dip coating with microalgae cells on a porous scaffold
Another straightforward strategy for immobilizing microalgae cells is to incubate porous scaffolds as substrates in a microalgae suspension [57,58,60]. Live microorganisms have been immobilized onto electrospun fibers made of biocompatible polymers, including chitosan, polysulfone (PSU), polyamide, polyacrylonitrile, and polypropylene [61–63]. For example, C. vulgaris cells were successfully immobilized on a porous chitosan nanofibrous membrane through an electrostatic interaction between the positively charged chitosan chains and the negatively charged surface of the C. vulgaris cells [64]. In another case, C. reinhardtii cells were successfully entrapped via physical absorption and grew well in a PSU nanofibrous web (PSU-NFW) (Figs. 3(a)–(e)) [61]. The combination of an electrospun fibrous mat and microalgae can take great advantage of the highly porous and ECM-mimicking structure of the fibrous network, which can be a good option for trapping microalgae. A mixture of a microalgae C. reinhardtii suspension supplemented with fibrinogen was used for seeding the microalgae in commercial bio-scaffolds (i.e., Integra matrix single-layer scaffolds); the additive fibrin ensured the immobilization of C. reinhardtii cells in the target position post-transplantation. As a result, a novel photosynthetic biomaterial with microalgae, named ‘‘HULK,” was obtained for local oxygen supply (Figs. 3(f) and (g)) [56,57]. After this, MBBM was transplanted for healing a full-skin defect in mouse. The microalgae cells survived within 5 days without inducing any significant inflammatory response; more importantly, they promoted the generation of chimeric tissues with murine cells in vivo [56]. These results revealed that MBBM with specific designs could be developed as photosynthetic bioscaffolds for autotrophic tissue engineering (e.g., wound healing, tissue repair), with the aim of providing an oxygen supply via microalgae photosynthesis to offset the hypoxia condition caused by poor vascularization in engineered tissues. Therefore, an early phase-1 clinical trial was performed to evaluate the safety and therapeutic efficiency of this C. reinhardtii-based scaffold in healing full-thickness skin wounds (Fig. 3(h)) [65]. This photosynthetic implant led to full-skin tissue regeneration in the wounds, without obvious immune responses during 90 days of post-surgery tracking observation.
《Fig. 3》
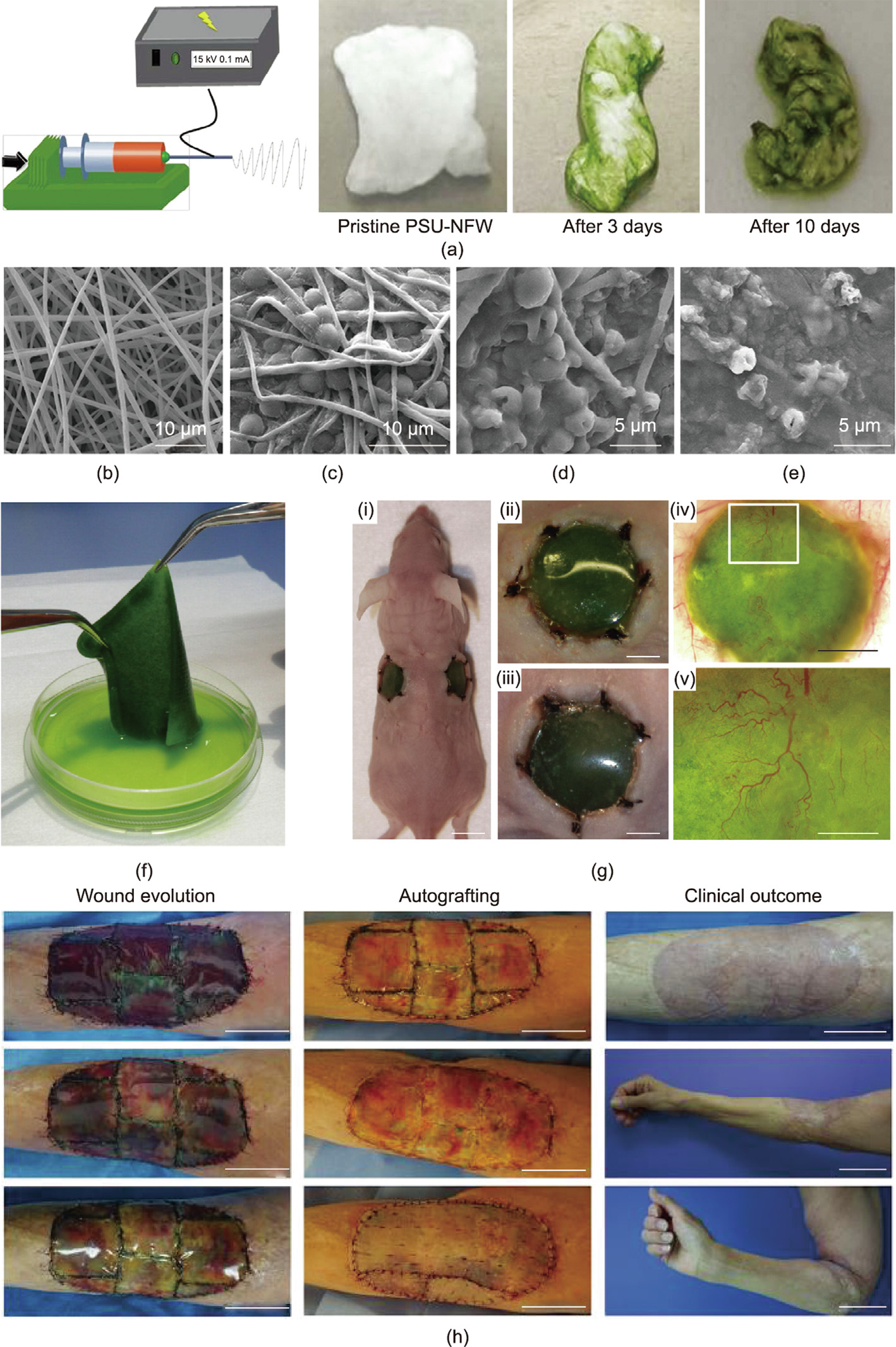
Fig. 3. Entrapment of microalgae in porous biomaterials. (a) A PSU-NFW prepared via electrospinning. Significant color changes are observed after several days’ incubation of the PSU-NFW in a microalgae solution to allow sufficient adhesion. (b–e) SEM images of PSU-NFW (b) without and (c) with attached microalgae, and biohybrid PSU-NFW with microalgae after 14 days’ decolorization for (d) Reactive Black 5 and (e) Reactive Blue 221. (f) Representative photos of the photosynthetic scaffold with microalgae. (g) Photos of the in vivo implantation of the microalgae-containing scaffolds in mouse: (i) The photosynthetic scaffolds remain green 5 days after implantation into bilateral full-skin defects in mouse; (ii, iii) the wound areas on both sides show no recognizable infection or inflammation; (iv, v) the scaffolds clearly promote vascularization in the defect regions. Scale bars: 1 cm in (i), 4 mm in (ii–iv), 1 mm in (v). (h) Wound evolution of patient at days 1, 7, and 13 (from top to bottom) post implantation; autologous splitthickness skin graft performed at day 21; clinical outcome at day 90. Scale bars: 5 cm in wound evolution, autografting, and the upper photo of the clinical outcome column; 10 cm in the middle and bottom photos of clinical outcome. (a–e) Reproduced from Ref. [61] with permission; (f, g) reproduced Ref. [56] with permission; (h) reproduced from Ref. [65] with permission.
3.1.3. 3D bioprinting for microalgae cell encapsulation
As a newly developed technique in recent decades, 3D printing can be used to prepare architectures by precisely depositing biocompatible materials layer by layer in a well-controlled manner. This technique has been demonstrated to have huge potential for tissue engineering [66–68]. By optimizing the formulation of hybrid bioinks such as alginate-based gels, 3D bioprinting can enable the spatially directed placement of biohybrid materials while enabling the encapsulated cells to retain their activities. For example, an ink composed of alginate, gelatin precursors, and hydroxyapatite was formulated for bioprinting and demonstrated excellent preservation of embedded human mesenchymal stem cells, with a viability of up to 85% 3 days post-plotting [62]. Similarly, living microalgae (C. vulgaris) cells were mixed in alginate hydrogels and printed onto a glass substrate in the form of viscous nanoscale sodium drops through a noncontact micro-dosage system; this was followed by a gelation process with Ca2+ ions or amino-functionalized silica sol [69]. The immobilized cells maintained their inherent bio-photoactivity over a prolonged period of 8 weeks. It was also reported that C. reinhardtii embedded in alginate-based scaffolds survived after 3D bioprinting and maintained their normal growth for 12 days. Moreover, a human osteosarcoma cell line (SaOS-2) was co-encapsulated in a spatially well-organized manner to create human/algae cell co-culture tissue constructs. This was achieved by using a multichannel printing protocol in which microalgae cells were plotted close to human SaOS-2 cells—a technique that demonstrated the microalgae’s promising participation in supplying oxygen and/or transporting metabolites for biomedical therapies (Figs. 4(a)–(c)) [70]. Trampe et al. [71] further expanded the functionalities of this co-culture scaffold by adding luminescent optical sensor nanoparticles into the bioink, which enabled the visualization of O2 concentrations, allowing spatiotemporal monitoring of O2 accumulation in the construct (Figs. 4(d)–(g)).
3.1.4. Enveloping microalgae cells with nanolayers
Enveloping microalgae cells with nanolayers can stabilize the cells to enable the sustainable use of their bioactivities, while endowing the composite structure with extra functions by tailoring the coating layers. For example, Wahid et al. [72] successfully achieved bio-hybridization via a vortex fluidic device (VFD) in two steps: the exfoliation of multilayer graphene sheets and hybridization with microalgae cells in water. The as-prepared MBBM exhibited a higher removal rate of initial nitrate content compared with free C. vulgaris cells and graphene alone. Furthermore, a similar biohybrid material but with a magnetic component in the coating layer was developed via the same VFD system by adding magnetic nanoparticles in a polyvinylpyrrolidone (PVP) matrix to the fabrication. This functional MBBM was used for applications in nitrate removal and separation under an external magnetic field (Fig. 5(a)) [72–74].
In the field of biomedicine, an ingenious design involved enveloping microalgae cells with a mammalian cell membrane, which worked as a stealth layer and as an efficient functionalization method to target tumor cells (Figs. 5(b)–(e)). In that work, C. vulgaris cells wrapped by a cloak of red blood cell membrane (RBCM) were used to target tumor tissue and relieve its hypoxia environment through in situ photosynthesis under red light irradiation. As a result, the resistance of the hypoxia tumor cells to radio therapy was markedly decreased. Moreover, the chlorophyll released from dead algae cells after X-ray treatment produced reactive oxygen species under 650 nm laser irradiation, further enhancing cell apoptosis via photodynamic therapy [25].
《3.2. Mobile microrobots》
3.2. Mobile microrobots
In recent decades, biohybrid materials have gradually attracted attention for the design of microrobots in which the embedded biological component functions as a bioactive filler or even actuates the movement of the microrobot through bioenergy rather than through external stimulation such as a magnetic field or light [24,75–78]. In particular, microalgae-based biohybrid robots (MBBRs) exhibit unique advantages for designing a transportable photosynthesis factory and targeted load delivery. First, the surface of microalgae cells (e.g., C. reinhardtii) enables functionalization, such as by loading nanoparticles or stimuli-responsive polyelectrolytes (PEs) via noncovalent interactions [24,26,79–81]. Second, the good biocompatibility and biodegradability of MBBR enable highly efficient transportation of cargos into the cytoplasm, as well as further degradation in a physiological environment for biomedical applications [76,80].
It is widely recognized that the cell wall structure of microalgae (i.e., C. reinhardtii) is constructed with multiple glycoproteins through either covalent or noncovalent interactions, leaving rich 4-hydroxyproline (4-HP) residues on the outer surface. Therefore, cargos were first decorated with biomimetic 4-HP polypeptides for binding to the microalgae cell surface by means of noncovalent interactions [79]. Further investigations revealed that—due to the negatively charged property of their 4-HP-rich surface—microalgae tend to attract positively charged molecules or nano-/micromaterials, probably via electrostatic interactions. Such noncovalent interactions can be used to avoid any damage to the microorganism’s natural bioactivity and motility during the biohybrid process. A positively charged PE with magnetic polystyrene (PS) microparticles [80], hybrid beads containing the antibiotic vancomycin (a highly active glycopeptide) [81], LbL-assembled magnetic iron oxide nanoparticle-decorated PS/cationic PDADMAC microbeads [24], and chitosan-coated iron oxide beads [26] were respectively utilized in bio-hybridizations with C. reinhardtii cells through this kind of bio-interfacial interaction (Fig. 6) [24,26,79,80]. By conjugating chemotherapeutic drugs to biohybrid carriers via photocleavable linkers, photodegradation-mediated cargo release has been demonstrated for on-demand delivery [26,79].
《Fig. 4》
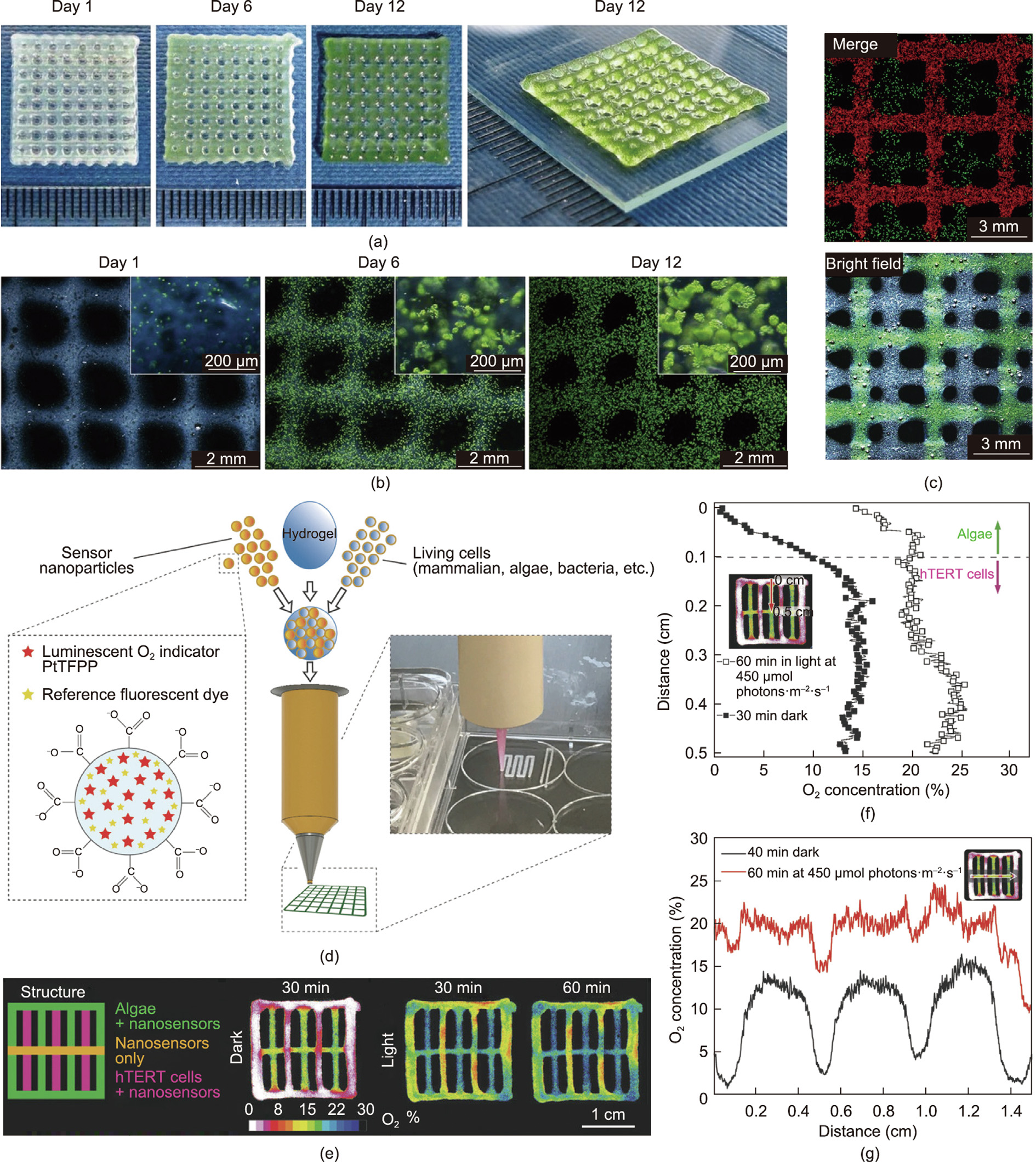
Fig. 4. Immobilization of microalgae for oxygen production through a 3D bioprinting process. (a) Four-layer constructs prepared via 3D bioprinting with algae-laden alginate bioink; (b) their corresponding microscopic images during 12 days culture. (c) Fluorescence and bright-field images of 3D bioprinted constructs for the co-culture of live microalgae and human SaOS-2; microalgae cells are represented by the red autofluorescence of chlorophyll and SaOS-2 are stained green by calcein. (d) Schematic of 3D bioprinting with multiple components such as live cells containing bioinks supplemented with O2-sensitive nanoparticles and reference fluorescent dye. (e) Illustration of a multilayered construct composed of various components in each layer: either sensing nanoparticles with/without microalgae or mammalian cells. Spatiotemporal dynamics images show the distribution of O2 in different strands of the construct under light or dark conditions. (f, g) Profiles of O2 concentration across the (f) mammalian cell + nanoparticles strand and (g) nanoparticle-only strand in the multilayered construct in the dark or after light irradiation. PtTFPP: platinum(II) meso(2,3,4,5,6- pentafluoro)phenyl porphyrin; hTERT: human telomerase reverse transcriptase. (a–c) Reproduced from Ref. [70] with permission; (d–g) reproduced from Ref. [71] with permission.
Theoretically, all the chemotaxis, phototaxis, geotaxis, and flagella-beating behaviors of microalgae cells could be utilized to design microrobotic actuation [82]. In 2005, Weibel et al. [79] were the first to report the proof-of-concept implementation of MBBR, which comprised a whole living microorganism with a controllable locomotion mode. Since then, researchers have made great efforts to investigate diverse movement guidance or various loading cargo patterns for promoting the development of MBBR.
3.2.1. MBBR driven by an external magnetic field
MBBRs containing magnetic substances have been suggested as an efficient tool for the targeted delivery of therapeutic and imaging agents, due to their highly precise locomotion under remote control by an external magnetic field, autofluorescence, and superparamagnetism of the magnetic component [24]. For example, helical-shaped Spirulina-based microswimmers that were decorated with magnetic (Fe3O4) nanoparticles were developed as biodegradable drug carriers and imaging agents with selective cytotoxicity to cancer cells [75,76]. The autofluorescence of Spirulina enabled the noninvasive tracking of the microrobot in superficial tissues, while the outer magnetic layer allowed robust navigation by means of magnetic resonance imaging [76]. Importantly, the Spirulina functioned as a template for further magnetization by preserving its chiral and helical morphology, which was important for achieving rotation-coupling propulsion—the most promising means of magnetic actuation. In addition, the degradation and cytotoxicity to cancer cells of this microswimmer relied on the thickness of the outer Fe3O4 shell, which could be tailored by controlling the dip-coating process. This one-step magnetization strategy was also demonstrated with spherical C. reinhardtii and ellipsoidal Tetraselmis subcordiformis. Similarly, terbium (Tb) was incorporated as the magnetic component when making microalgae-based microswimmers. Rather than forming a magnetic shell, Tb3+ was added to a culture of C. reinhardtii and was uptaken by the algae cells. As a result, the tailored C. reinhardtii cells showed a directional motion in response to an external magnetic field. They also held promise for use as a bioimaging agent due to the inherent autofluorescence of the algae cells and the photoluminescence of internalized Tb3+ [77].
3.2.2. MBBR driven by microalgae autokinetic movement
The autokinetic movement of dinoflagellates is generally achieved by pulling fluid from their head to the outer surface. Utilizing the tropism of these microalgae to light, chemicals, or gravity can trigger motion, steer the locomotion trail of MBBR in a specific environment, and endow them with potential carrying capacities for delivering drugs. For example, directional swimming of C. reinhardtii cells was observed when varying the light signal in a microfluidic chip [78]. The algae cells exhibited obviously random and light-parallel motion trajectories in the absence and presence of light, respectively; even a zigzag/triangle movement was induced under precisely controlled optical guidance with a novel algae-guiding system (AGS). Similarly, Yasa et al. [80] reported a biohybrid microswimmer containing C. reinhardtii as the actuator. Magnetic PS microparticles functionalized with a positively charged PE shell were loaded onto the microalgae cell surface via noninvasive electrostatic interaction. The microswimmer exhibited traveling abilities under both illumination and dark conditions, with a swimming speed of (135.92 ± 4.82) μm∙s–1 in a 3D fashion, while its motility in biological fluids was significantly hampered by the high viscosity. The microswimmer also moved more slowly than free microalgae due to the bonded microparticles, although the existence of superparamagnetic PS particles promoted the speed to a comparable value ((156.13 ± 9.66) μm∙s–1 ) and guided the propulsion trajectory under an external magnetic field. Deep investigation of the swimming behaviors of C. reinhardtii after loading the microbeads revealed decreased swimming velocity, as well as a motion mode change from a linear forward motion to a helical motion, resulting from the attached cargo suppressing the flagella movement [83]. Hydrosoluble polysaccharide – fluorescent isothiocyanate (FITC) – dextran molecules, as a visible cargo model, were then successfully transported into cervical cancer cells (HeLa) by this swimmer (Fig. 6(b-vi)) [80].
《Fig. 5》
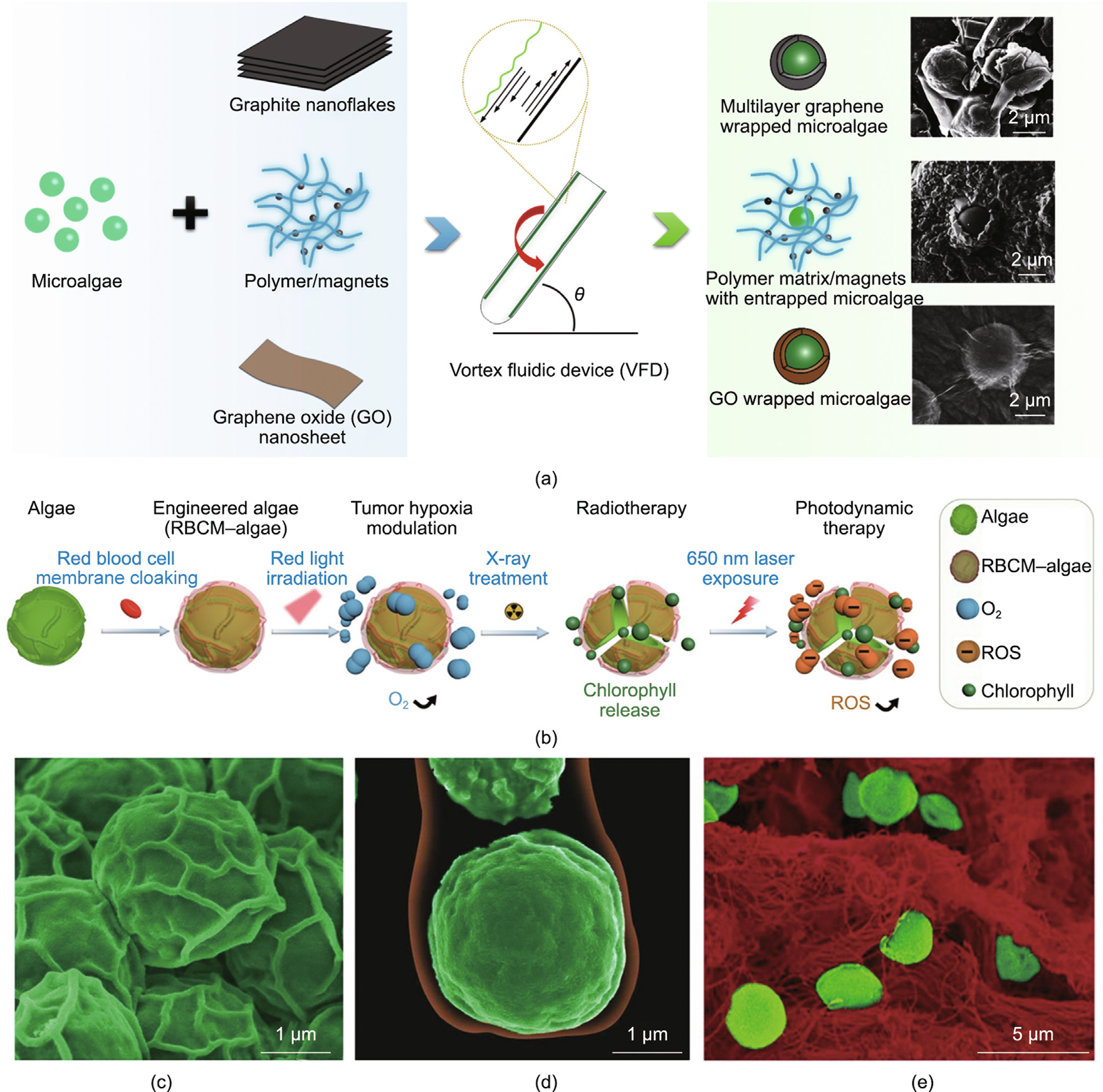
Fig. 5. Enveloping microalgae cells with nanolayers. (a) Schematic of fabrication and corresponding SEM images of the bio-hybridization of C. vulgaris cells with graphene/graphene-oxide/PVP-magnetic materials via a VFD platform. (b) Illustration of the fabrication process of engineered microalgae enveloped in a red blood cell membrane (RBCM), and an as-developed novel strategy for eliminating hypoxic conditions in the tumor microenvironment by generating oxygen under external light modulation. (c, d) SEM images of (c) pristine algae and (d) algae enveloped by an RBCM, where the algae cells and RBCM are pseudocolored green and red, respectively. (e) SEM image showing RBCM–algae (pseudocolored green) were transported to tumor tissue (pseudocolored red) in 2 h, after intravenous injection. ROS: reactive oxygen species. (a) Reproduced from Refs. [72–74] with permission; (b–e) reproduced from Ref. [25] with permission.
Despite the straightforwardness of the strategies that have been developed for loading microscale cargos onto the microalgae surface via noncovalent electrostatic interactions [24,80,81], the actual efficiencies of these strategies have been far from satisfactory. The population of microalgae cells carrying payloads is limited, probably due to the low efficiency of random collisions between the cells and the micrometer-scale particles of the payload. To overcome this drawback, a molecular assembly approach was proposed in which the algae cell surface was first coated by a thin layer of naturally bioactive polymers (e.g., chitosan), which acted as a binding agent to increase the attachment of particles or therapeutic molecules [26]. The chitosan thin layer provided commendable preservation of the viability and phototactic ability of the microalgae. This biohybrid microswimmer was used for ondemand drug delivery to tumors through conjugation with a chemotherapeutic drug, doxorubicin, via a photocleavable linker (Figs. 6(c-i) and (c-ii)). Aside from being bound through electrostatic adhesion, cargos such as the antibiotic vancomycin can be bound to the cell surface of C. reinhardtii by means of oligohydroxyproline anchors (Fig. 6(c-iii)) [81]. However, it should be noted that possible internalization and cell toxicity arises with decreased cargo size or specific functionalization, which is likely to be dependent on concentration, incubation time, microalgae cell density, and so forth.
《Fig. 6》
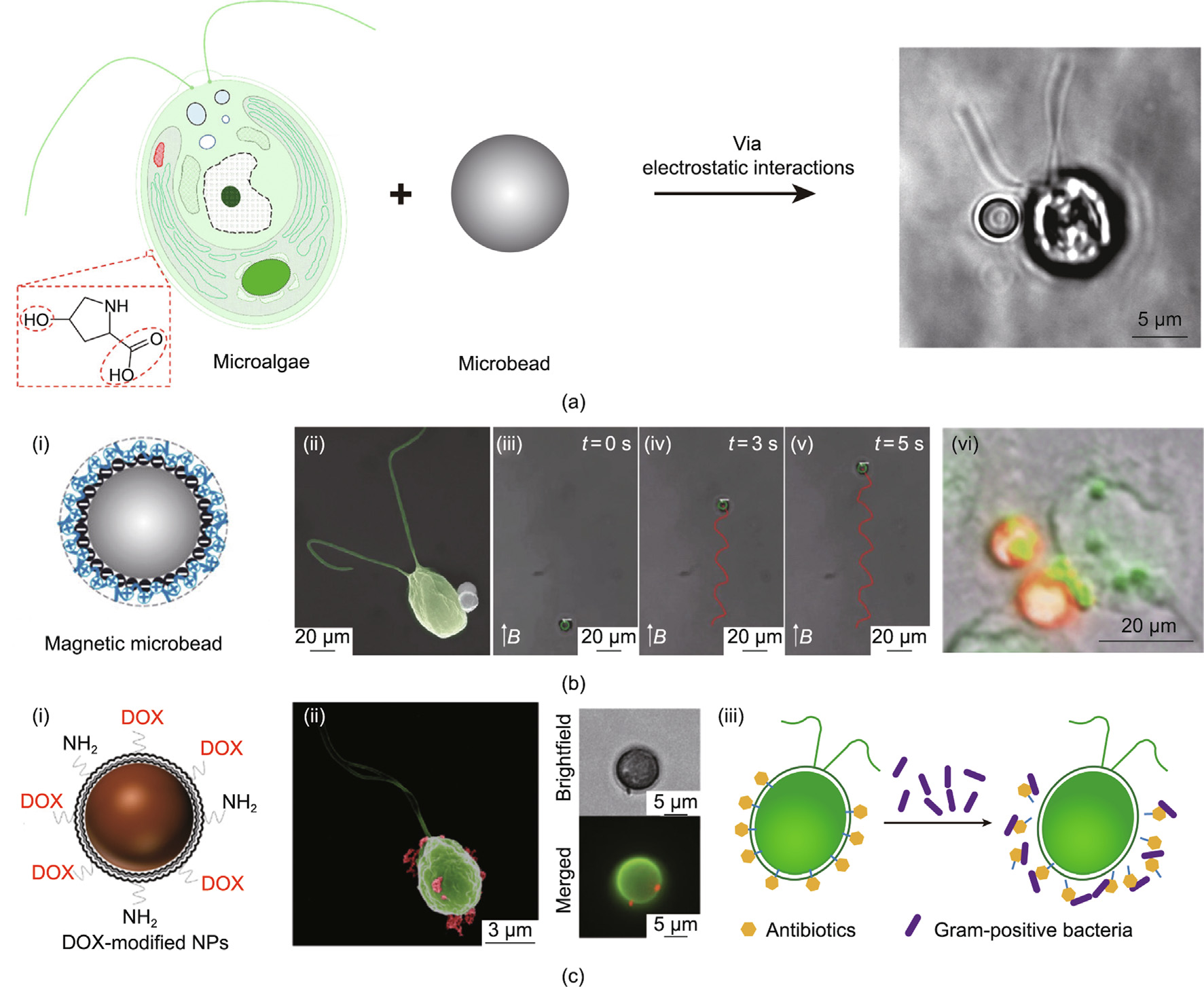
Fig. 6. Schematics of flagellum- or magnetism-driven MBBR. (a) Schematic of MBBR generation process. (b) MBBR loaded with magnetic macromolecules can (i–v) move in a superparamagnetic field and (vi) transport a model drug (fluorescent isothiocyanate (FITC)–dextran) to HeLa cells in 24 h. (c) MBBR loading with micromolecules such as (i, ii) chitosan-coated iron oxide nanoparticles, and (iii) schematic of the process of antibiotic-modified microalgae killing Gram-positive bacteria. DOX: doxorubicin; NP: nanoparticle. (a) Reproduced from Refs. [24,79] with permission; (b) reproduced from Refs. [24,80] with permission; (c) reproduced from Ref. [26] with permission.
《4. Genetically engineered microalgae》
4. Genetically engineered microalgae
During the last few decades, the potential of microalgae in biotechnological research has been demonstrated, based on their ease of cultivation and rapid growth. Since the complete genome sequences of many microalgae species have been revealed, molecular biology and transformation approaches—such as transcription activator-like effectors (TALEs) and clustered regularly-interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)—have been gradually utilized for the genetic manipulation of microalgae to improve their harvestability, robustness, nutritional composition, and so forth [84]. Genetically engineered microalgae strains, such as those possessing truncated antennae and so forth, have been widely investigated, mostly for the sake of improving their photosynthetic efficiency and biofuel production [85]. Moreover, the potential of genetically modifying microalgae to favor biomedical applications has been explored. Nanoporous biosilica, which can be derived from diatom microalgae, has a highly porous structure and is considered to be a promising carrier for delivering therapeutic drugs. To endow this natural nanoporous carrier with cell-targeting capability, a genetic engineering method known as live diatom silica immobilization (LiDSI) was used to modify the diatom Thalassiosira pseudonana by placing an immunoglobulin G (IgG)-binding domain of protein G on the biosilica surface. As a result, a specific antibody—p75 neurotrophin receptor (p75NTR)—was bound to the biosilica carrier, and the targeting capability and therapeutic effect of the carrier loaded with chemotherapeutic drugs were demonstrated both in vitro and in vivo [86].
Many recombinant proteins, such as growth factors, cytokines, and other bioactive molecules, can now be produced in microalgae by means of well-established methodologies. Therefore, in addition to efficiently providing an oxygen supply, genetically engineered living microalgae cells that can express certain recombinant growth factors or therapeutic molecules have been demonstrated to provide supplementary chemical cues for promoting the therapeutic efficacy of MBBM in several biomedical applications. For example, by including genetically modified C. reinhardtii expressing angiogenic recombinant protein (i.e., vascular endothelial growth factor, VEGF), functionalized MBBMs that could accelerate angiogenesis were constructed and showed promise for enhancing skin tissue regeneration [55]. Similarly, seeding genetically modified C. reinhardtii microalgae cells on the surface of commercially available sutures resulted in novel versatile photosynthetic sutures that could locally release both oxygen and human growth factors at the wound sites [87].
Considering possible host immune responses toward microalgae cells, appropriate designs of MBBM in which these heterologous cells are well encapsulated in biocompatible biomaterials can lead to successful immune evasion. This innovative incorporation of genetically engineered microalgae into biomaterials or medical materials opens up a novel route toward more efficient therapy. The genetic engineering of microalgae still has limitations regarding sequencing the genomes of some strains for genetic manipulation [88]; it also presents difficulties in efficiently delivering genetic materials across the cell wall [89]. The stability of genetically modified algae—especially when they are embedded in biomaterials—is another concern. Moreover, the in vivo safety of such microalgae must be carefully evaluated, which requires an in-depth understanding and detailed characterization of foreign gene expression for precise modulation in clinic usage. Although these MBBM concepts with genetically engineered algae cells remain to be demonstrated before being translated to the clinic, ongoing research shows that this combination may promote and diversify the clinical use of genetically engineered algae in several biomedical fields, including advanced drug/vaccine delivery systems, tissue engineering, and tumor therapy.
《5. Conclusions and perspective》
5. Conclusions and perspective
Natural active compounds extracted from microalgae biomass, such as cytotoxic, antibiotic, antioxidant, antifungal, antiinflammatory, and anti-helminthic compounds, were applied as prophylactic and therapeutic nutrients in the original exploration stage of biomedicine [90,91]. However, in recent decades, increasing attention has gradually been focused on exploring the extended biomedical applications of biohybrid materials comprising live microalgae cells and biocompatible nanomaterials. Such applications include ion adsorption, oxygen production, ondemand drug delivery, tissue repairing, in vivo tracing elements, and more. Depending on whether locomotivity is applicable, these as-developed MBBM can be sorted into two major categories— namely, immobilized or motile materials—aiming toward various applications. Many biocompatible and biodegradable materials (e.g., sol–gel, PS, PVP, tetramethoxysilane (TMOS)/tetrakis (2- hydroxyethyl) orthosilicate (THEOS), silk fibroin, and graphene) are feasible as supporting substrates for microalgae adhesion and immobilization, and integration can be achieved via various manufacturing techniques. The movement of MBBM-based microrobots is driven by chemotaxis, phototaxis, geotaxis, flagellabeating behaviors, or external magnetic field, endowing them with good motilities for cargo transportation; thus, they show promise for the formulation of future biomedical therapy.
Despite the increasing usage of microalgae in developing functional biohybrid materials with promising biomedical applications, the production of possible toxins or metabolites from live algae in the complex physiological environment of the body must be fully investigated. Photosynthesis ability is regarded as one of the unique properties of MBBM with live microalgae and can introduce more possibilities in several applications, such as in reversing the hypoxic environment in tumors. However, this ability could be hindered in deep-tissue cases, as the penetration depth of light in various biological tissues is limited. Thus, incorporating upconversion luminescent materials or integration with a wireless light-emitting device that can supply the necessary light source to initiate photosynthesis might be taken into the design. In addition, the microscale size of microalgae may limit their applications as drug carriers due to difficulties in penetrating biological barriers. Furthermore, a deep understanding of the correlations between disease pathogeneses and microalgae bioactivities must be gained to enable biohybrid design for therapeutic strategies. Extensive in vitro and in vivo investigations are worth carrying out in order to improve the functional design of MBBMs and explore their biomedical applications.
《Acknowledgments》
Acknowledgments
This work was supported by EU H2020 RISE (MNR4SCell project) (734174) and the European Union’s Horizon 2020 research and innovation programme (SENTINEL project) (812398).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Zhongyang Zhang, Yumeng Chen, Lasse Hyldgaard Klausen, Sebastian Amland Skaanvik, Dan Wang, Jianfeng Chen, and Mingdong Dong declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




