《1. Introduction》
1. Introduction
《1.1. Analysis of current energy supply status》
1.1. Analysis of current energy supply status
Energy is essential to human beings, and its stable supply is crucial. Traditional energy sources such as coal, gas, and oil are still playing significant roles in the energy sector, with an ongoing expansion to other sources such as renewables and biomass [1]. The demand for energy will continue to increase (Fig. 1), with a forecasted value of 16.2 gigatonnes of oil equivalent (Gtoe) in the year 2030 by the International Energy Agency (IEA) [2]. There are obvious gaps between the source of energy supply and its increasing demand. Thus, investment in the energy sector is maintained at a very high level worldwide, with a reported record of 6.9 billion USD (venture capital investment) from energy technology companies in 2018, mostly in transport areas (5.5 billion USD) [3].
《Fig. 1》
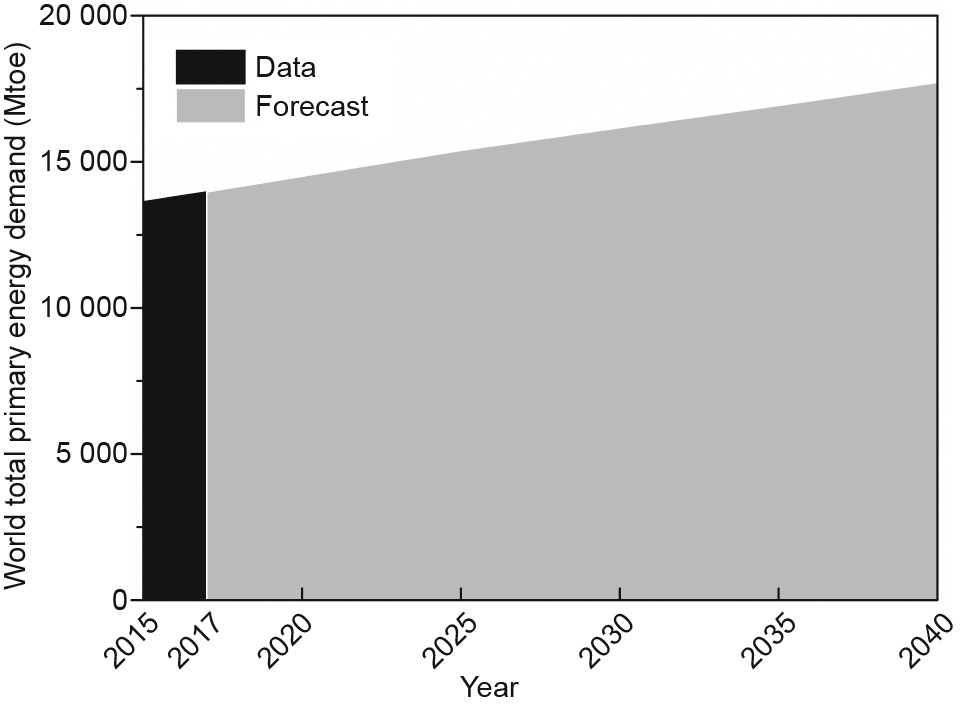
Fig. 1. Global primary energy demand (new policies scenario) in 2015, 2017, and forecasted until 2040. Mtoe: million tonnes of oil equivalent.
Meanwhile, environmental problems including air pollution, global warming, and climate change are attracting increasing attention. These problems have been largely attributed to the combustion of fossil fuels and the emissions of carbon dioxide (CO2) and other greenhouse gases. It has been reported that the greenhouse gas level in the atmosphere is greater than 480 parts-per-million (ppm) carbon dioxide equivalent (CO2e)—a measure that comprises CO2 (> 400 ppm) and other greenhouse gases including methane (CH4), nitrous oxide (N2O), fluorinated gases, and so forth [4]. Carbon capture and storage (CCS) from coalfired power plants has been a popular topic for decades [5–7], and it must be recognized that CCS can reduce the emission of CO2 and many other pollutants, including fly ash, mercury, SOx, and NOx. However, CCS is still high in energy consumption and cost. The fuel consumption and land and water requirements of CCS net delivered energy are about 25% greater than those without CCS [8]. In addition, the supply chain of fossil fuels has attracted concern regarding energy security, due to the increasing difficulty of fossil fuel extraction and its nonrenewable nature [8].
According to the IEA [2], the shares of fossil fuels have been changing over the years. We have observed an obvious decline in the share of coal [2] due to the competitive price of oil and gas, availability of renewables, and stricter regulations on pollutant emissions. Natural gas is playing more important roles in the energy sector due to the shale gas revolution in the United States and increased supply in other major natural gas exporters such as Russia and Australia. It has been forecasted that the portion of natural gas will further increase from 22% to 24% in the global energy market by 2035 [9].
《1.2. Roles of renewables in the energy sector》
1.2. Roles of renewables in the energy sector
Renewables, especially solar and wind, have been developed significantly over the past decades. As shown in the 2018 IEA renewable energy market report [2], the share of renewables is expected to increase to a historically high rate of 12.4% of global energy consumption in 2023. More specifically, the share of renewables in the electricity sector will increase from 24% in 2017 to 30% in 2023 [10]. Although these numbers may vary depending on the assumptions and configurations of different models, it is noteworthy that the deployment of renewables (Fig. 2), especially solar and wind, is playing an increasing role in energy supply [11], as well as in reducing greenhouse gas emissions.
《Fig. 2》
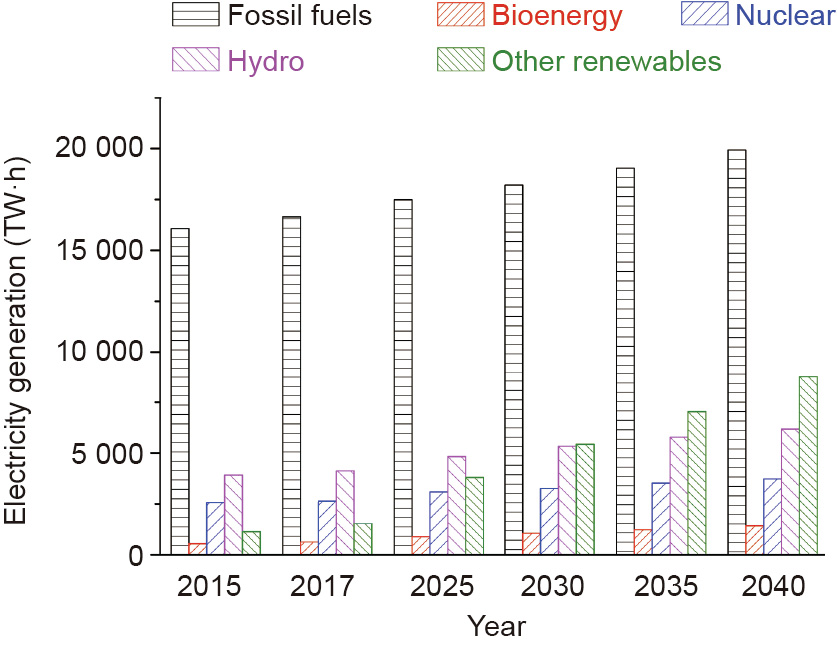
Fig. 2. Electricity generation by technology (new policies scenario) in 2015, 2017, and forecasted until 2040 (other renewables include solar, wind, marine, geothermal, concentrated solar, and thermal).
The employment of renewables greatly relies on the revolution of science and technology. Breakthroughs such as materials innovation, energy conversion efficiency improvements, and costs reduction are all crucial to the increased adoption and use of renewables. Although the distribution of renewables may be a limitation, the source of renewables (i.e., the world as a whole) is not a concern. Taking wave energy as an example, although its distribution is greatly restricted geographically, its potential is huge. The net wave resource was estimated to be about 3 TW globally [12], which is abundant compared with the global electricity consumption of 2.3 TW in 2018 [13]. Although there are still technical barriers (e.g., fluid corrosions, energy transmission and fluctuations) to overcome before large-scale deployment, the potential of wave energy has been manifested through many active demonstrations by companies such as Carnegie Clean Energy Ltd. (formerly Carnegie Wave Energy, Australia) and Wavestar® Energy (Denmark).
As a secondary energy, electricity is a flexible way to distribute energy from renewables. However, as the voltage values of electricity grids are strictly restricted to ±10% of the nominal value, the intermittent supply of renewables such as solar and wind present a difficulty in plugging renewable electricity into existing grids [14]. Therefore, appropriate energy storage and management methods must be developed to enable continual and wide employment of renewables.
As one of the leading countries in both renewable technology development and its implementation, Germany is playing a significant role in the renewable energy sector. It was reported that renewables (wind and solar only) contributed more than 51% (Fig. 3) to electricity generation in Germany in 2018 [15]. This value is expected to be further enhanced in 2019 and onward. It should be noticed that fossil fuels are still an important contributor to electricity generation. That is because coal- and gas-fired power plants are most effective in complementing the intermittent renewable energy to maintain a stable and reliable electricity supply (Fig. 4). Although Germany is an electricity exporter (gross) [16], it also imports electricity as a supplement in certain areas; these numbers are not reflected in Fig. 4.
The availability of renewables strongly depends on weather conditions and seasonal changes (Fig. 4). The supply of solar-sourced electricity (Fig. 4(a)) is limited to the daytime and peaks in summer (Fig. 4(c)). Wind-sourced electricity (Figs. 4(a) and (b)) may shift significantly on a daily or even hourly basis, while its capacity peaks in winter. The gap between the highest and lowest electricity supply for solar and wind combined can be as large as 40 GW, which is about 57% of the annual electricity consumption in Germany. These huge shifts lead to significant problems due to the lack of appropriate energy storage solutions. As shown in Fig. 5, Germany has utilized negative electricity prices to encourage consumers to use electricity in off-peak hours. On the other hand, surplus electricity during these times may be curtailed due to the limitation of energy storage methods [17].
In another scenario, countries such as Australia have abundant renewables, which are much greater than their domestic energy demand. Thus, an effective and smart energy management system must be implemented to ensure better storage, transportation, and international trade of variable renewable energy while satisfying the domestic need [18].
《Fig. 3》
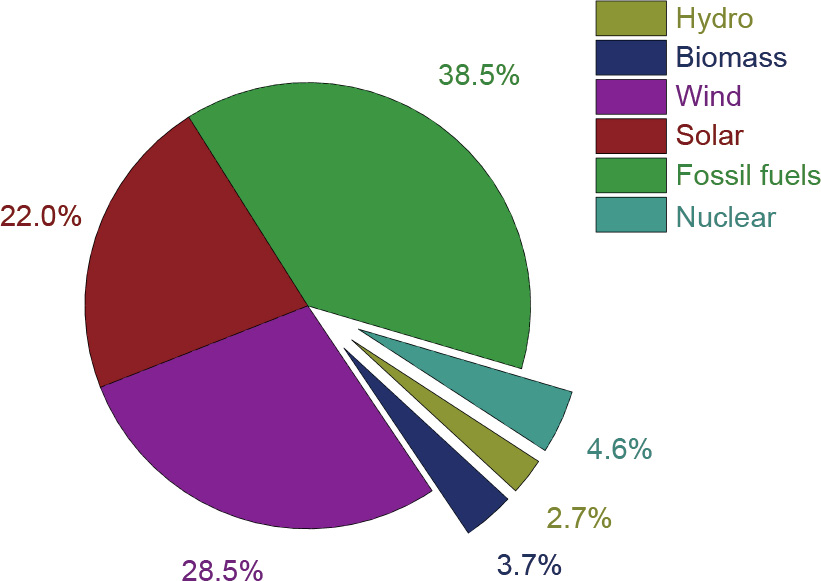
Fig. 3. Net electricity generation in Germany in 2018.
《Fig. 4》
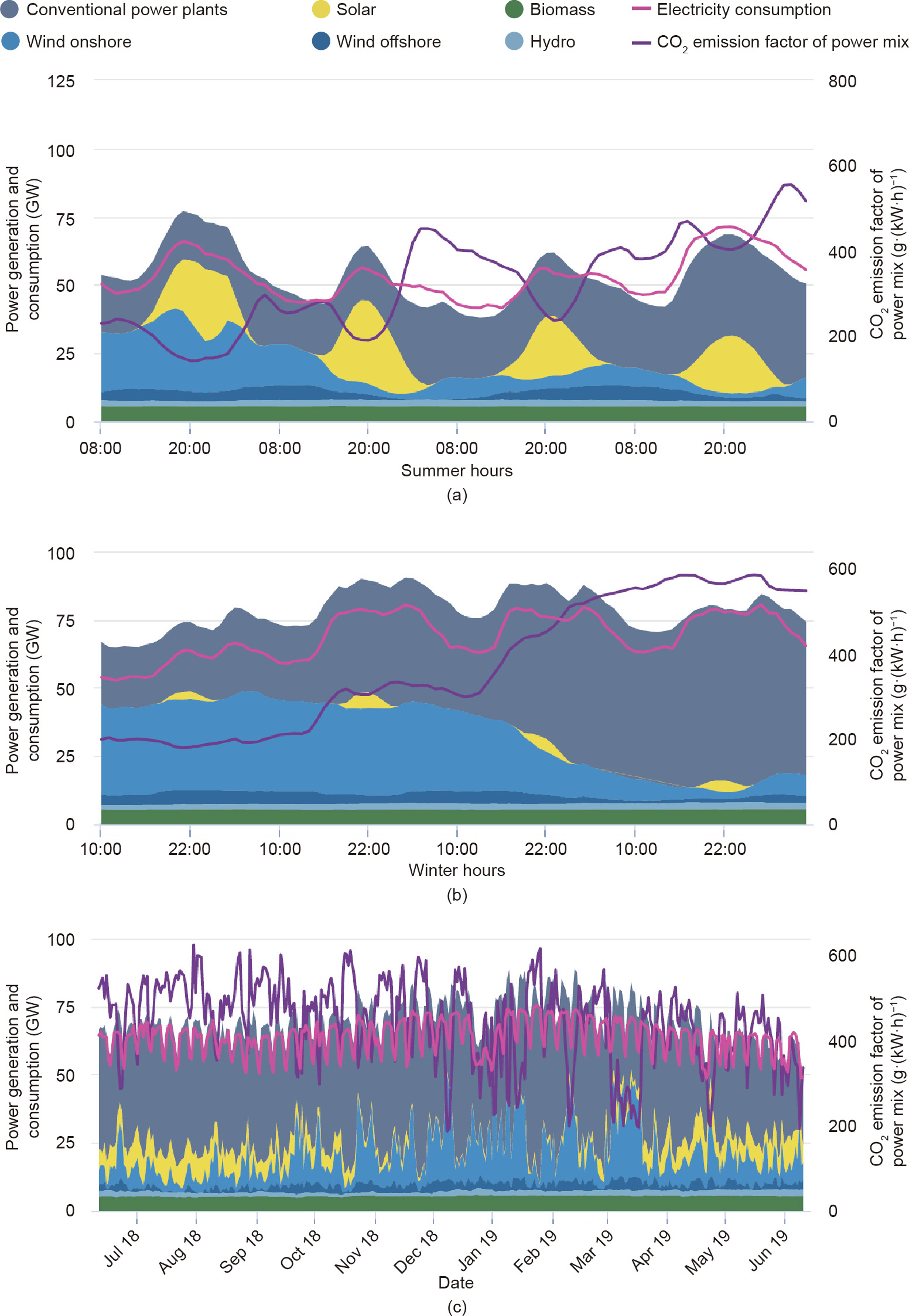
Fig. 4. Power generation and consumption in Germany. (a) Summer (8–11 June 2019); (b) winter (9–12 December 2018); (c) year round (June 2018 to June 2019). Source: Agora Energiewende (https://www.agora-energiewende.de/en/service/recent-electricity-data/), with permission.
《Fig. 5》
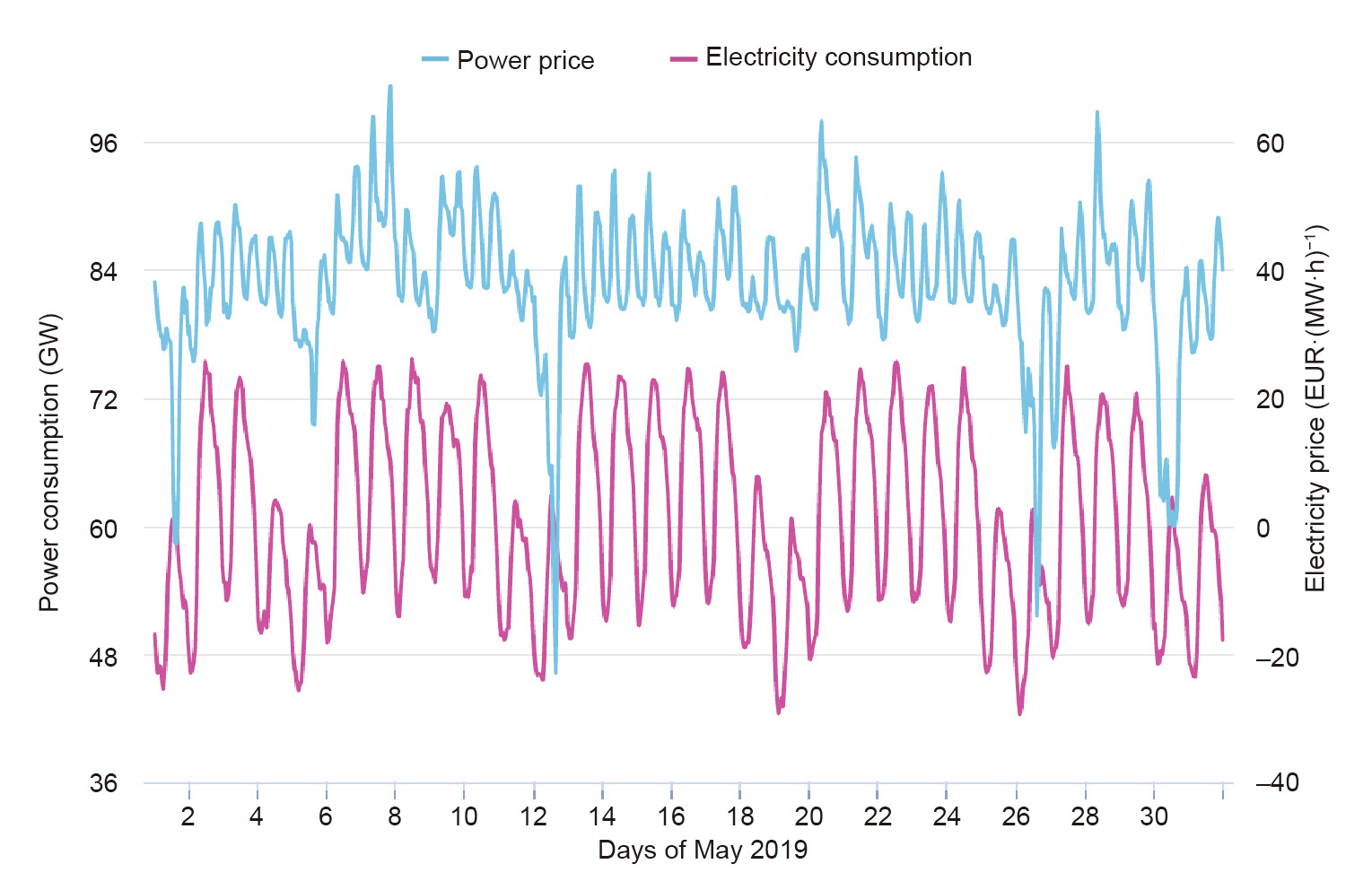
Fig. 5. Price of electricity in Germany (May 2019). Source: Agora Energiewende (https://www.agora-energiewende.de/en/service/recent-electricity-data/), with permission.
《1.3. Why hydrogen?》
1.3. Why hydrogen?
As the share of renewables continues to grow rapidly, energy storage systems are crucial to the successful integration of intermittent renewable energy into existing energy supply networks. Hydrogen (H2) is an ideal medium for the storage of renewable energy at various scales for a few reasons. First, hydrogen has the highest gravimetric energy density (120 MJ·kg-1 ), even though it has a lower volumetric energy density (2.7 MJ·L-1 for 350 bar (1 bar = 105 Pa) compressed hydrogen, 4.7 MJ·L-1 for 700 bar compressed hydrogen, and 2.36 MJ·L-1 for liquid hydrogen), compared with other common liquid fuels such as ethanol, propane, and gasoline (Fig. 6 [19]). Second, hydrogen can store energy almost permanently, if proper storage methods are provided [8], compared with energy storage by batteries. Finally, hydrogen can be used in various industries.
《Fig. 6》

Fig. 6. Gravimetric energy density and volumetric energy density (based on lower heating values) of fuels [19]. JP-8: jet propellant 8; E-10: ethanol-blended fuel; liq: liquid. Credit: US Department of Energy Fuel Cell Technologies Office.
The traditional pathways for hydrogen production include hydrocarbon reforming, gasification [20], hydrocarbon pyrolysis, biomass, and water splitting (through electrolysis, solar thermolysis, or photocatalysis) [21]. Current annual hydrogen production is 0.1 Gt, which is mainly consumed onsite [21] for refinery and metal treatment. The current emerging demand of hydrogen for fuel cells requires a significant enhancement in its production. Fig. 7 provides a new pathway to produce hydrogen from renewables, which can contribute to both an increased supply of hydrogen and the continual reduction of greenhouse gases caused by the consumption of fossil fuels via traditional processes.
Hydrogen is an important industrial chemical that is used in applications such as ammonia synthesis, refining (i.e., converting crude oil into diesel and jet fuels), and gas purification (i.e., removing sulfur and nitrogen from fuel) [18]; it is also used to replace carbon monoxide in iron making [18]. Furthermore, hydrogen is an important clean energy that is technically feasible, economically acceptable, and environmentally friendly [22] for multiple purposes such as heating [17,23], power generation, fuel cells, and transportation (trucks and trains) [18,24]. Taking California, USA, as an example, it was reported that more than 3000 fuel cell electric vehicles were sold or leased as of September 2017, 40 hydrogen refill stations were operating as of 22 May 2019 [25], and over 17 million passengers took hydrogen fuel cell buses in 2017. There has also been evidence showing that the addition of hydrogen to traditional fuels may benefit energy efficiency, fuel consumption, and carbon emission reduction [26]. The prolonged lifetime of fuel cells (60 000–90 000 h, which remains to be proved [27], and is targeted at 60 000–80 000 h set by the US Department of Energy (DOE) [28]) may also add confidence to the overall deployment of fuel cells, with a range of automobile companies stepping into this sector such as Hyundai, Toyota, Honda, and Mercedes-Benz [29].
Due to its diverse sources and utilization pathways, as well as its low levels of carbon/pollutant emissions, hydrogen may play an important role in a clean and secure energy future. As an energy carrier for short (hourly), intermediate (daily), and long-term (seasonal) energy storage, the importance of hydrogen is also very well recognized in maintaining the energy supply stability from inherently variable and seasonal renewables (especially solar and wind) [27]. Therefore, many countries, including Germany and other European countries [30–32], the United States [18,33,34], and Australia [35,36], have already proposed strategic plans in the hydrogen sector.
《1.4. Objectives of this review》
1.4. Objectives of this review
In the power to hydrogen (P2H) process (Fig. 7) using electrolysis [37], surplus electricity is converted into hydrogen [38] by consuming electricity using water electrolysis to reduce the peak loads on the grid [17,18,39,40]. Thereafter, hydrogen can be used for heating, transportation, the chemical industry, and so forth [41]. With an extremely short response time (4 min from start up to maximum loading [32]), P2H could act as an important connection between the inter-seasonal surplus electricity and hydrogen production.
P2H is a very popular research topic and a great deal has been published in the literature on its technical details such as electrolysis, hydrogen distribution, and hydrogen purification. However, to the best of our knowledge, there is still a lack of a review of its research advances, barriers, and solutions. The present review aims to fill this literature gap from the perspective of process engineering. In the following parts of this paper, we will review the status and research advances of P2H and hydrogen distribution, analyze the related technical barriers and solutions, and propose potential opportunities for future research and industrial demonstrations.
《Fig. 7》
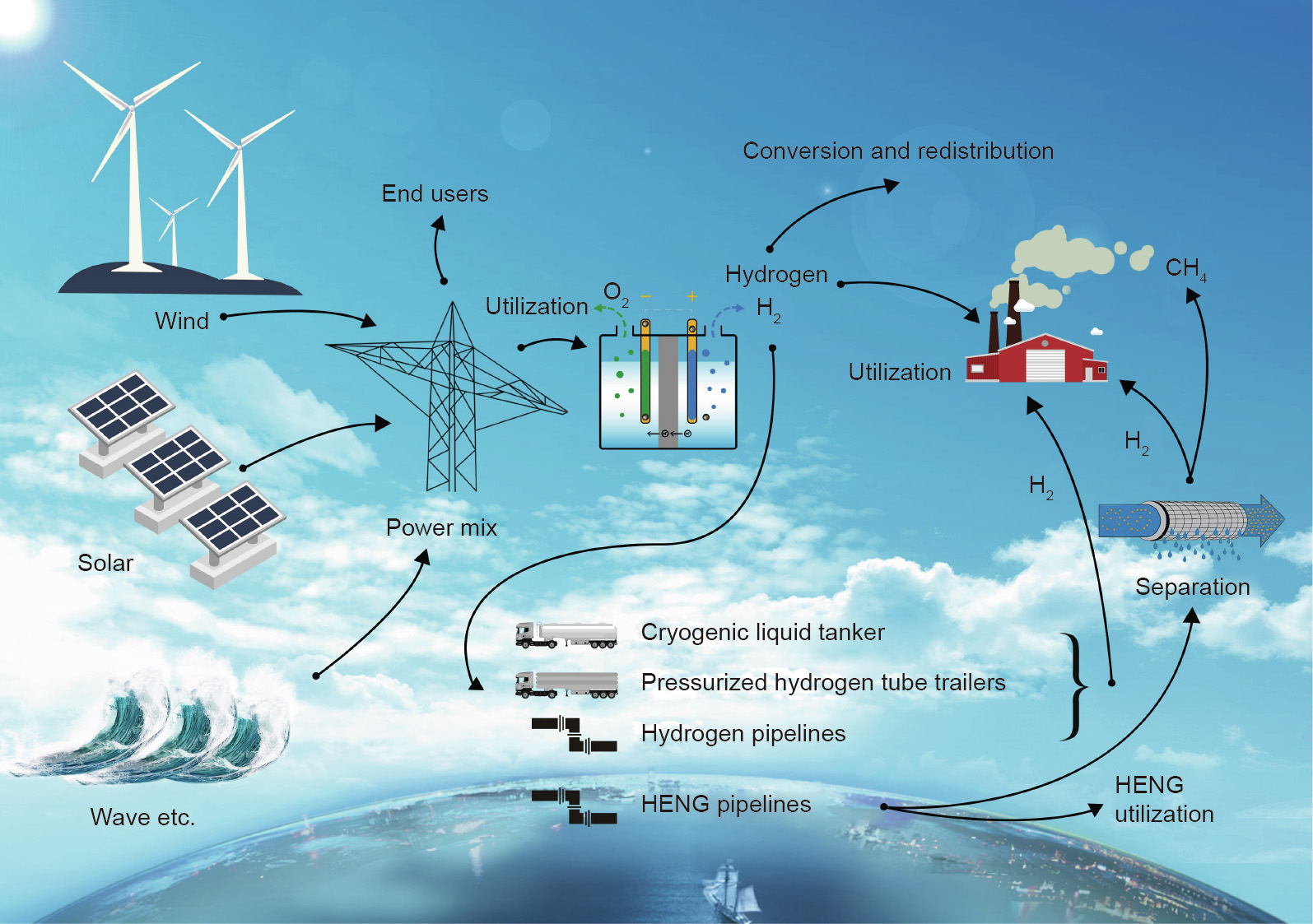
Fig. 7. The roadmap of power to hydrogen (P2H) technology. HENG: hydrogen-enriched natural gas.
《2. P2H demonstration projects》
2. P2H demonstration projects
Germany is one of the leading countries in the area of P2H. It sources a large portion of its electricity from renewables (mainly solar and wind), with strong commitment and support from the government and efforts from a range of high-tech companies. Its P2H strategy platform, named the German Energy Agency (Deutsche Energie-Agentur GmbH, DENA), was launched in 2011 and is behaving as a significant window showing progress in stabilizing the fluctuating power supply (both seasonal and spatial) from renewables. There are more than 30 active P2H pilot projects in Germany at present, with an electrolysis capacity of about 25 MW. Furthermore, an important online database [31] displays active P2H projects worldwide. Some of the P2H demonstration projects are listed in Table 1 [42].
《Table 1》
Table 1 Examples of P2H demonstration projects.
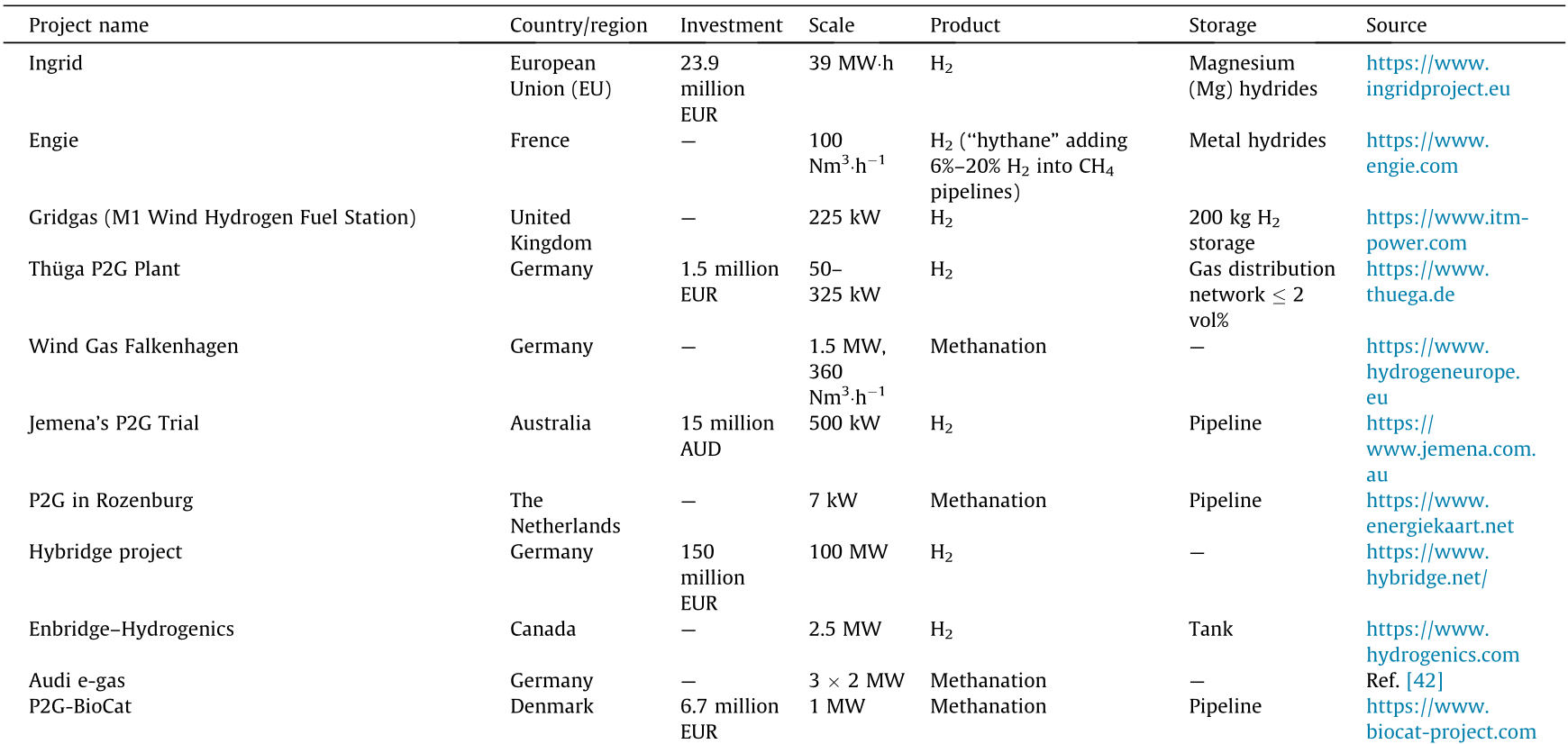

P2G: power-to-gas; Nm3 : the volume of the gas under standard condition (0 °C, 1.01 × 105 Pa).
A feasibility study [14] on installing an electrolyzer to produce hydrogen as an alternative to a network expansion in a German electrical distribution grid showed that hydrogen production from P2H (4–7.5 EUR·kg-1 ) was cost competitive for the hydrogen supply to refueling stations in Germany (9.5 EUR·kg-1 ) as of 2011. A case study in South Carolina, USA [43] also proved that electrolysis can be profitable for supplying hydrogen for fuel-cell-powered vehicles, with a hydrogen price of 12.5–16 USD·kg-1 (production and delivery), as reported by Sutherland and Joseck [44] in 2015. The US DOE Fuel Cell Technologies Office further set up a target of 7 USD·kg-1 by 2025 [45], with about 5 USD of this coming from delivery/dispensing [46] and 2 USD coming from production [47].
The capital costs of P2H facilities are mainly from the electrolyzers, while the operating costs are mainly from the electricity [48]. When P2H is used as an energy storage solution, the cost of electricity may be disregarded, as excessive renewable electricity must be curtailed when appropriate energy storage methods are absent. The investment costs of polymer electrolyte membrane (PEM) electrolyzer (3100–6600 USD per kilowatt hydrogen (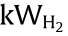 ) and alkaline electrolyzer (2100–5700
) and alkaline electrolyzer (2100–5700 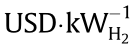 ) have been reported to vary widely with the scale of plants (by as much as 3–4 times) [11,43]. It was also concluded that alkaline electrolyzer is more mature and cost effective, albeit slightly lower in efficiency than the PEM electrolyzer from a long-term perspective [43]. Robinius et al. [14] recently summarized the reported data in the literature and provided an equation (Eq. (1)) as a reference for the calculation of investment costs (in EUR·kW-1 ).
) have been reported to vary widely with the scale of plants (by as much as 3–4 times) [11,43]. It was also concluded that alkaline electrolyzer is more mature and cost effective, albeit slightly lower in efficiency than the PEM electrolyzer from a long-term perspective [43]. Robinius et al. [14] recently summarized the reported data in the literature and provided an equation (Eq. (1)) as a reference for the calculation of investment costs (in EUR·kW-1 ).

where  is the flowrate of hydrogen (Nm3 ·h-1 ) (Nm3 : the volume of the gas under standard condition (0 °C, 1.01 × 105 Pa) and I is the current density (kA·m-2 ).
is the flowrate of hydrogen (Nm3 ·h-1 ) (Nm3 : the volume of the gas under standard condition (0 °C, 1.01 × 105 Pa) and I is the current density (kA·m-2 ).
To date, the scale of P2H pilots is very small (in terms of MW). Many questions remain to be answered before P2H can take on an even larger share of the energy market, including the availability of cheap renewable power, the need for low capital costs of electrolyzers, efficiency shifts with the scale of the electrolyzers and loading, the feasibility of high-pressure electrolysis, and so forth. It can be seen from Table 2 [32,37,49–51] that the overall longterm efficiency of P2H energy is still quite low, regardless of the end user of the hydrogen. It has been suggested that the integration of electricity generation and heat generation (known as combined heat and power (CHP)) from hydrogen fuel cells may lead to a much higher total energy efficiency (80%–95%) [27]. However, this solution is not always applicable. Thus, many researchers are dedicated to improving the overall efficiency of P2H.
《Table 2 》
Table 2 Energy efficiency by employing P2H for different purposes.

HHV: higher heating value.
《3. Discussion》
3. Discussion
Due to the mismatch between the supply and demand of hydrogen, hydrogen’s distribution methods and infrastructures are crucial for increasing the overall size of the hydrogen market [52]. However, the volumetric energy density of hydrogen is relatively low and the energy requirement for hydrogen liquefaction is extremely high. Thus, the storage and transportation of hydrogen are recognized as the most difficult phases for the redistribution of hydrogen [29].
《3.1. Hydrogen conversion and redistribution》
3.1. Hydrogen conversion and redistribution
A range of technologies have been investigated to convert hydrogen produced from P2H into other forms for hydrogen storage and redistribution, such as CO2 hydrogenation into fuels [53,54] (e.g., methanol, ethanol, formic acid, or even gasoline)— which was favored due to its low modification requirement for existing fuel systems—as well as ammonia synthesis [55] and methanation.
Hydrogen production from renewable energy sources paves the way for many storage systems and for the downstream synthesis of valuable chemicals. For example, Morgan et al. [55] investigated ammonia fuel production from wind power, with H2 from electrolysis as a key intermediate in the process. Recently, Nayak-Luke et al. [56] reported the concept of "green” ammonia produced from variable renewable energy, in which the intermittency of energy sources is largely mitigated by ramping the electrolyzer for hydrogen production. Hydrogen also plays an important role in CO2 hydrogenation, as demonstrated by the studies of Van-Dal and Bouallou [57], Pérez-Fortes et al. [58], and Chen et al. [53]. CO2 methanation from hydrogen provides a promising alternative for reducing carbon emissions and generating fuels. The materials for catalytic CO2 methanation include noble metals, such as rhodium-, ruthenium-, and nickel-based catalysts [59].
The synthesis of derivatives for reversible H2 uptake and release—namely, liquid organic hydrogen carriers (LOHC) [60,61] such as ethylene glycol [62], dibenzyltoluene [63], and 1,2- dimethylindole [64]—can be considered as a cascade storage of renewable energy to meet different requirements of storage size, duration, and management. The synthesized high-energy chemicals can be converted back into hydrogen to meet energy demand when required. As an example, the Marquardt Group (Germany) has proposed a conceptual design of an energy storage system with pressurized reversible solid-oxide fuel cells for power conversion, coupled with external ammonia synthesis/decomposition processes and a steam power cycle, resulting in a round-trip efficiency of 72% [65]. Although LOHC technology is still in its early stage [60], it has demonstrated significant potential for industrial implementation due to its mild transport conditions (atmospheric temperature and moderate pressure) and high hydrogen uptake, with a reported value of 6.5 wt% using ethylene glycol and 5.23 wt% using 1,2-dimethylindole [64].
《3.2. Direct distribution of hydrogen》
3.2. Direct distribution of hydrogen
A range of hydrogen-transportation pathways are available, including cryogenic liquid tankers (moderate to large stations), pressurized hydrogen tube trailers (early market and small stations), gas pipelines (mature market and large stations) [66], and other storage forms such as hydrogen carriers [67]. However, these methods have different shortcomings [67], such as high operating costs for large-quantity transportation, energy inefficiency for liquefaction, and high capital and time costs for pipeline construction. The construction costs of hydrogen delivery pipelines can vary significantly from one case to another, with some estimations reported at 329–590 USD per meter for 2 and 4 in (1 in = 2.54 cm) pipelines (69 bar) [68]—a mean value of 854 USD·m-1 for 30 cm (in diameter) pipelines [69], which is around 10%–20% more expensive than natural gas pipelines [70]. The US DOE Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration (MYRD & D) Plan set a 2020 target of 432 USD·m-1 for hydrogen pipelines at a transport pressure of 100 bar for a lifetime of 50 years [71].
As the existing natural gas pipelines are very well established (Fig. 8) [9], another strategy is to combine existing natural gas pipelines, with supplemented new pipelines as needed, to transport pure hydrogen [70]. However, there are still difficulties in using natural gas pipelines (which are mostly composed of ferritic stainless steel [72,73], with a small portion using plastic and cast or wrought iron [74]) for hydrogen transport, especially when dealing with high-pressure hydrogen gases. These difficulties include hydrogen blistering, hydrogen embrittlement, and hydrogen-induced fracture [29,75].
《Fig. 8》
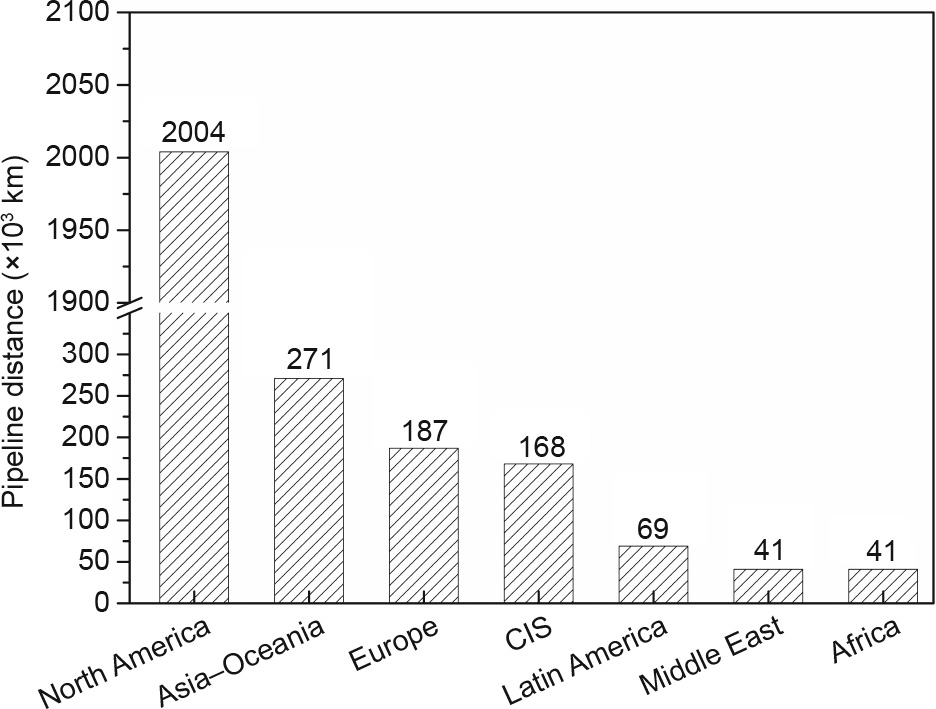
Fig. 8. Existing natural gas pipelines on a country/region basis in 2016. CIS: Commonwealth of Independent States.
Mixing hydrogen with natural gas and using existing natural gas pipelines for transportation has been demonstrated and discussed in various aspects [76]. In 2010, Pinchbeck and Huizing [77] suggested different safe hydrogen addition limits into existing natural gas facilities, such as 6% and 10% for natural gas pipelines at 40 and 8 bar, respectively; 12% for burners; and 18% for in-house appliances. Later, in 2013, Altfeld and Pinchbeck [78] summarized that a hydrogen mixture of 10% in natural gas is possible in some parts, but has different requirements in different areas. Therefore, they stated that case-by-case studies for specific projects are essential. Melaina et al. [79] concluded that a hydrogen concentration of 5%–15% (vol) seems to bring no significant risks to household appliances, public safety, and existing natural gas pipelines in delivering renewables (hydrogen) to the market. However, it should be noted that the heating value of the gas mixture (British thermal unit (BTU) value) will decrease with the addition of hydrogen due to its low BTU value, such that modification of natural gas appliances may be required when the addition of H2 is greater than 11% [75]. Another very important reason for encouraging the transportation of hydrogen using existing natural gas pipelines is that the future of natural gas pipelines must be revisited. The uncertainty of the depletion of natural gas wells makes investors extremely cautious to invest in new facilities, due to the uncertainty of natural gas storage. If existing natural gas pipelines could be modified (through coating, etc.) and converted into hydrogen pipelines, it might help investors to decide to build better developed pipeline networks, which will benefit the transportation of hydrogen.
In summary, the addition of hydrogen into existing natural gas pipelines is a feasible short-term solution for the following reasons. First, an addition of about 10% into natural gas pipelines is technically feasible, with minor ignition risks [79], no increase in pipeline fatigue [23], and no increased leakage risks [79,80]. Second, transporting hydrogen via existing natural gas lines is economically acceptable, as it can be treated as an alternative way to decrease the overall high construction cost and to save time during the early market-development phase [79]. Third, the transportation of flammable gases through existing natural gas pipelines is already accepted by the public. Last but not least, the operating practice of such cases will be important in order to explore the further utilization of existing natural gas pipelines. On that basis, the transportation of hydrogen using existing gas pipelines may be an interim solution, and the downstream hydrogen/natural gas mixtures may be used for direct heating, electricity generation, or pure hydrogen sources via the downstream separation of hydrogen from natural gas.
《3.3. End-user purification》
3.3. End-user purification
The transportation and redistribution of bulk and decentralized hydrogen produced by P2H projects are currently facing practical issues due to a lack of extensive hydrogen delivery networks. Building large-scale and dedicated hydrogen infrastructures is currently regarded as economically inviable; thus, injecting a low concentration of hydrogen into existing natural gas pipelines is an interim method. Based on research conducted by various institutions to date, blending about 10% hydrogen based on gas volume would barely induce minor issues, depending on the specific conditions of the pipelines and the composition of the carrying natural gas [79]. In such cases, the hydrogen blended with natural gas in the pipelines, sometimes called hydrogen-enriched natural gas (HENG), can be directly used as a fuel for boilers or furnaces in order to extract the heating value and for a gas turbine to generate electricity [17]. An ongoing two-year trial of P2H (Jemena Powerto-Gas Demonstration) in Australia and the HyDeploy project in the United Kingdom are injecting part of the hydrogen into the local natural gas network for household appliances [81,82].
Aside from direct utilization, hydrogen-blended gases could be an effective way to move bulk hydrogen from production sites to the potentially vast amount of renewable energy end users, if downstream separation techniques to obtain pure hydrogen from HENG are proved to be technologically feasible and economically viable. One of the key issues here is the separation of lowconcentration H2 from CH4 mixtures to produce pure hydrogen [83].
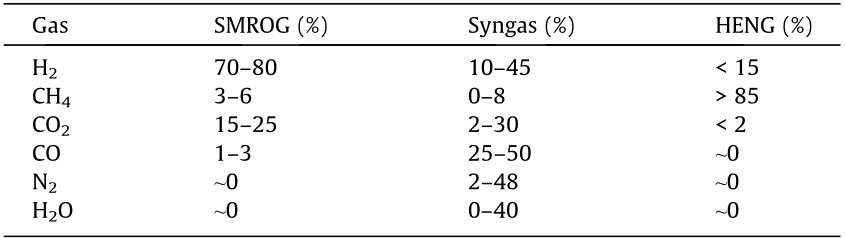
However, most current hydrogen separation technologies are customized for steam methane reforming (SMR), SMR off-gas (SMROG), or syngas, which may contain up to 80% hydrogen, balanced with CO2, carbon monoxide (CO), and methane [17,79,84], under high temperatures. These streams have different compositions from HENG, with less than 15% hydrogen. A comparison of the feed stream specification for these applications is shown in Table 3 [17,79,84].
《Table 3》
Table 3 Typical compositions of gas mixtures [17,79,84].
It has been reported that current separation costs are comparatively high (2–7 USD·m-1 for 100–1000 kg·d-1 ) [85]. The separation of hydrogen from low-concentration mixture sources suitable for HENG at low temperatures is not yet well investigated. Therefore, developing inexpensive, highly efficient separation techniques suitable for low concentrations of hydrogen in natural gas at low temperatures is of great significance for ensuring that overall hydrogen prices are competitive.
Many technologies are available to achieve the target of efficient, affordable separation technologies with compact facilities to acquire pure hydrogen (≥ 99.99%, as set by the US DOE [86]). In this section, we provide an overview of the technologies for separating H2 from CH4.
3.3.1. Membranes
Membrane technology has been commercialized for decades and is widely implemented in natural gas sweetening [87], hydrogen recovery from ammonia purge gas [88], and carbon capture [89,90]. Hydrogen transport across membranes may follow one or a combination of the following mechanisms: viscous flow, Knudsen diffusion, molecular sieving, solution–diffusion, and surface diffusion. The description and application of each mechanism are well established in the literature [91–93], although they are out the scope of this work and will be discussed in another paper [76].
The membrane performance for H2/CH4 separation can be expressed via the permeability (P) of H2 and the selectivity (α) of H2 versus CH4 (see Eqs. (2) and (3)). The driving force for hydrogen transport across the membrane barrier relies on the partial pressure difference of hydrogen on the two sides of the membrane. The partial pressure of hydrogen on the feed side of the membrane must be higher than that on the permeate side in order to support the permeate flow. As a result, the hydrogen separation efficiency (i.e., H2 concentration in the permeate/H2 concentration in the feed) is limited by the pressure ratio of the feed side to permeate side (see Eq. (4)). This suggests that a high pressure ratio is required to obtain high-purity hydrogen from a dilute hydrogen feed gas mixture. However, this limit is not the case when separating H2/CH4 in transmission pipelines, which commonly operate at around 68 bar [79].

where Qi is the gas permeance of the gas component i; l is the membrane thickness; J is the gas flux; and  is the partial pressure difference of the gas component on two sides of the membrane.
is the partial pressure difference of the gas component on two sides of the membrane.

Membrane studies in the literature are mostly aimed at mixtures with high hydrogen concentration (≥ 50%) to obtain a pure hydrogen product (≥ 99.99%) at high temperatures (≥ 300 °C). A range of membranes have been investigated for performance in hydrogen separation (Table 4 [92,94–98]), including dense metallic membranes [79,92,99,100], porous inorganic membranes [79,92,101,102], metal organic framework (MOF) membranes [103,104], and polymeric membranes [105–124]. Among these, dense metallic membranes and porous inorganic membranes are probably the closest to industrialization for hydrogen purification. Palladium membranes are able to separate hydrogen from methane with 99.9999999% purity; however, they also show low recovery and need a high operation temperature of 300 °C, which is not suitable for the purpose of separating hydrogen from natural gas pipelines [79]. Molecular sieve (MS) membranes [86,125,126] have generally low costs, show high permeability, and can be operated at low temperatures [127], but can only achieve a limited hydrogen purity. For example, a carbon molecular sieve membrane was reported to achieve a hydrogen purity of 98% [79] for the separation of hydrogen (~20%) from methane. Recently, the fabrication of graphene-based carbon molecular sieve (CMS) membranes has drawn attention due to its low thickness (single-atom thickness), high stability, and high H2 separation performance (e.g., 1 mol·m-2 ·s-1 ·Pa-1 H2 permeance and 108 H2/CH4 selectivity) [102]. Polyimide-based membranes such as 4,4' -(hexafluoroisopropylidene)diphthalic anhydride (6FDA) and Matrimid® have also been introduced at the industrial scale, and showed better H2 transport than conventional polysulfone and cellulose acetate membranes [87,92]. However, a significant limitation in polymer membranes is the tradeoff between gas permeability and selectivity, also known as the Robeson’s upper bound (Fig. 9) [97,98,105–124]. Several approaches have been applied to overcome this upper bound, including fabricating ultrathin membranes, synthesizing new polymers, and incorporating a polymeric membrane with other materials such as MOF materials. Many laboratory-scale membranes (Table 5 [97,105–124]) were reported to cross the upper bound; examples include polymer of intrinsic microporosity (PIM) membranes [105–107] and thermal rearranged (TR) membranes [108]. However, membrane-based hydrogen separation is still in the research and development (R&D) stage and more research in this area is expected before large-scale deployment.
《Table 4》
Table 4 Common membranes for hydrogen separation [92,94–98].

a H2/CH4 selectivity unless otherwise specified;
b H2/N2 selectivity.
《Fig. 9》
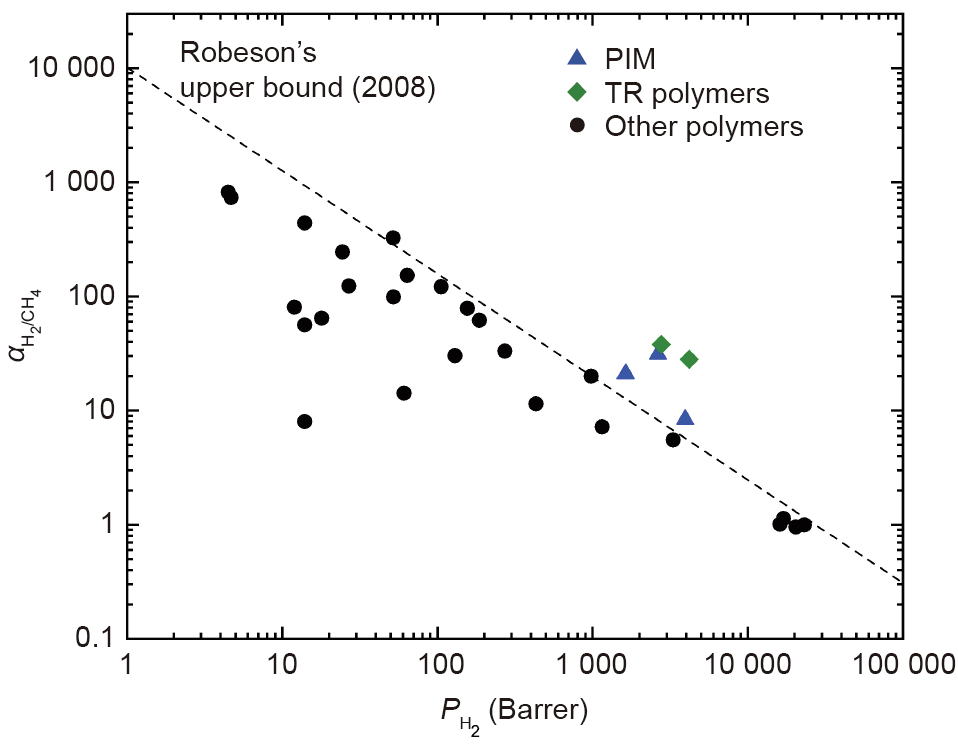
Fig. 9. Robeson’s upper bound (2008) for H2/CH4 separation, and some recent advanced membranes. PIM: polymer of intrinsic microporosity; TR: thermal rearranged; Barrer: unit for gas permeability through membranes, 1 Barrer = 3.35 × 10-16 mol·(m2 ·s·Pa)-1 .
《Table 5》
Table 5 A shortlist of polymeric membranes for hydrogen/methane separation.

3.3.2. Sorbents
Hydrogen purification was the very first large-scale application of pressure swing adsorption (PSA) technology (Fig. 10), and hydrogen purification via PSA is a well-established technology in the industry, with most examples servicing SMR processes [128]. Most researchers are currently focusing on further improving the performance of hydrogen PSA for the SMR process. Activated carbons and zeolites are the most frequently used adsorbents for H2 purification as equilibrium separation processes, with a reported selectivity (CH4/H2) of 10 and 13.5 for active carbon and zeolite 5A, respectively [129]. It is difficult to achieve high purity and recovery of hydrogen with the use of activated carbon as the sole adsorbent. However, 99.999% hydrogen can be obtained easily through PSA with a high recovery using zeolite 5A as the adsorbents through multiple columns and inter-column purging steps [129]. An earlier approach [130] to separate a lower concentration of hydrogen from a gas mixture via PSA was intended to recover hydrogen from the waste gas of SMR, which is known as gas polishing. Zeolites 5A are also known for their broadband adsorption capability for various impurities in hydrogen such as CO2, CO, H2O, N2, and so forth. Sircar [130] managed to use a threestage PSA process to improve the hydrogen recovery of the SMR process from 87% to 95%; in this process, the third stage of PSA with two adsorption columns was designed to treat feed gas with a lower hydrogen concentration. However, to the best of our knowledge, no published study has had the precise objective of extracting dilute hydrogen from methane via PSA thus far.
《Fig. 10》
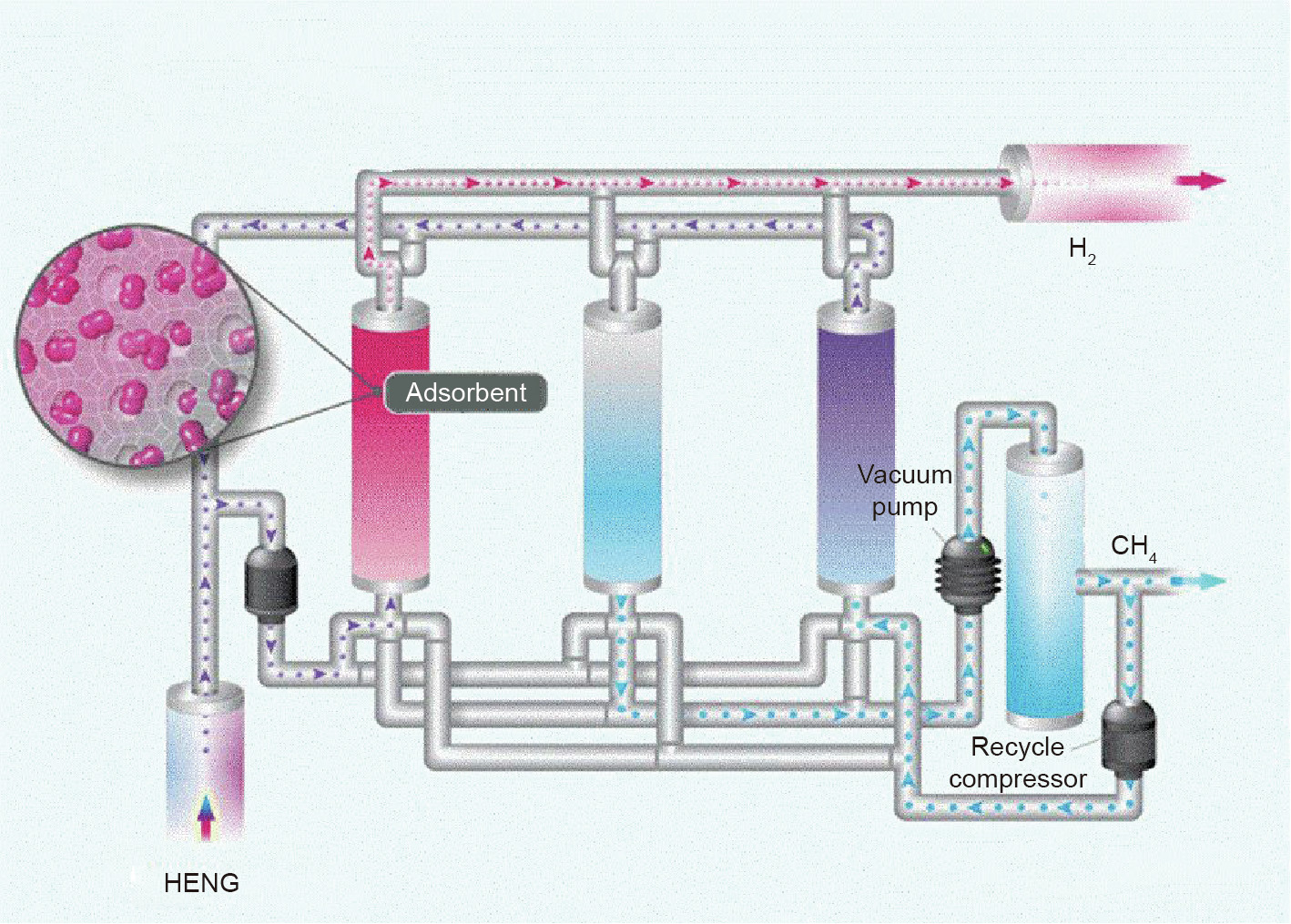
Fig. 10. Schematic diagram of a PSA. Images courtesy of CO2CRC.
Adsorbents are key to the production of high-purity hydrogen via PSA. Several researchers have focused on the development of novel materials for CH4/H2 separation, such as MOF materials. A range of (CH4/H2) selectivity data have been reported, such as 20 for copper (Cu)–benzene-1,3,5-tricarboxylic acid (BTC), 5 for MOF-5 [131], 2.5 for zeolitic imidazolate framework (ZIF)-70, 4 for ZIF-68 [132], 15 for ZIF-3 [133], and 10.5 (500 kPa) for MIL101_R7-benzene dicarboxylic acid (BDC) (by adding one more benzene ring into each carboxylate ligands of MIL-101) [134]. Kinetic separation processes have also been investigated for the separation of CH4 and H2, as the latter has a much faster mass transfer rate through MS beds. On strontium-modified sodium exchanged Engelhard titanosilicate (NaETS-4), the kinetic selectivity of H2/ CH4 was as high as 8.91 [135]. Most of these materials are at a very early stage of utilization, however, due to their high manufacturing costs.
PSA requires a large facility and more frequent adsorption– regeneration cycles due to the high concentration of methane in HENG (because sorbents are often methane-selective); thus, it has high overall costs. Because PSA technology is an intensive site-specific process, the gas feed composition and product specification may impact many design factors. One important factor to consider is the PSA column size against the tolerable level of methane. With more impurities in the gas feed, more adsorbents and larger PSA columns are needed in order to achieve high purity and recovery of hydrogen. Thus, a PSA bed for extracting pure hydrogen from HENG will be much larger than that in SMR processes. Any other differences in the process parameters, such as source gas pressure, sub-product gas pressure, and specification, will inevitably change the PSA design accordingly. Therefore, the experience of designing PSA for SMR cannot be directly adopted for the scenario of HENG purification.
In conclusion, there are two issues to be overcome in separating a low concentration of hydrogen from natural gas with PSA. First, as PSA requires a large facility and more frequent adsorption– regeneration cycles due to the high concentration of methane, it has high overall costs. Second, recovering hydrogen from a PSA unit is likely to involve the recovery of the main constituent at a pressure as low as a few bars; thus, huge recompression costs will be needed for further transportation.
Furthermore, an integrated membrane-adsorption process (Fig. 11) may be considered to produce high-purity hydrogen in order to overcome the shortcomings of low selectivity for membranes at low temperatures and the low methane-adsorption capacity of most adsorbents.
《Fig. 11》
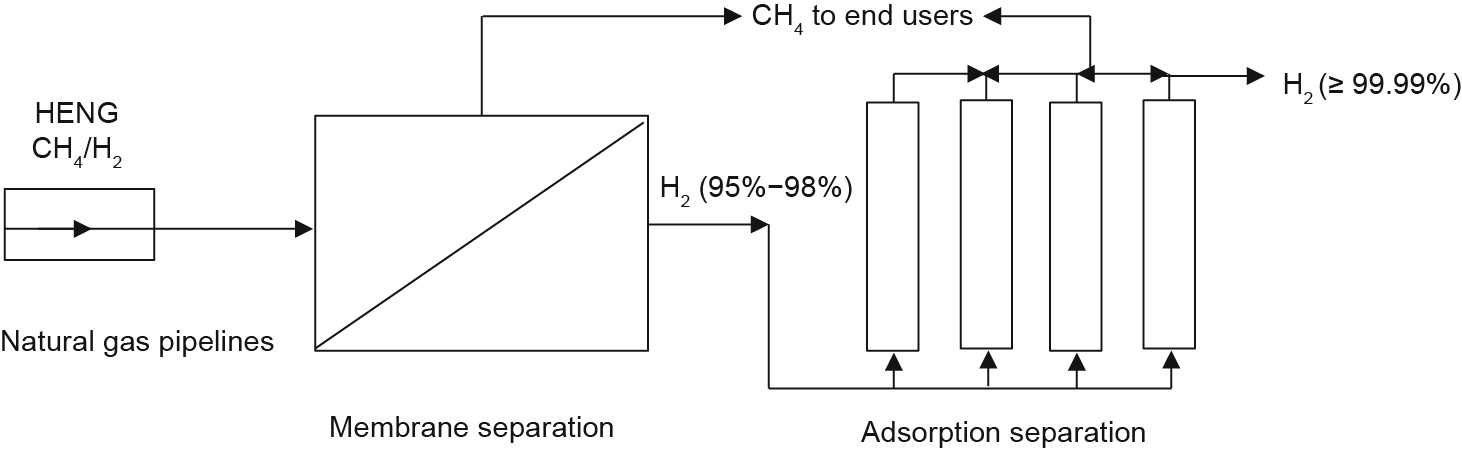
Fig. 11. An integrated membrane-adsorption process for the separation of H2 from CH4.
3.3.3. Hydrogen pump
Electrochemical separation is another choice for selectively capturing and pressurizing hydrogen from bulk methane mixtures. Pressure (i.e., partial pressure) is not the driving force in this technology (Fig. 12); instead, the electric potential drives the process by imposing voltage to dissociate H2 to H+ at a protonconducting anode (step 1, oxidation), transporting H+ through proton-conducting membranes/materials (step 2, transportation), and then re-associating protons to H2 at another protonconducting cathode (step 3, reduction) [136,137]. Recently, Wagner et al. [138] reported an electrochemical cell for the selective extraction of hydrogen from its mixtures with methane at a concentration as low as 5%. The advantage of the hydrogen pump is that it can separate hydrogen gas from methane and other gases [139] (CO2, H2O, CO, etc.) and has a very high hydrogen product purity, so that no further purification is needed. Although various electrolytes including ceramics [137] and polymers [139] have been reported as proton conductors, it is critical to find suitable materials with higher proton conductivity and lower mass transport resistance. The minimum electrical work to be supplied to the cell is the theoretical work of compression, as described by the Nernst equation [136]. The hydrogen pump process, however, is not yet ready for large-scale industrial deployment.
《Fig. 12》
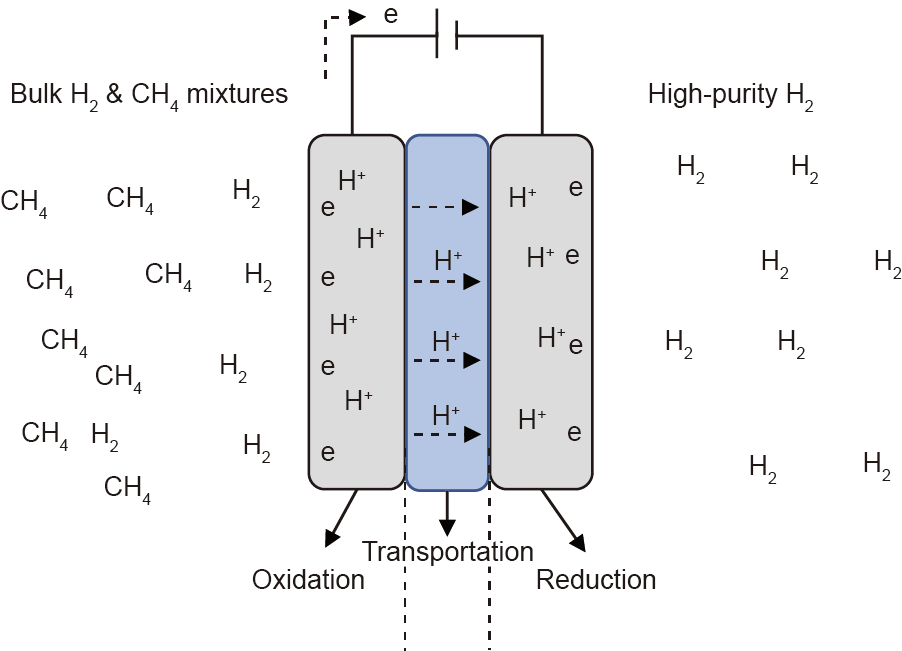
Fig. 12. Schematic showcase of the hydrogen pump process.
3.3.4. Solvents
The separation of hydrogen from methane using solvents has been a huge challenge during past decades, in comparison with other effective methods such as the use of membranes and adsorbents to separate gas mixtures. Suitable absorbents require a low solvent power for hydrogen but a high solvent power for methane in order to achieve high selectivity of methane over hydrogen. Solvents with a high solvent power for hydrogen could result in a significant loss of hydrogen and make hydrogen difficult to regenerate from rich absorbents, whereas absorbents with a low solubility of methane could have a negative impact on the purity of the final products. In addition, low vapor pressure and degradation of absorbents are required to avoid the loss of solvent.
Considering that both methane and hydrogen are non-polar and inert, their interactions with most solvents are very weak. Physical absorption under harsh operating conditions such as high pressures and sub-cooling temperatures is likely to be the only way to separate these two gases via absorption. For example, Palazzo and Schreiner [140] filed a US patent for the separation of methane and hydrogen using liquefied C2 and C3 hydrocarbons at –180 to –160 °C and about 7 bar. This process is not cost effective due to its high energy consumption. In addition, a portion of purified hydrogen was injected to regenerate the rich solvent, compromising the productivity of the hydrogen recovery.
Kim et al. [141] evaluated the solubility of methane in common liquid solvents in the form of the Henry’s constant using a molecular simulation method named the conductor-like screening model for realistic solvents (COSMO-RS) [142,143]. The solubility is calculated based on the chemical potentials between the solute and solvent, which are expressed by the σ-profile (Eq. (5)).

where x is the mole fraction of the solute;  (kJ·mol-1 ) is the pseudo-chemical potential of the solute when x = 1, a pure liquid state;
(kJ·mol-1 ) is the pseudo-chemical potential of the solute when x = 1, a pure liquid state; 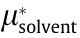 (kJ·mol-1 ) is the pseudo-chemical potential of the solvent; kB is the Boltzmann constant; and T (K) is the temperature.
(kJ·mol-1 ) is the pseudo-chemical potential of the solvent; kB is the Boltzmann constant; and T (K) is the temperature.
The simulation was conducted at 300 K and 1 bar, with  (methane) = –26.8 kJ·mol-1 [144,145]. Calculation details are provided in Ref. [141].
(methane) = –26.8 kJ·mol-1 [144,145]. Calculation details are provided in Ref. [141].
Trinh et al. [146] investigated the solubility of hydrogen in 42 organic solvents at different temperatures, including alcohols, carboxylic acids, esters, ethers, aldehydes, glycols, n-alkanes, and water. They calculated the Henry’s constant of hydrogen using the following rigorous equation:

where Hi (MPa-1 ) is the Henry’s constant of the solute; 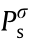 (MPa) is the vapor pressure of the solvent; and
(MPa) is the vapor pressure of the solvent; and  is the fugacity coefficient of the solute in the liquid mixture at an infinite dilution state, which is evaluated using the Peng–Robinson (PR) equation of state (EoS) with binary parameters.
is the fugacity coefficient of the solute in the liquid mixture at an infinite dilution state, which is evaluated using the Peng–Robinson (PR) equation of state (EoS) with binary parameters.
In total, 838 experimental data points from these 42 organic solvents were used to regress the binary parameters in the PR EoS with a deviation of 5%–10%. The regressed results were further used to validate the simulation using a Monte Carlo molecular simulation via an anisotropic united atom (AUA4) force field [147–151]. It was found that hydrogen has the highest solubility in n-alkanes, followed by ethers, aldehydes, esters, and alcohols, with diols having the lowest solubility.
To compare the selectivity of hydrogen over methane, we chose the Henry’s constants of hydrogen and methane using identical solvents from the above resources—namely, n-hexane, n-octane, methanol, ethanol, 1-propanol, 1-butanol, acetone, diethyl ether, acetic acid, and water—at 300 K; the results are shown in Fig. 13.
《Fig. 13》

Fig. 13. CH4/H2 molar selectivity versus CH4 Henry’s constant in different liquid solvents at T = 300 K.
It was found that all these liquid solvents have relatively high selectivity (≥ 5) of CH4/H2 at 300 K except for water, with 1-butanol showing the highest selectivity at 8.34. However, the Henry’s constants for both gases are extremely low at this temperature, which means that there is very limited solubility even for the best solvent, 1-butanol. For example, if the partial pressure of CH4 is 1 MPa, the mole fraction of CH4 loaded in n-octane is only 0.035. Optimizing the operating conditions (temperature and pressure) will require high energy input, resulting in economic concerns. Therefore, the use of solvents for separating hydrogen and methane is not favorable for industry.
3.3.5. Cryogenic separation
Cryogenic technology (Fig. 14) is one of the commercial techniques for gas separation [152], particularly for H2/CH4 mixtures. The recovery and purity of hydrogen can be as high as 95% and 90%–98%, respectively, with a feed gas H2 composition of 30%– 80% using cryogenic technology [153]. With an extremely low boiling point of 20.4 K, hydrogen is typically separated out of other gases as the gaseous phase in cryogenic processes [154]. Although it is applied widely in industrial processes, cryogenic technology is a costly and energy-intensive process [92,155]. Baker [87] recommended that the appropriate gas capacity for a cryogenic plant to be economical was in the range of 1.8 × 106 –8.8 × 106 m3 ·h-1 . This suggests that cryogenics may not be suitable for small-scale separation facilities in the downstream of the H2/CH4 transmission process. Another technical concern for the cryogenic processing of H2/ CH4 mixtures is the formation of methane clathrate at high pressures and low temperatures, which might block the processing pipelines [156]. Furthermore, insulation of the diffraction columns and liquefied gas containers are factors that affect the energy and economic efficiency of the cryogenic process [155,157]. Recently, hybrid systems that combine membrane and cryogenic systems [87,158] have been introduced and hold promise for reducing the land and economic footprints of the cryogenic process. It is worth noting that cryogenics may be an ideal option when the end user requires liquefied natural gas (LNG) on a relatively large scale (millions of cubic meters per hour).
《Fig. 14》
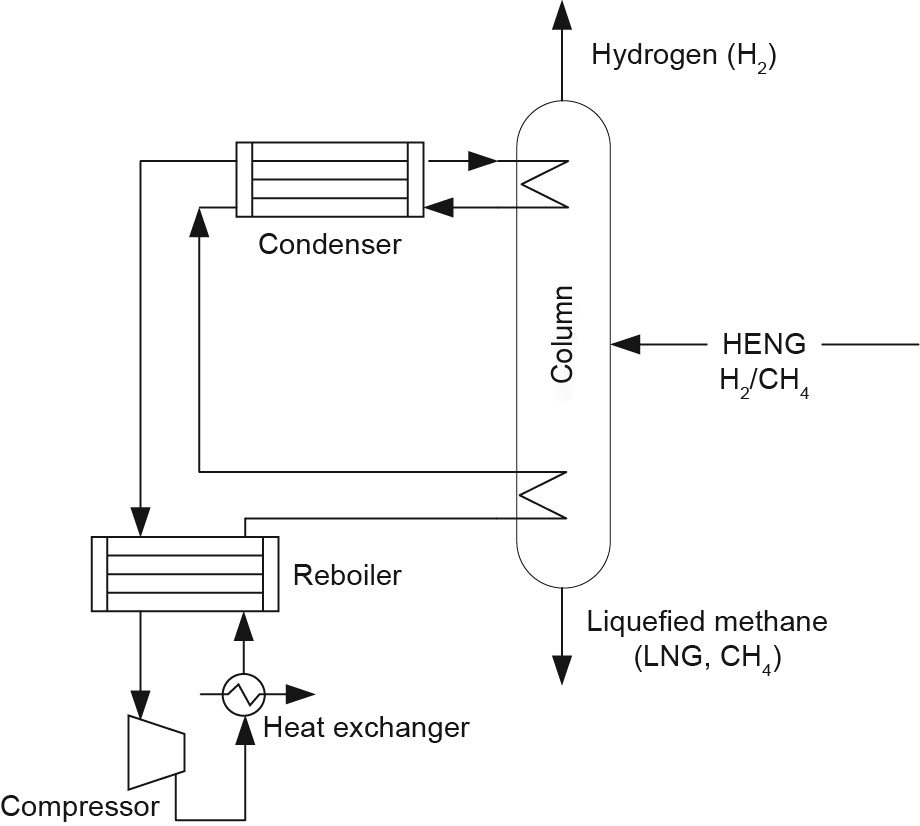
Fig. 14. Schematic graph of a single-column cryogenic process for the separation of H2 from CH4. LNG: liquefied natural gas.
《3.4. Other barriers and alternatives》
3.4. Other barriers and alternatives
Although there are still barriers for the P2H technology, we are quite confident that the development of this technology will be able to contribute to a clean and secure future. Other than the above technological barriers, other barriers also need to be addressed in order to ensure the large-scale deployment of P2H; these include the availability of water, government regulations, environment impacts, and so forth.
3.4.1. Supply of water for electrolysis
Availability of water is a key to P2H technologies, as water is essential for electrolysis and hydrogen generation. However, there may be a mismatch between the availability of renewable power and a source of clean water. Taking solar power as an example, drought areas are often indicative of a large amount of solar energy, whereas the availability of water is poor. In this case, either the transmission of electricity or the transportation of a large amount of water will be necessary. Therefore, it is worth investigating the local availability of water and analyzing the economic tradeoff between the transmission of electricity and the transportation of water and hydrogen.
3.4.2. Utilization of oxygen
Oxygen is a byproduct when producing hydrogen via electrolysis. However, the utilization of this byproduct is still not well explored. As the production of hydrogen increases, the quantity of oxygen produced will become significant as well. It is worth revisiting the possibility of utilizing high-concentration oxygen in industry. The following list provides some directions that may be considered:
(1) Using oxygen in fuel cells to enhance the overall efficiency and initiation speed;
(2) Using oxygen in combustion to enhance the temperature and fuel efficiency;
(3) An oxy-fuel combustion process followed by CCS with low costs;
(4) Steel or oxygen acetylene welding.
3.4.3. Environmental impacts
Many research publications have assessed the environmental effects of hydrogen utilization [26,73,159–163]. Bicer and Dincer [72] compared the effects of different vehicles (hydrogen, methanol, and electric) from the perspective of environmental impact ranging from global warming potential and ozone layer depletion, to human toxicity indicators, and covering the whole industry chain including vehicle production, operation, maintenance, and disposal. They concluded that hydrogen is the most environmentally friendly option. However, this conclusion can differ if the source of the hydrogen is taken into consideration. The amount of greenhouse gases emitted during the hydrogen production phase can vary significantly, depending on what type of fuel or energy is consumed. Renewable hydrogen may lead to fewer greenhouse gas emissions than hydrogen derived from fossil fuels. Other environmental impacts of different hydrogen production technologies, especially the consumption of resources (e.g., the large amount of water consumed by electrolysis or shifting processes), are yet to be investigated.
3.4.4. Safety, regulations, and public acceptance
Regulation is another factor that needs to be considered [75]. Taking China as an example, hydrogen is defined as dangerous (chemical) goods. Therefore, hydrogen refill stations must be operated in chemical plant areas or far away from urban areas. This makes the large-scale deployment of hydrogen in in-house appliances, self-used fuel cell cars, and so forth almost impossible. Whether this situation can be changed soon is still a big question. However, we have also observed some positive signs related to hydrogen deployment very recently. On 14 June 2019, the IEA released a special report [164] on hydrogen for the upcoming Group of Twenty (G20) meeting, followed by a joint statement issued by the Ministry of Economy, Trade, and Industry (METI) of Japan, the European Commission (EC) Directorate-General for Energy (ENER), and the US DOE to promote the development of hydrogen and fuel cells. This will accelerate the speed of hydrogen deployment, with potential deregulation of hydrogen in certain aspects.
3.4.5. Alternatives/competitors
P2H may not be competitive with fossil fuels from the perspective of current overall costs yet, and its cost reduction in large-scale deployment and in the long run will be highly dependent on the availability of excessive power, the scale of the electrolyzer, and the number of electrolyzer units [11]. Meanwhile, there are many competitors in the renewable energy sector, such as energy storage batteries, the conversion of renewables into fuels, and more, which can also contribute to a clean and secure energy future.
Converting renewable energy into electricity is one of many pathways for utilizing renewables in the energy sector. Various projects and research are underway, such as concentrated solar thermal energy and the production of hydrogen via direct photolysis or thermolysis of water [165–169]. Although electrolysis is a relatively mature technology, there is as yet no winner in the competition among solutions for renewables. Innovation in technology and engineering solutions will be the key for the growth of such technologies in the market, and it may take a few decades or even longer to make final decisions.
The storage of electricity using large-capacity batteries and supercapacitor systems [8] provides another choice for energy storage. Although hydrogen is much better in terms of energy storage density (volumetric/gravimetric) compared with current lithium (Li)-ion batteries [49], it is very likely that the energy density of batteries will be improved over time, given the large increase in battery R&D, and their price will be further reduced. It can be seen from Fig. 15 that the costs of batteries have dropped significantly over the last decade, and this decline in costs is believed to be continuing [170]. In addition, although problems such as the availability of resources and the economical recycling of used batteries remain unsolved, battery storage is a relatively mature technology with a vast manufacturing capacity (~70 GW·h in 2018) [3]. Furthermore, there are very strong indications that big corporations are willing to push battery storage development, with about 0.2 billion USD invested in batteries R&D in 2018 and a planned increase in the production capacity of batteries to about 400 GW·h by the mid-2020s [3]. Andrews and Shabani [8] recommended that hydrogen should play a role, together with electricity and other energy storage options, in future sustainable energy systems. Indeed, batteries are playing an important role in the energy storage sector, and it is believed that they will continue to do so in the future.
《Fig. 15》
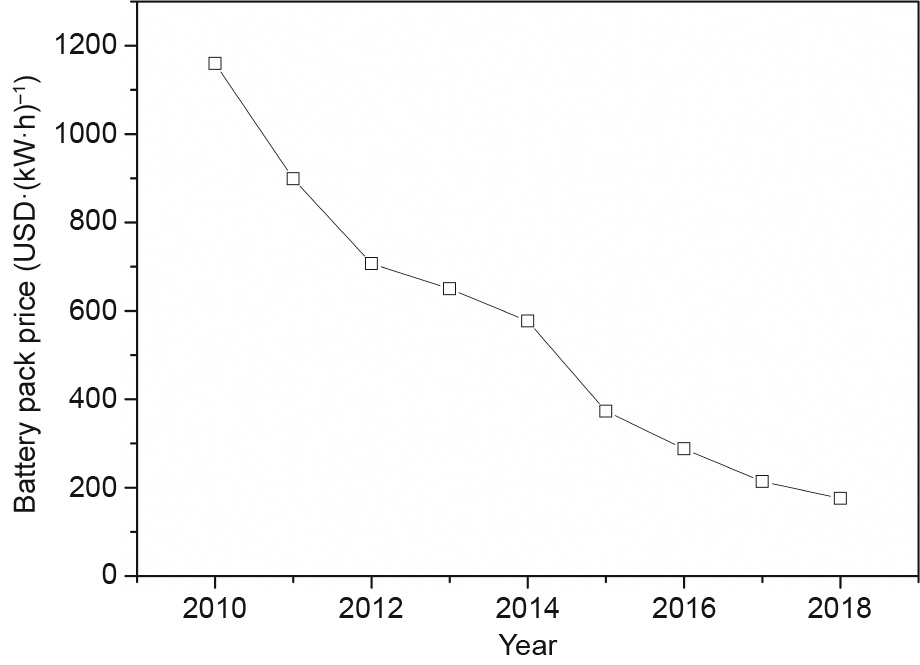
Fig. 15. Price of lithium batteries by year.
《4. Summary and outlook》
4. Summary and outlook
《4.1. Summary》
4.1. Summary
This article reviewed the overall process of the P2H value chain, from energy demand and power generation from renewables, to hydrogen production from electricity via electrolysis, and eventually to hydrogen redistribution, utilization, and re-separation from low-concentration hydrogen–methane mixtures. We showed that the share of renewables in the energy sector has increased dramatically in recent years and has been recognized as an important source of energy supply in the future. Energy storage pathways are essential, and P2H provides a promising solution to this issue. The redistribution of hydrogen using existing natural gas pipelines is well studied, and the recommendation of approximately 10% addition into natural gas pipelines has been shown to have negligible effects on pipelines and utilization appliances; this may also provide a solution for natural gas pipelines after natural gas has been depleted. Current research on end-user hydrogen purification mainly focuses on SMROG or syngas, often with higher hydrogen concentration and other impurities besides methane.
We reviewed the progress of a range of techniques for the separation of hydrogen from natural gas mixtures, including solvent absorption, membranes, adsorption, hydrogen pumping, and cryogenics. Solvents are not recommended due to their low absorption capacity. Cryogenics are too energy intensive to be cost effective, unless LNG is one of the final products. Membranes, adsorption, and hydrogen pumping are potential candidates with different advantages and degrees of maturity.
《4.2. Future R&D outlook》
4.2. Future R&D outlook
P2H is a relatively new area in the energy sector. This review discussed its technical advances and barriers, along with potential solutions in the P2H roadmap. With this review, we hope to promote promising areas for future R&D in order to advance this field. By overcoming these challenges, P2H will contribute significantly to a clean and secure energy future.
A key barrier to the large-scale deployment of P2H is its low overall energy efficiency and high cost. In the value chain of P2H, electrolysis, compression, and liquefaction are relatively mature in terms of technological readiness, but there is still a great deal of room for technological improvement in the following areas: hydrogen redistribution, transmission, and transportation; hydrogen purification and separation; conversion between hydrogenloaded and unloaded carriers; hydrogen utilization; and cost reduction.
4.2.1. Gas separation
Membranes and adsorption are key technologies in separating hydrogen from HENG. Due to the current limitations, the development of novel membranes and adsorbent materials with high selectivity and capacity, the advance of an integrated membraneadsorption process, and the feasibility study of hydrogen pumping utilization on a large scale are both necessary and promising. In Fig. 15. Price of lithium batteries by year. addition, research on the removal of low-concentration gas odorant blends (parts-per-billion (ppb) to ppm) such as tetrahydrothiophene, dimethyl sulfide, methyl ethyl sulfide, tetrahydrothiophene, and other mercaptans remains very rare, yet is crucial to the future deployment of hydrogen separation from HENG by end users.
4.2.2. Energy efficiency assessment
This review provides a rough energy efficiency assessment in Table 2 [32,37,49–51]. A more rigorous energy efficiency model will be essential for the comparison of new technologies and in order to determine the cost threshold of P2H.
4.2.3. Others
The conversion of hydrogen into liquid fuels (e.g., methanol, ethanol, methane, formic acid, or even gasoline) will remain a popular research topic, as these hydrogen-sourced fuels require little modification of existing fuel systems. This approach may play a significant role in promoting the progress of P2H in light of carbon dioxide utilization. Other aspects, such as the cost reduction of electrolyzers, the redistribution and utilization of oxygen, environmental impacts, the availability of water, and the readiness of regulations are still in their early stages and require considerable efforts to explore.
《Acknowledgements》
Acknowledgements
The authors acknowledge the support of Global Innovation Linkage (GIL) awarded by Department of Industry, Innovation and Science entitled "Development of Unconventional Gas Technologies for Sustainable Energy Security” (GIL54444), Early Career Researcher Grants Scheme awarded by the University of Melbourne entitled "Production of HighPurity Hydrogen from Mixed Pipeline Gases” (1858821), and Future Fuels Cooperative Research Centre (CRC) "Novel Separation Technology development forhydrogen and future fuels systems” (RP3.2-08). G. Hu also would like to acknowledge Mr. J. Liu for preparing the table of art.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Guoping Hu, Chao Chen, Hiep Thuan Lu, Yue Wu, Congmin Liu, Lefu Tao, Yuhan Men, Guangli He, and Kevin Gang Li declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




