《1. Introduction》
1. Introduction
Hydrogen-bearing systems are currently of intense interest due to their desirable physical properties and possible application as hydrogen-storage materials. Physical properties such as superconductivity at high temperatures have been reported in the hydrogen–sulfur system [1], while the storage capabilities of these systems are best exemplified by the hydrogen amassed in methane–hydrogen, CH4(H2)4 [2], and more recently in the hydrogen–iodane system, HI(H2)13 [3]. Despite these prospects and intensive research activity in this field, many hydrogen-bearing systems are yet to be explored, with the noble metals remaining as a notable hydride gap in the periodic table.
The noble metals, Cu, Ag, and Au, are relatively inert under ambient conditions, as shown by their reluctance to form oxides under ambient conditions—an attractive quality for their usage in ancient coinage and electronics. High pressure has become an indispensable tool in modifying chemical affinities and thereby creating exotic materials [4–7]. When such materials are synthesized, tantalizing properties can be induced, exemplified by the hydride formation of other noble metal systems, such as rhodium hydride (RhH2) [8] and platinum hydride (PtH) [9]. The former, RhH2, is a prototypical example of polyhydride formation and the latter, PtH, exemplifies a material with potential induced superconducting properties. For hydride formation in d-metals, the barrier for molecular dissociation is typically driven down by pressure, resulting in atomic hydrogen permeating freely through the metallic lattice and tending to reside at interstitial sites. The presence of atomic hydrogen in the metal can lead to changes in the crystalline structure ranging from simple lattice expansion to reconstructive phase transitions and changes in space group symmetry [10].
Despite numerous attempts using complex synthesis techniques such as high pressures coupled with resistive and laser heating [11,12], the definitive formation of Au–H and Ag–H compounds has remained elusive, likely due to the large reduction potentials of these group-11 metals. On the other hand, Cu, with a reduction potential approximately half that of Ag, has been known to form a binary system with hydrogen for over 100 years, making it the first metal hydride to be discovered [13].
The Cu–H system has been found to have an extensive chemistry with some very unusual chemical pathways, such as sonification [14]. Copper’s reactivity with hydrogen offers a testing bed for further experimental and theoretical studies of the group11 hydrides. The first binary compound was formed by the reduction of copper sulfate with hypophosphorous acid to form the stoichiometric monohydride, CuH [13]. CuH was determined to have a wurzite structure with a significantly modified mechanical response, as a consequence of Cu–H bonding—a highly unusual characteristic in d-metal hydride systems [15]. However, wurzite-CuH, the only stoichiometric group-11 hydride, is unstable at ambient temperatures and readily decomposes above 60 °C [16]. Calculations predict that pressures in excess of 30 GPa are required to stabilize it at room temperature [16].
Since this early pioneering work, high-pressure experimental campaigns have been conducted in an attempt to form new hydrogen-bearing Cu compounds. Direct compression of Cu in a H2 atmosphere results in the formation of a low-H-content hydride, isostructural to Cu and exhibiting a slightly expanded lattice; in accordance with the literature naming scheme, this phase is called γ0-CuH0.15. Compressing this phase above 10 GPa leads to further hydrogen entering the lattice, forming the second γ1-CuH0.5 phase [11]. In both phases, the lattice expansion is a consequence of hydrogen filling the octahedral interstitial sites. A subsequent study used synchrotron X-ray diffraction (XRD) to identify an ε-Cu2H phase [12] that is distinctly different from the previously reported wurzite-CuH and c-phases, synthesized at around 18.6 GPa. This phase adopts an anti-cadmium iodide (CdI2) type structure and its stoichiometry is constrained due to significantly reduced volumes when compared with the formerly identified phases. As Cu–H has the highest chemical affinity with hydrogen of all the group-11 metals, it is imperative that we endeavor to fully describe its chemistry and perhaps reveal higher hydrogen stoichiometries, as have been reported for other d-metal systems [17,18].
Here, with the use of high-pressure, in situ laser heating and synchrotron XRD, we explore the Cu–H system up to pressures of 50 GPa and temperatures in the range of 1000–3500 K. We report the previously identified high-pressure phases, γ0-CuH0.15, γ1-CuH0.5 [11], and Cu2H [12], and constrain their pressure evolution up to 50 GPa, respectively. Most importantly, we identify a new c-phase synthesized by laser heating Cu in a dense hydrogen atmosphere—namely, γ2-CuH0.65. Unusually, this phase decomposes into γ1-CuH0.5 rather than the ε-Cu2H phase previously found to be stable at these pressures. This work illustrates the complexities that may be found in the noble-metal hydride phase diagrams when explored with the latest experimental techniques.
《2. Experimental methods》
2. Experimental methods
The diamond-anvil cell (DAC) is the workhorse in most static high-pressure experiments. Its compact and simple design has proven to be powerful and adaptable, making it a principal driving force in high-pressure science for the last 30 years. The concept of a DAC is simple: By coupling very small sample chambers with the hardness of diamond, pressures can be generated that are orders of magnitude greater than what can be achieved with conventional mechanical presses. In this study, high-pressure measurements were conducted in a symmetric-type DAC with Boehler–Almax cut diamonds on tungsten-carbide seats with large opening angles (~50°). The diamond culets varied from 200 to 300 μm in diameter. Samples were loaded in a laser-milled rhenium-foil gasket chamber with initial dimensions of 20– 30 lm in thickness and a diameter of approximately 75% the culet size. No pressures higher than 50 GPa were probed, as we were limited by the mechanical instability dictated by the geometry of the diamond anvils. Pressures were determined during the experiment via the known equation of state of a particulate of gold and/ or the calibrated shift of the R1 florescence of a ruby sphere placed in the sample chamber [19,20].
High-purity Cu grains (Alfa Aesar, 99.9%), approximately 10 μm in size, were placed so that they were centered on a diamond anvil. The DAC was calibrated such that it hermetically sealed in a 200 MPa hydrogen atmosphere (research grade 99.9995%). The loading procedure resulted in significant excess of hydrogen, in order to promote hydride formation and provide quasihydrostatic conditions for synthesized samples.
The Cu sample was heated in situ from both sides uniaxially by directly coupling to pulsed-infrared (IR) lasers (10–100 μs). The double-sided laser heating system is a dedicated part of the GeoSoilEnviroCARS (GSECARS) beamline at the Advanced Photon Source (APS), and a thorough description can be found in Ref. [21]. The flat-topped 1064 nm laser spot of 15 μm is comparable to the incident X-ray beam, negating temperature gradient effects. Over the course of laser heating, temperature was determined spectroscopically, via a Planck-fit to blackbody emission.
Powder XRD data were collected at the beamline at the APS, USA. The diffraction from 0.3344 Å wavelength X-rays was recorded using a Pilatus 1M image-plate detector, after which it was integrated using DIOPTAS software [22] to a twodimensional dataset. The data were subsequently indexed and further underwent Le Bail refinement using Jana2006 [23].
《3. Results and discussion》
3. Results and discussion
Although now a mature field in its own right, modern highpressure chemistry dates back to the invention of the pressure devices built and used by Bridgman, which earned him the Nobel Prize in Physics in 1946. Since then, high pressure has been widely applied to the formation and study of d-metal hydrides, first by Baranowski and Bochen´ ska [24] over 50 years ago (up to 0.3 GPa), and then by the work of Ponyatovskiĭ et al. [25] in the 1980s (up to 9 GPa). In recent times, primarily due to the advent of new extreme condition techniques along with key developments in diagnostics, we are in a prime position to routinely investigate and expand our understanding of hydrogen chemistry at high densities. This study provides an important example, by outlining the techniques, diagnostics, and synthesis pathways in the Cu–H system; in doing so, we report a noble metal-hydride material with the highest reported hydrogen stoichiometry at ambient temperature.
The use of synchrotron radiation has greatly expedited the exploration of high-pressure systems, with their brilliance and tight focus finding great applicability with sample sizes on the order of tens of microns. In this study, harnessing synchrotrongenerated light, we have ascertained structural insight into the Cu–H system using conventional powder XRD. As these studies only require a fraction of accumulation time when compared with conventional in-house sources [11], we present a thorough mapping of phases in the Cu–H system. Through the high-quality spectra obtained (Figs. 1 and 2), we have identified the appearance of four phases in the Cu–H system: γ0-CuH0.15, , Cu2H, γ1-CuH0.5 , and γ2-CuH0.65, the latter two of which require high temperatures (discussed later). As discussed previously, the Cu–H system has been well characterized at ambient temperatures up to 50 GPa in previous studies [11,12] and our data up to 25 GPa at room temperature are in agreement, upon observing the direct reaction between Cu and its surrounding H2 atmosphere. The reaction product is readily identified by the appearance of diffraction peaks (Fig. 1), which correspond to previously reported ε-Cu2H crystallizing in space group  , where lattice parameters a = 2.5229(2) Å and c = 4.1984(8) Å at 25 GPa.
, where lattice parameters a = 2.5229(2) Å and c = 4.1984(8) Å at 25 GPa.
《Fig. 1》
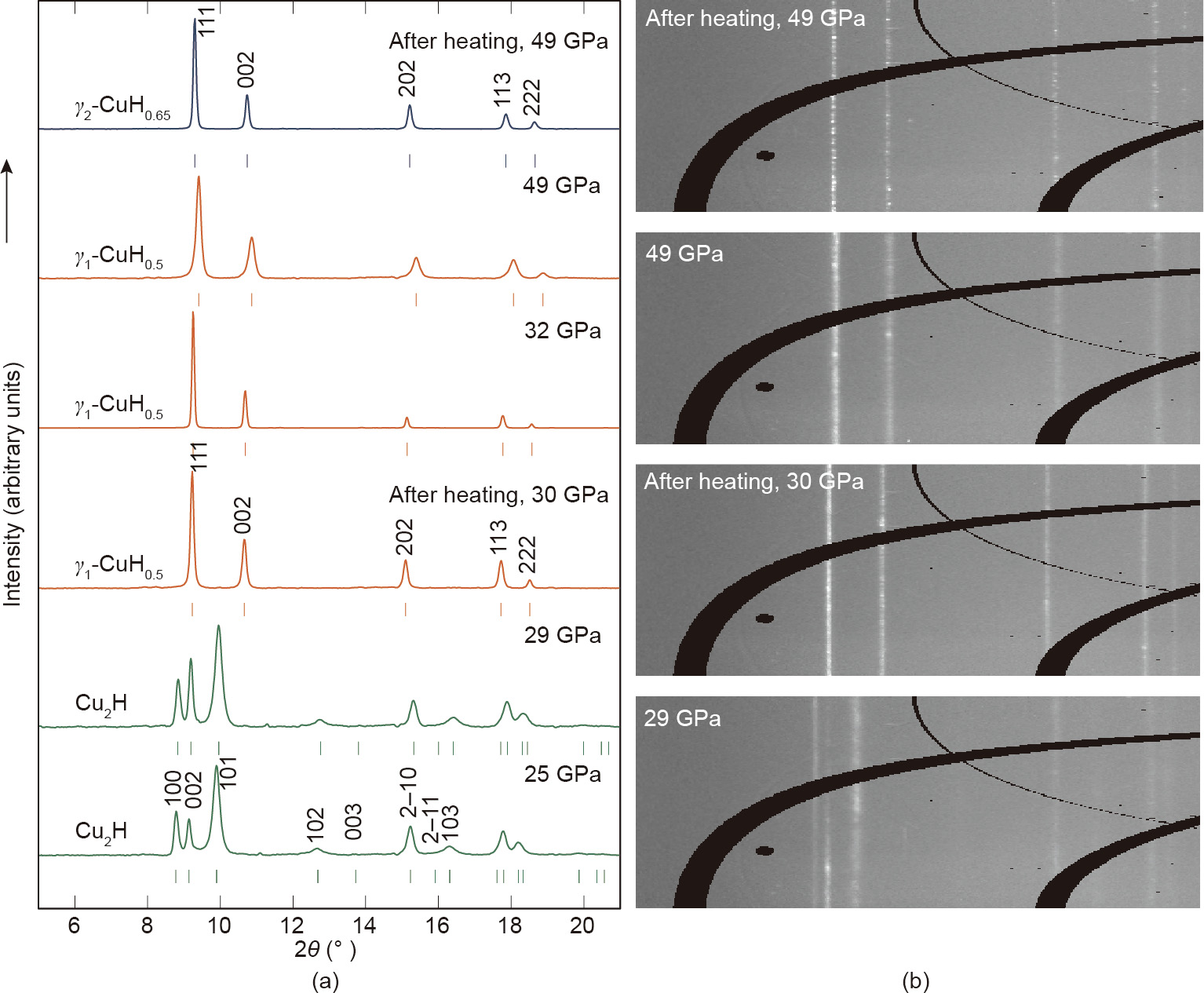
Fig. 1. (a) High-pressure XRD patterns (λ = 0.3344 Å) and (b) raw image plates showing the stepwise synthesis of γ1-CuH0.5 (orange) and γ2-CuH0.65 (blue) by sequential laser heating at 30 GPa and 49 GPa. Tick marks indicate the positions of Bragg reflections from the noted phases.
《Fig. 2》
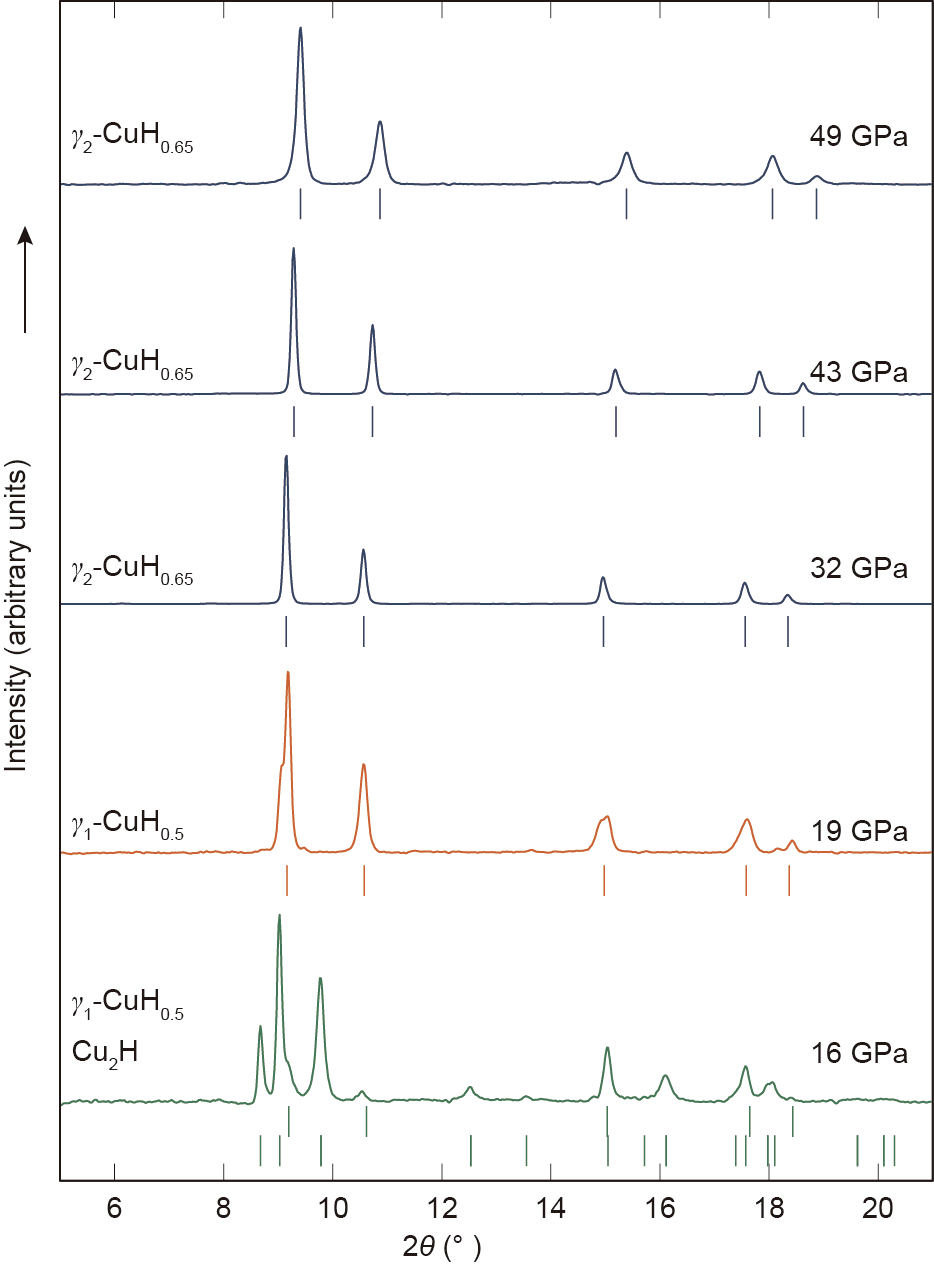
Fig. 2. High-pressure XRD patterns (λ = 0.3344 Å) taken on decompression showing the stepwise decomposition of γ2-CuH0.65 into γ1-CuH0.5 and Cu2H. Tick marks indicate the positions of Bragg reflections from the noted phases.
Temperature has always been an essential parameter in chemistry, particularly when high temperatures serve as a pathway to overcome potential energy barriers and thereby promote reactivity. In DAC work, specialist heating techniques are required, as large amounts of thermal energy must typically be deposited over very small surface areas. Laser heating provides an optimum solution [26], as high-powered IR lasers (~100 W) can be tightly focused, resulting in an energy density of roughly 20 W·μm-2 and—if incident on strongly coupling metallic foils—temperatures in excess of 3000 K are possible. Therefore, in order to promote further reactions, samples that were first compressed to 30 GPa were further subjected to laser heating to temperatures in the range of 1000–3500 K. This treatment resulted in radical changes to the observed diffraction patterns. The peaks due to the aforementioned ε-Cu2H phase disappeared and were replaced by the peaks of a new phase, γ1-CuH0.5, that was readily indexed to a face-centered cubic structure with a = 3.5976(1) Å at 30.1 GPa. Once transformed, the sample was further compressed in order to explore the possible synthesis of other Cu–H compounds. After heating at 50 GPa, we observed another abrupt increase in unit-cell volume consistent with the formation of an isostructural phase with greater hydrogen content: γ2-CuH0.65.
Unfortunately, despite the brilliance of synchrotron light, hydrogen has a weak X-ray scattering cross-section and is therefore essentially invisible to the XRD technique. Although this makes it impossible to directly infer the stoichiometry of synthesized hydrides, it is still possible to ascertain the content in interstitial hydrogen systems, as the presence of hydrogen distorts the structure of the metallic host, which is often observed as a volume expansion. Therefore, examination of the atomic volume (V) per Cu atom with pressure allows us to estimate the hydrogen contents of these new copper hydride phases. Fig. 3 [11,12] and Table 1 illustrate the observed volumes for Cu2H and the two new phases induced through laser heating—γ1-CuH0.5 and γ2-CuH0.65—as previously discussed. In face-centered cubic (fcc) metal hydrides (γ-phases), hydrogen atoms occupy the octahedral vacancies; their incorporation results in a volumetric expansion proportional to the hydrogen content. As is empirically observed in d-metal systems [11,12,28,29], The volume expansion ( ) of the host metal’s unit cell is 2.9 Å3 per hydrogen atom incorporated. In the absence of neutron diffraction data, this convention provides an assigned estimate of the hydrogen content in a metallic hydride [28,29].
) of the host metal’s unit cell is 2.9 Å3 per hydrogen atom incorporated. In the absence of neutron diffraction data, this convention provides an assigned estimate of the hydrogen content in a metallic hydride [28,29].
《Fig. 3》
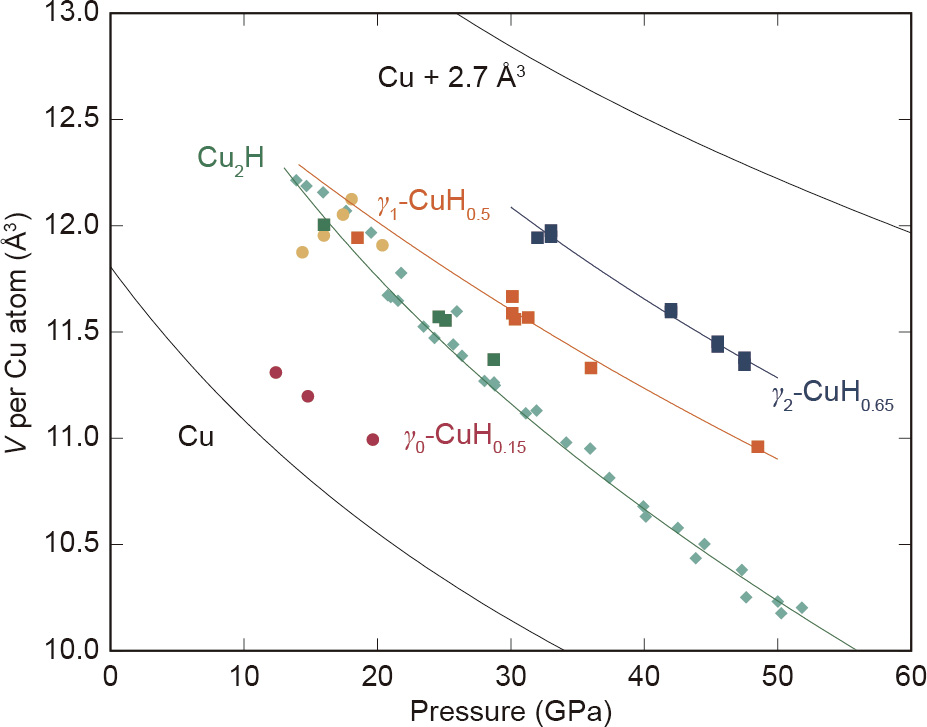
Fig. 3. Atomic volume as a function of pressure for Cu–H compounds. Data from this study are shown with squares; data on Cu2H from Ref. [12] are shown with diamonds; and γ0-CuH0.15 and γ1-CuH0.5 from Ref. [11] are shown with circles. Equations of state (Table 1) are shown with solid lines.
《Table 1》
Table 1 Birch–Murnaghan equation-of-state parameters for Cu, Cu2H, γ1-CuH0.5, and γ2-CuH0.65.

V0: volume at ambient pressure; B0: bulk modulus at ambient pressure; B0’: first derivative of the bulk modulus.
As is common with interstitial hydrides, none of the high-pressure phases in the Cu–H system—that is, γ0-CuH0.15, γ1-CuH0.5, or γ2-CuH0.65—display modified mechanical properties when compared with the host material, Cu. As can be seen in Fig. 3 and Table 1, both synthesized γ-phases show similar compressibility to that of pure Cu over this pressure range. The average increase in volume versus pure Cu for “γ1-CuH” is 1.44(10) Å3 per Cu atom, which establishes a stoichiometric assignment of γ1-CuH0.5. The second isostructural γ-phase formed after laser heating γ1-CuH0.5 shows an average volume expansion of  = 1.85(5) Å3 , implying the formula γ2-CuH0.65. However, although there is not a marked change in their mechanical properties, these materials’ electronic properties can be altered profoundly. As reported for the Pd–H system, the absorption of hydrogen to form the monohydride PdH results in an enhanced critical temperature for superconductivity when compared with elemental Pd (greater than three orders of magnitude) [30]. The same alteration has been proposed for other transition metal hydrides, such as PtH [31,32]. Studying the impact on the electrical properties of noble metalhydrides through the incorporation of hydrogen would be of particular interest, as these materials already find widespread industrial and commercial application due to their desirable conductive attributes.
= 1.85(5) Å3 , implying the formula γ2-CuH0.65. However, although there is not a marked change in their mechanical properties, these materials’ electronic properties can be altered profoundly. As reported for the Pd–H system, the absorption of hydrogen to form the monohydride PdH results in an enhanced critical temperature for superconductivity when compared with elemental Pd (greater than three orders of magnitude) [30]. The same alteration has been proposed for other transition metal hydrides, such as PtH [31,32]. Studying the impact on the electrical properties of noble metalhydrides through the incorporation of hydrogen would be of particular interest, as these materials already find widespread industrial and commercial application due to their desirable conductive attributes.
From the highest pressure point of 50 GPa, our samples were decompressed in order to determine the stability range of the newly formed hydride phases. γ2-CuH0.65 was found to be stable to 32 GPa (Fig. 2), where—interestingly—it decomposed to γ1-CuH0.55 rather than to the ε-Cu2H phase, which is the favored phase under room-temperature compression [12]. In turn, the γ1-CuH0.5 phase was stable down to 19 GPa, where weak peaks due to ε-Cu2H could be observed; below this pressure, the sample completely transformed to the known ε-Cu2H phase.
Laser heating is a powerful technique to synthesize new phases, particularly with unreactive metals. The observation of a new stable copper hydride with enhanced hydrogen content, coupled with the known existence of analogous d-metal monohydrides, such as CoH [18] and NiH [33], strongly suggests that under further pressurization and high temperatures, a copper monohydride species could be stable. These results should encourage the re-examination of many metal-hydrogen systems, particularly in light of the moderate pressures used here. This method is applicable to any metal-hydrogen system and may be of interest in the study of high-performance alloys under extreme conditions.
《4. Conclusions》
4. Conclusions
In summary, using high-pressure powder XRD, we have explored the Cu–H system up to pressures of 50 GPa. In agreement with previous studies, we have verified the synthesis of ε-Cu2H, γ0-CuH0.15, and γ1-CuH0.55 compounds, further constraining their structural evolution with pressure. We find that a second, more hydrogen-rich, γ2-CuH0.65 phase is formed after laser heating above 30 GPa, and is the highest hydrogen-content system known to exist for the group-11 metals that is stable at room temperature. This study and its incorporated understanding will reinvigorate interest in H-rich hydrogen-bearing materials and in the realization of the other noble metal hydrides, Au–H and Ag–H.
《Acknowledgements》
Acknowledgements
Philip Dalladay-Simpson and Ross T. Howie acknowledge their respective ‘“1000 Talents” awards. Miriam Peña-Alvarez acknowledges the support of the European Research Council (ERC) Grant HECATE (695527) secured by Graeme Ackland. Parts of this research were conducted at the APS facility under proposal No. 51037 at the GSECARS beamline. Finally, the authors would like to thank Eran Greenberg and Vitali Prakapenka for their assistance during the course of the data collection.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jack Binns, Miriam Peña-Alvarez, Mary-Ellen Donnelly, Eugene Gregoryanz, Ross T. Howie, and Philip Dalladay-Simpson declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




