《1. Introduction》
1. Introduction
The energy cost of separation processes in industry occupies 10%–15% of total energy consumption [1]. Membrane separation requires significantly less energy than the adsorption process and cryogenic distillations, and thus is a key component of sustainable processing and manufacturing. Moreover, unlike extraction and crystallization processes involving solvents, membrane processes eliminate the possibility of generating secondary contamination during the recycling and reprocessing of organic solvents. Therefore, membrane separations are ideal processes for green chemical engineering.
The working principle of membrane separation is based on the selective transport of different species through the membrane under a driving force. The selective sorption of one particular molecule over another and the different diffusion rates through the pores can both contribute to the selectivity of membrane separation [2]. Accordingly, precise functionalization within the membrane pores and fine-tuning of pore dimensions are desirable in order to achieve high selectivity while maintaining large permeance, which always appears to be a trade-off. The specific application of membranes depends on the pore sizes. Membranes with nominal pore sizes larger than 0.1 μm (or a Mw cut-off > 5000 kDa) are typically used in microfiltration for particles, large bacteria, blood cells, and yeasts. Ultrafiltration membranes possess nominal pore sizes ranging from 2 to 100 nm (or a Mw cut-off of 5– 5000 kDa), and are effective for the separation of viruses, albumin proteins, and colloidal solids. Membranes with nominal pore sizes of 1–2 nm are often classified as nanofiltration membranes, and are used for separating synthetic dyes, sugars, and molecules larger than 5 kDa. The pore sizes of reverse osmosis (RO) membranes are generally smaller than 1 nm, leading to the effective removal of aqueous salts and small organic molecules (< 100 Da) [3]. Membranes with ultramicropores (pore sizes < 0.7 nm) are useful for gas separation, pervaporation, and vapor permeation [4,5].
Although this fact is often overlooked, common fabrication methods for membrane materials involve a wide variety of organic solvents, most of which are not environmentally benign. Solventless synthetic methods offer greener solutions for membrane fabrication. In recent years, vapor-phase approaches have been reported for synthesizing membrane materials. Ultrathin selective layers directly deposited on membrane substrates and precise pore engineering in membrane structures (Fig. 1) have enabled many sustainable separation processes such as gas separation, nanofiltration, desalination, and water/oil separation. This perspective covers the recent development of solvent-less deposition processes for advanced membrane materials.
《Fig. 1》

Fig. 1. Schematic illustration of solvent-less vapor-phase deposition for the fabrication of a thin-film composite membrane comprised of (a) a blanket layer over a porous support and (b) a conformal coverage penetrating into interior pore surfaces.
《2. Initiated chemical vapor deposition for membrane surface modification and fabrication》
2. Initiated chemical vapor deposition for membrane surface modification and fabrication
The initiated chemical vapor deposition (iCVD) polymerization method holds great promise for developing improved membranes for a multitude of separation processes (Table 1 and Fig. 2 ) [6–26]. Ultrathin free-standing membranes have been fabricated by means of iCVD alone (Fig. 2(a)) for envisioned biomedical applications and biomedical microelectromechanical systems (bioMEMS) applications, such as the fabrication of a lung-assist device [8]. However, iCVD is more commonly utilized to modify base membrane supports. This enables independent optimization of the surface and bulk properties. As a vapor-phase method, iCVD eliminates the potential for solvent-induced swelling or damage to the base membrane. In addition, iCVD allows surface tension effects, such as dewetting, to be avoided, making the formation of pinhole-free surface modification layers of uniform thickness possible, even over complex porous geometries.
《Table 1》
Table 1 Selected examples of membranes fabricated utilizing iCVD.



a Signifies a crosslinking monomeric unit for added durability.
b Sacrificial layer.
c Base membrane.
《Fig. 2》

Fig. 2. (a) Schematic of iCVD polymer thin-film growth from monomer and initiator vapors [24]; (b) cross-sectional transmission electron microscopy (TEM) image for an ultrathin blanket iCVD antifouling layer on a commercial RO membrane [25]; (c) conformal iCVD coating on the interior of the cylindrical pore wall [14]; (d) schematic of the on–off switching of a nanovalve fabricated with a conformal iCVD hydrogel layer [14]; (e) the complex porous architecture of a poly(vinylidene fluoride) membrane before (left) and after (right) conformal iCVD surface modification [17].
In iCVD [27], monomers and a free-radical initiator are introduced as vapors into a reactor held at modest vacuum (~0.1 to ~1 Torr, 1 Torr ≈ 133.3224 Pa). The array of wires inside the reactor is heated to 250–300 °C, which decomposes the initiator but not the monomer. The reactive species adsorb and polymerize on a cooled surface, typically held at around 25 °C. The low temperature of the growth surface is ideal for avoiding damage to fragile substrates, such as porous polymeric membranes.
The large library of iCVD monomers enables the synthesis of a diverse array of homopolymers and copolymers (see Table 1 for examples). Selection of specific iCVD polymers is a key design parameter for achieving fouling-resistant surfaces, stimuliresponsive layers, and highly efficient separations based on specific molecular interactions. The organic functional groups displayed by iCVD polymers include both simple moieties, such as hydroxyl (–OH), carboxyl (–COOH), and amine (–NH2) groups, and more complex structures, such as zwitterionic moieties.
For membrane applications, copolymerization commonly utilizes crosslinking monomers containing two or more vinyl bonds. With the iCVD approach, crosslinking occurs simultaneously with polymerization and thin-film formation. There is no need for any post-deposition annealing or ultraviolet (UV)-curing steps to achieve crosslinking by iCVD. The crosslinker imparts durability and low surface roughness to the resulting iCVD film. Using the iCVD vapor-phase approach permits the copolymerization of monomers that do not have a common solvent. Indeed, mixing the vapors of a fluorine-containing monomer with a hydrogelforming monomer enabled random iCVD copolymerization, leading to the formation of an amphiphilic surface resistant to protein fouling [28] and proton-exchange membranes for fuel cells [20].
Novel membranes result from combining iCVD layers with a variety of membrane supports (Table 1). Demonstrated iCVD compositions can impart hydrophobic, hydrophilic, responsive, or fouling resistance to membranes as either blanket layers (Fig. 1(a)) or conformal coatings (Fig. 1(b)).
A blanket anti-biofouling iCVD layer robustly grafted to an underlying commercial RO desalination membrane (Fig. 2(b)) can reduce the frequency of costly shutdowns for membrane cleaning [25]. iCVD copolymer films exhibit excellent antifouling performance, mainly due to two reasons: First, iCVD copolymers and zwitterionic coatings can generate a hydrophilic surface/hydration layer to reduce protein and bacteria adsorption [10,17]; second, iCVD random copolymers possess compositional heterogeneities in the same dimension as the foulant, resulting in thermodynamically unfavorable interaction between the biofoulant and the surface [28]. As ultrathin films (< 30 nm thick), the antifouling layers have a limited impact on water permeability. Combining blanket iCVD layers with an anodized aluminum oxide (AAO) support results in novel composite membranes in which the iCVD hydrogel films provide permselectivity [9].
Applying iCVD conformally, rather than as a blanket layer, modifies the surface chemistry over the entire pore architecture of the base membrane, and can systematically decrease pore diameter (Fig. 1(b)). Covering the interiors of the cylindrical nanopores of a base track-etched polycarbonate with conformal hydrophobic iCVD coatings altered the surface chemistry inside the pores and narrowed their mean diameter down from 50 to < 5 nm. At this small final diameter, the separation of similarly sized molecules was achieved based on the difference in their hydrophobicity [12]. Conformally coating AAO membranes with a responsive iCVD layer create a smart membrane that switches on and off the passage of protein [14]. In the collapsed state, the iCVD layer has a thickness of about 30 nm (Fig. 2(c)) and the pore is open. Changing the temperature causes the iCVD layer to swell with water, effectively closing off the pores (Fig. 2(d)). Smart membranes have also been fabricated with pH-responsive iCVD layers [13]. Figs. 2(e) and (f) demonstrate that tortuous pore architectures can also be conformally modified by iCVD [18] to create membranes for oil/water separation.
The macroscale openings of steel mesh supports provide high permeability. Conformal hydrophilic zwitterionic iCVD coatings encasing the mesh structure allow the rapid passage of water through the openings in the mesh, leaving oil behind [29]. Using the inverse strategy, hydrophobic iCVD-coated meshes allow the rapid passage of algae lipids, which are desired as a biofuel, from water-based emulsions [22]. Nucleic acid separation has also been demonstrated with iCVD-coated steel meshes [21].
For water desalination by means of membrane distillation, only water vapor should pass through the pores, causing brine to concentrate on one side of the membrane (Fig. 3(a) [16]). Optimal operation requires a narrower pore size distribution than is typically available with commercial hydrophobic membranes. Nylon membranes can provide the desired narrow pore-size distribution, but are hydrophilic. However, a nylon membrane can be made hydrophobic by conformal iCVD hydrophobic surface modification [16]. Other types of membrane substrates have also been iCVD surface modified for potential application in membrane distillation [30,31]. Using a blanket hydrophobic layer on one side of the base membrane with a blanket hydrophilic layer on the other produces Janus membranes [23] and Janus films [32], which are used for oil/water separation, wound healing, and other applications. Fig. 3(b) shows a responsive Janus membrane for the directional transport of protein [33]. On each side of the membrane, a pH-responsive iCVD layer was coated in a partially conformal manner at a P/Psat of about 0.3 to create keyhole-shaped pores. These pores open and close as gates when the pH is switched in succession, in order to produce unidirectional mass transport.
《Fig. 3》
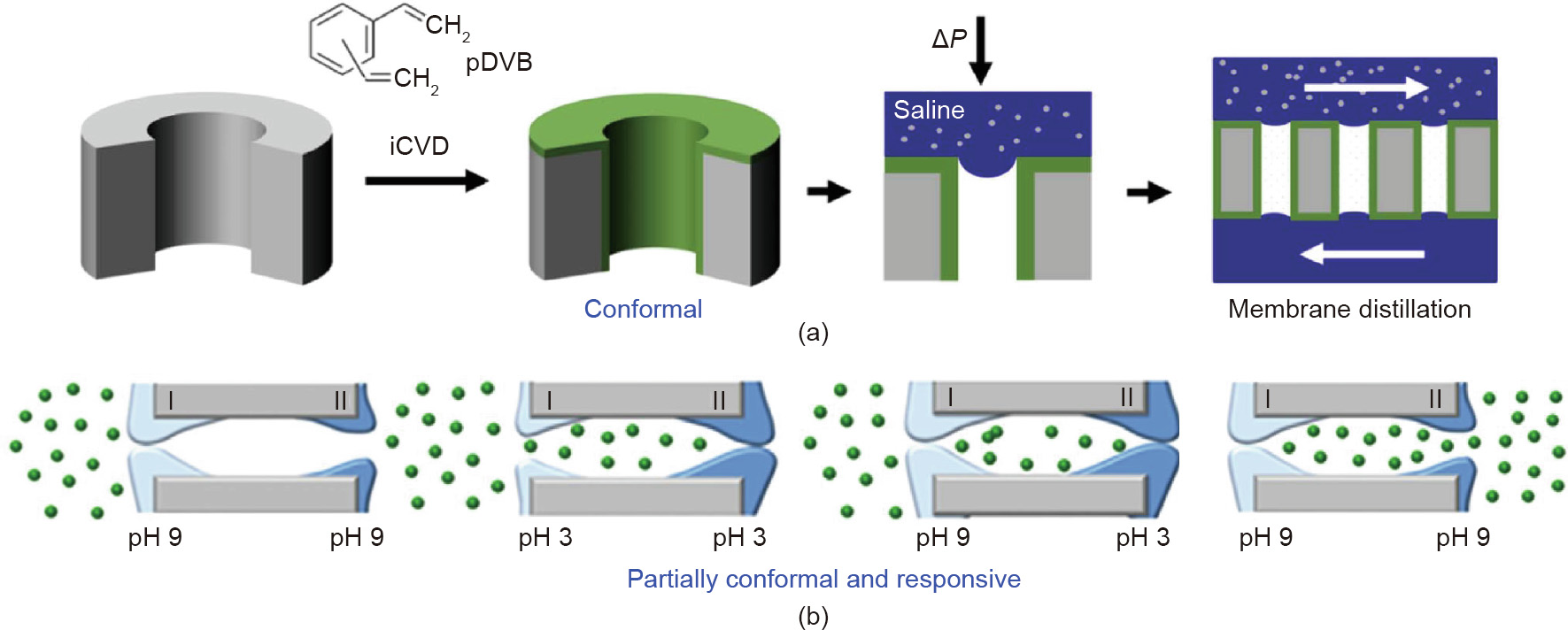
Fig. 3. Separation processes using novel membranes fabricated via iCVD. (a) A fluorine-free hydrophobic conformal coating permits only water vapor from the saline to pass through the pore [16]; (b) keyhole-shaped pores from partially conformal iCVD pH-responsive layers produce the directional transport of protein as pH is varied on each side of the smart membrane [33]. pDVB: poly(divinylbenzene).
The consumption rate of the monomer to form films relative to the monomer’s diffusion rate, as quantified by the Thiele modulus (θ), is key to determining the final architecture of the iCVD modified porous media [34]. The value of θ is proportional to pore length squared and inversely proportional to pore radius. The precise equation for θ depends on the underlying kinetic mechanism of the deposition process.
For θ >>1, iCVD polymerization is rapid compared to the rate at which monomer vapor diffuses into the pores. Hence a blanket iCVD layer forms over the top surface of the membrane. In order to function as the selective layer of thin film composite membrane, the blanket layer must be free of pinhole defects. Additionally, the blanket layer must be ultrathin in order to maintain high permeation rates across the composite structure. Optimization to achieve the sub-1 nm roughness levels enables ultrathin pinhole free iCVD layers to form. In contrast, surface tension effects make defect-free ultrathin layers difficult to form by liquid-based methods.
For θ << 1, the monomer is able to rapidly diffuse down the length of the pore before reacting, allowing all of the internal pore wall surfaces to be uniformly covered. Conformal deposition on cylindrical pores causes their diameter to shrink uniformly down their length. Under optimized conditions, iCVD has achieved conformal coverage of cylindrical pores 4000× longer than their diameter. Conformal iCVD coverage is also possible on tortuous pore geometries. In contrast, the ability to achieve conformal coverage by liquid-based coating methods is often limited by the effects of surface tension. For θ << 1, the iCVD layers formed at the pore mouth will be thicker than in the interior of the pore, resulting in bottleneck-shaped pores.
Since the iCVD layer starts at the surface of the substrate, it is possible to engineer the formation of covalent bonds across the interface as the iCVD process commences. The goal is to promote the functional groups present on the surface of the substrates to undergo chemical reactions with complementary functional groups in the iCVD film. The grafting reduces the probability of the surface modification layer delaminating, and is particularly valuable for enhancing the durability of thin-film composite membranes. The geometry of conformal surface modification has reduced probability to fail by delamination.
《3. Initiated plasma-enhanced chemical vapor deposition for porphyrinic metal–organic covalent network membranes》
3. Initiated plasma-enhanced chemical vapor deposition for porphyrinic metal–organic covalent network membranes
Their microporous structure and narrow pore-size distribution make porphyrin polymers attractive for the adsorption, separation, and sensing of gases. While a variety of porphyrin-based metal– organic frameworks (MOFs) and covalent organic frameworks (COFs) have been reported, it is still challenging to fabricate pinhole-free large-area membranes with nanoscale thickness. Taking advantage of the solvent-less initiated plasma-enhanced chemical vapor deposition (iPECVD) process, Boscher et al. [35,36] and Wang et al. [37] developed a series of ultrathin (20–100 nm) porphyrin-based metal–organic covalent networks (MOCNs). This iPECVD process has been shown to be a scalable, facile approach to synthesize free-radical polymerized porphyrin networks in a single step over a large substrate area (175 cm2 ). In addition, the soft radio frequency (RF) plasma employed in the iPECVD process allows the use of delicate substrate materials such as the porous polymer support for MOCN membranes.
In a typical iPECVD process (Fig. 4(a)), low-power capacitive coupled Ar plasma cleaves the O–O bonds in tert-butyl peroxide (TBPO) and generates free radicals. Metalloporphyrin monomers are evaporated from a heated crucible (275 °C) and polymerize on the substrate surface with the presence of tert-butoxy radicals. Optional crosslinkers such as divinylbenzene (DVB) can also be delivered into the vacuum chamber and copolymerize with the metalloporphyrin, offering additional tunability in the design and synthesis of MOCN structures.
《Fig. 4》
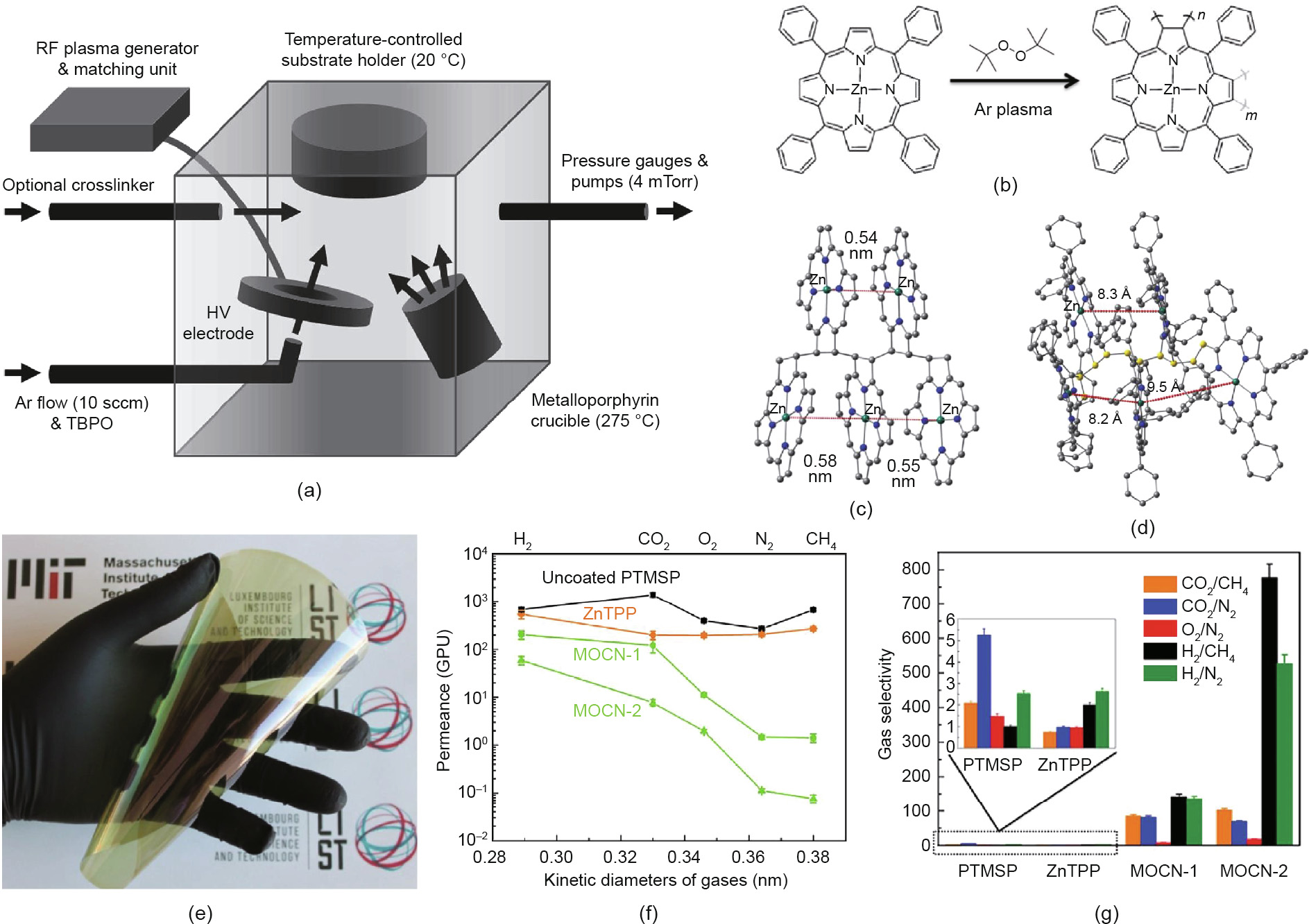
Fig. 4. (a) Illustration of the iPECVD reactor for the solvent-less synthesis of MOCN membranes [36]. (b) Reaction schematic for forming poly(Zn(II) meso-tetraphenylchlorin) (poly(ZnTPC)) MOCN via iPECVD free-radical polymerization. (c, d) Density functional theory (DFT)-optimized pentamer structure of [ZnTPC]5 (hydrogen omitted; color code: grey for carbon, blue for nitrogen, green for zinc) [35,37]. Phenyl rings were truncated for DFT calculation in (c), while (d) shows the full structure of the [ZnTPC]5 pentamer (carbon atoms in the polymer backbone are color-coded yellow). (e) Photo of a large-area flexible poly(1-trimethylsilyl-1-propyne) (PTMSP) membrane coated with an iPECVD MOCN selective skin layer. (f, g) Gas-separation performance of an uncoated PTMSP membrane and of PTMSP membranes coated with evaporated Zn(II) meso-tetraphenylporphyrin (ZnTPP) film and iPECVD MOCN films. Film thickness is 47 nm for MOCN-1 and 67 nm for MOCN-2 [35]. HV: high voltage; sccm: standard cubic centimeter per minute; GPU: gas permeation unit.
Spectroscopic analyses reveal that Zn(II) meso-tetraphenylporphyrin (ZnTPP) is reduced to Zn(II) meso-tetraphenylchlorin (ZnTPC) during the iPECVD process, indicating that the free-radical polymerization occurs at the exo-pyrrole double bonds (Fig. 4(b)). Less pronounced π–π stacking of the porphyrin structures was also found in iPECVD MOCN from the spectral results. As illustrated in Figs. 4(c) and (d), the molecular structures of [ZnTPC]5 pentamers optimized via density functional theory (DFT) show the pore size and geometry. The truncated model shows an average Zn–Zn distance of about 0.56 nm, while the full model reveals the Zn–Zn distance to be about 0.87 nm with consideration of all phenyl rings at meso-positions [37]. The actual pore size of the iPECVD MOCN film, which was determined using ellipsometric porosimetry, is as low as 0.4 nm, which is in the perfect range for separations via molecular sieving.
Large-area MOCN membranes have been deposited on poly(1- trimethylsilyl-1-propyne) (PTMSP) substrates (Fig. 4(e)) [35]. Effective molecular sieving of MOCN membranes suppresses the permeance of gases with a large kinetic diameter (i.e., CH4 and N2), while the high flux of smaller gas molecules is maintained (Fig. 4(f)). Single gas permeation tests reveal that the separation selectivity of uncoated PTMSP and thermal-evaporated ZnTPP membranes is generally below 5, regardless of the gas pairs. The low selectivity of evaporated ZnTPP membranes is due to major defects being present in the ZnTPP layer [35]. This metalloporphyrin tends to crystallize during thermal evaporation, so it may leave a significant number of defects at the grain boundaries in ultrathin polycrystalline films. In contrast, the separation selectivity for H2/CH4 and H2/N2 gas pairs reaches over 130 with a defectfree iPECVD poly(ZnTPC) membrane, while H2 permeance maintains over 200 gas permeation units (GPU). Excellent separation performance has also been obtained for CO2/CH4 and CO2/N2. Separation of these gas pairs has significant industrial implication in H2 recovery, pre-combustion CH4 purification, and CO2 capture from flue gas.
Boscher et al. [36] further expanded the iPECVD chemistry for MOCNs by introducing multi-vinyl crosslinkers during the free radical polymerization of metalloporphyrins. Separation selectivity for CO2/CH4 over 150 has been achieved with poly(DVB-co-ZnTPC) membranes. Wang et al. [37] investigated the role of the center metal ion of porphyrinic MOCNs in gas separation performance. Poly(meso-tetraphenylchlorin), poly(Mn(III) (meso-tetraphenylchlorin) chloride), and poly(Co(II) meso-tetraphenylchlorin) membranes have been synthesized using the iPECVD process and compared with poly(ZnTPC). DFT calculations show that the center metal ion does change the packing structure of the MOCNs. Consequently, high performance for gas separation has been demonstrated for all four MOCN membranes.
《4. The solvent-less vapor deposition followed by in situ polymerization process for polyimide membranes》
4. The solvent-less vapor deposition followed by in situ polymerization process for polyimide membranes
Polyimide (e.g., Matrimid®) is commonly used for gas separation membranes, and has been widely studied in the past [38]. Developed by the Lawrence Livermore National Laboratory (LLNL), the solvent-less vapor deposition followed by in situ polymerization (SLIP) process is capable of forming polyimide thin films on substrate membranes [39,40]. During the SLIP process, a dianhydride (e.g., pyromellitic dianhydride (PMDA) and 5,5' -[2,2,2,- trofluoro-1-(trifluoromethyl) ethylidene] bis-1,3-isobenzofuranedione (6FDA)) and a diamine such as oxydianiline (ODA) are evaporated separately and delivered into the vacuum chamber simultaneously (Fig. 5(a)). After passing through a mixing nozzle, a polyamic acid thin film is deposited onto the substrate surface. A final in situ imidization step is required to convert the polyamic acid into polyimide for the thin-film composite membrane (Fig. 5(b)). The growth rate was found to be 11 nm·s-1 for PMDA-ODA and 2.5 nm·s-1 for 6FDA-ODA [40]. This deposition rate is promising for high-throughput manufacturing if the SLIP process can be scaled up.
《Fig. 5》
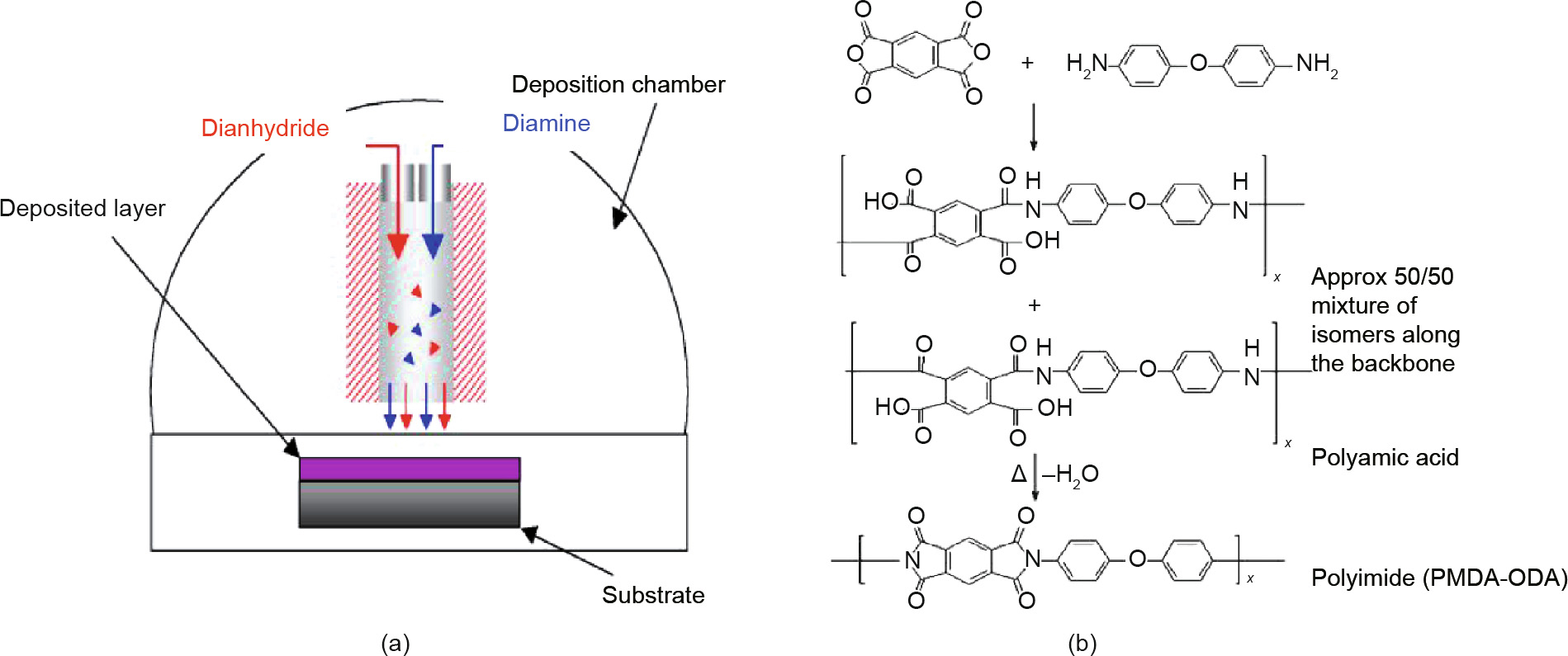
Fig. 5. (a) Reactor scheme for the SLIP process for polyimide membranes; (b) reaction route for the synthesis of polyimide (PMDA-ODA) [40].
The use of delicate polymer substrates actually limits the temperature that can be used for the thermal imidization reaction in SLIP. Spadaccini et al. [40] reported lowering the imidization temperature from 300 to 180 °C in order to adopt the process for the amorphous fluoropolymer (perfluorodioxole) substrate. Although complete imidization has been achieved for PMDA-ODA, the limited degree of imidization for 6FDA-ODA makes this SLIP polymer unqualified for gas separation. The PMDA-ODA membrane fabricated via the SLIP process enables a CO2 permeance of 79 GPU (calculated from Ref. [39]) with a CO2/N2 selectivity of 24.5 [39]. Further optimization of the SLIP process is needed to develop new polyimide membranes with enhanced separation performance.
《5. Atomic/molecular layer deposition for MOF membranes》
5. Atomic/molecular layer deposition for MOF membranes
Atomic layer deposition (ALD) is a vapor-phase deposition process that is widely used in semiconductor manufacturing. Due to the self-limiting effect of precursor chemisorption, ALD is powerful in its precise control of film thickness at a sub-nanometer scale and its exceptional conformality in high-aspect-ratio nano-structures [41]. In addition, ALD processes have been developed for a wide variety of materials including metals, oxides, nitrides, sulfides, and many other compounds [42]. These advantages of ALD have given rise to extensive research efforts in tuning membrane pore structures for separation applications [43–47]. Fig. 6(a) depicts a typical ALD process involving the cycling processes of sequential precursor doses and inert purge steps [48]. Taking ALD Al2O3 as an example, trimethylaluminum (TMA) reacts with surface –OH groups in the first half reaction and creates a –CH3 terminated surface. In the second half reaction of the ALD cycle, H2O vapor reacts with the –CH3 terminated surface via ligand exchange, thus changing the surface into –OH groups again. Purge steps between the half reactions ensure the complete removal of unreacted precursors and byproduct CH4. Monolayers of Al2O3 are added sequentially onto the surface by repeating these process cycles.
《Fig. 6》
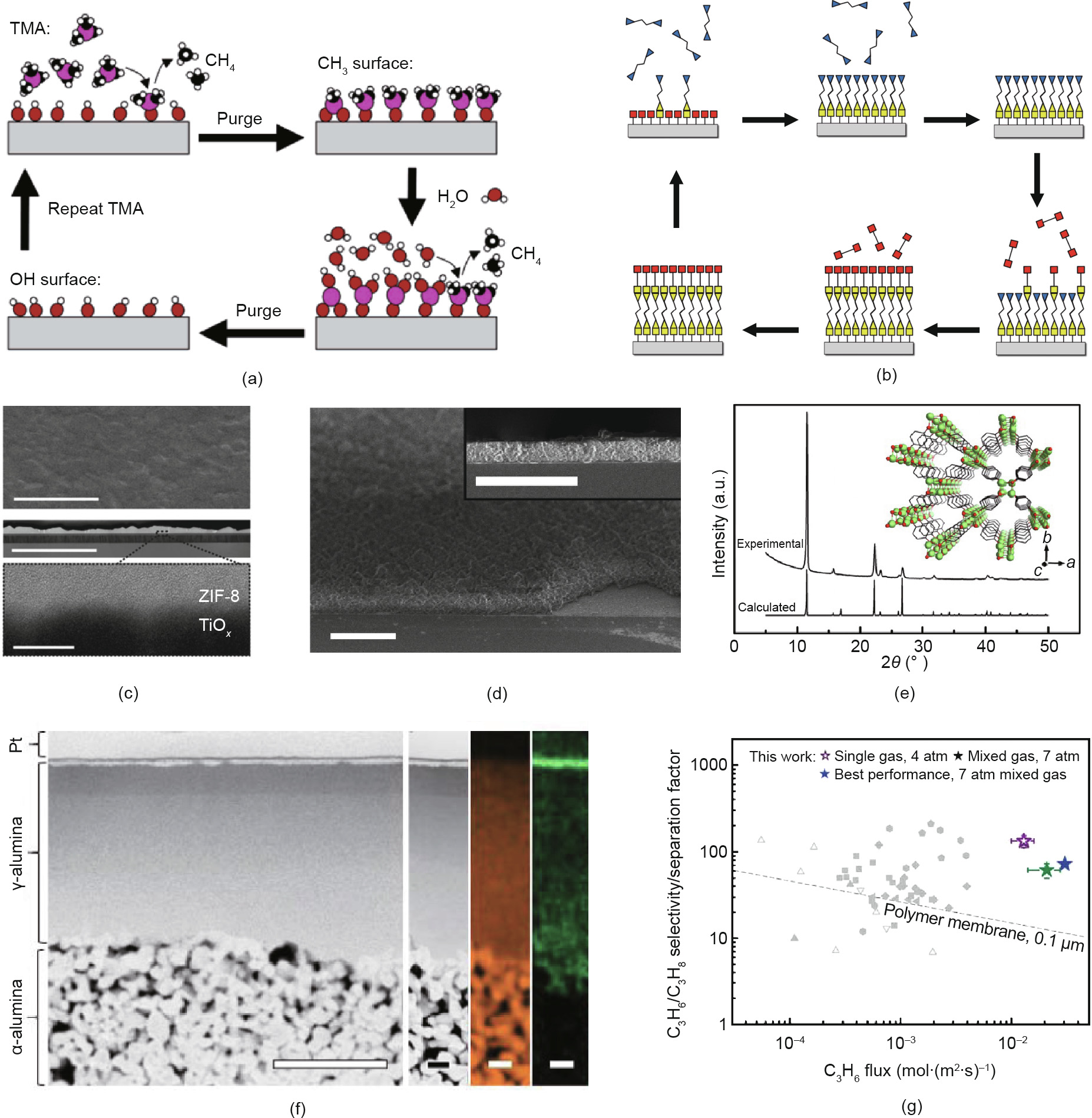
Fig. 6. Process scheme for (a) a typical ALD process [48] and (b) an MLD process [49]. (c) Scanning electron microscope (SEM) and cross-sectional TEM images of zeolitic imidazolate framework (ZIF)-8 thin film deposited on a TiOx substrate via the ALD-chemical vapor deposition (CVD) process [61]. (Scale bar in top and middle images: 2 μm; scale bar in the inset: 20 nm.) (d) SEM images of UiO-66 thin film synthesized through modified ALD [63]. (Scale bar represents 2 μm.) (e) Experimental and calculated X-ray diffraction (XRD) patterns of Li2[p-C6H4O2] thin film deposited via the ALD/MLD process; inset shows the crystal structure of Li2[p-C6H4O2] [68]. (f) Cross-sectional annular dark-field scanning transmission electron microscopy (ADF-STEM) imaging with energy dispersive X-ray spectroscopy (EDX) mapping for Al (orange) and Zn (green) for a ZIFfilled γ-alumina membrane on top of an α-alumina substrate [70]. (The large scale bar represents 2 μm; the small scale bar represents 400 nm.) (g) Separation performance for C3H6/C3H8 using the ZIF-filled alumina membrane [70]. TMA: trimethylaluminum. 1 atm = 101 325 Pa.
Molecular layer deposition (MLD) is analogous to ALD and is based on self-saturating surface reactions in repeating cycles (Fig. 6(b) [49]). This solvent-less vacuum deposition can be used to create organic polymer thin films including polyamide, polyimide, copolymers of polyimide–polyamide, polyurethane, and polyurea. In addition, using organometallic precursors and bifunctional ligands such as ethylene glycol, diamines, and dithiols, MLD can be employed to deposit "metalcone” hybrid organic–inorganic materials [49]. Similar to ALD, MLD exhibits great advantages in tuning pore size and internal functionality for membrane structures [50,51].
The use of ALD and MLD for membrane fabrication has been recently reviewed by Weber et al. [4]. Here, we simply highlight recent research efforts in synthesizing MOF thin films using ALD and MLD. Assembled from metal-containing secondary building units and organic ligands, MOFs are characterized by ordered pore structures, a large surface area, and amenability for post-synthetic modification. These characteristics result in the exceptional gas separation performance of MOF membranes, which exceeds Robeson’s upper bound defined for traditional membrane materials [52]. The motivation for developing vapor-phase deposition methods to prepare MOF membranes is to obtain ultrathin MOF skin layers with a high mass-transfer flux, which is very challenging for traditional solvothermal synthesis, and to avoid the use of organic solvent so that post-synthetic activation processes are no longer necessary.
The development of ALD/MLD processes for MOF thin films includes two main thrusts. One of these thrusts is based on the facile conversion from a metal oxide precursor to the MOF structures. Previous research has found that ALD metal oxides can act as interfacial layers for tuning MOF nucleation [53–55], form hydroxyl double salt intermediates and convert into MOFs [56– 58], or react directly with organic linkers to generate MOF films [59–61]. For example, ALD ZnO can react with 2-methylimidazole vapor to form zeolitic imidazolate framework (ZIF)-8 thin films in an ALD-chemical vapor deposition (CVD) process (Fig. 6(c)) [61]. The other thrust is to directly adapt the ALD/MLD process to synthesize MOF structures. Thus far, MOF thin films that can be directly deposited via ALD/MLD include MOF-5 [62], UiO-66 (Fig. 6(d)) [63], UiO-66-NH2 [64], Cu-benzenedicarboxylic (BDC) [65], Ca-BDC [66], Fe-BDC [67], and Li2[p-C6H4O2] (Fig. 6(e)) [68]. Ongoing research aims to further explore ALD/MLD chemistry in order to expand these processes for MOF thin films.
Taking advantage of the vapor-phase conversion from ZnO to ZIF-8, Ma et al. [70] recently reported a membrane fabrication method using ALD ZnO and subsequent ligand vapor treatment. This MOF was chosen because it possesses aperture (pore window) sizes of about 3.4 Å, which is in the appropriate range for the kinetic separation of propylene and propane [69]. A ZIF-filled γ-alumina membrane on top of an α-alumina substrate (Fig. 6(f)) enables a C3H6/C3H8 selectivity over 100 with a C3H6 permeance as high as 3.81 × 10-8 mol∙(m2 ∙s∙Pa)-1 (or 114 GPU) [70]. This excellent gas separation performance is among the best data reported for ZIF-8 and ZIF-67 membranes, and surpasses the Robeson’s upper bound for C3H6/C3H8 (Fig. 6(g)) [70], which demonstrates the great promise of ALD processes for making high-performance MOF separation membranes.
《6. Conclusion and future outlook》
6. Conclusion and future outlook
Solvent-less vapor-phase deposition methods have shown great promise in precisely modifying the nanoscale pores in membranes as well as in synthesizing ultrathin selective layers for thin-film composite membrane structures. These membranes have been applied to a wide variety of sustainable separation processes including gas separation, water filtration, oil/water separation, membrane distillation, separation of biomolecules, antifouling RO membranes, and proton-exchange membranes. In addition, membranes modified or fabricated using solvent-less vapor-phase techniques have enabled new applications in catalytic membrane reactors and biomedical sensors and devices. Table 2 summarizes the features and membrane applications of these vacuum deposition approaches used in membrane fabrication and modification. It is worth noting that these advanced synthesis methods cover a wide variety of materials ranging from organic polymers to inorganic materials. Furthermore, inorganic–organic hybrid materials such as metalcones, MOFs, and MOCNs have been successfully grown using these solvent-less vapor-phase approaches. The wide range of materials available for vacuum deposition processes offers great flexibility for the design and fabrication of membranes.
《Table 2》
Table 2 Summary of solvent-less vapor-phase deposition processes for membrane fabrication.

Most of the processes discussed here can be performed at low chamber/substrate temperatures, enabling the use of delicate substrate materials including polymers with a low glass transition temperature and melting point. Since a cooling stage is used in the iCVD and iPECVD processes, the substrate materials can be kept at room temperature or even lower temperatures. ALD and MLD process conditions usually depend on the materials to grow in order to achieve self-limiting growth in each cycle, while a great number of ALD and MLD processes are available for mild temperature conditions that can be easily applied for membranes. As mentioned above, the SLIP approach at low temperatures may affect the degree of imidization and, therefore, may affect the membrane properties. Careful selection of substrate materials may be required for the SLIP fabrication of asymmetric membranes.
Conformal deposition processes are highly desirable for precise modification of internal functionalities within pores and finetuning of pore dimensions. iCVD, ALD, and MLD have been demonstrated to produce excellent conformality in non-planar structures, such as nanoscale trenches with very large aspect ratios and tortuous pore structures. In iPECVD, the directionality of the plasma source affects the conformality of the deposited films. However, employing ultra-short plasma pulses and matching the plasmaoff time with the lifetime of the free-radical polymerization have been reported to be effective means of generating good conformality for iPECVD polymer films [71,72]. The conformality of SLIP process has not been demonstrated.
The future development of these advanced manufacturing processes should focus on new membrane materials for enhanced separation performance, novel applications beyond separations and filtrations, and reactor scale-up for continuous highthroughput production.
New membrane materials enabling high separation selectivity and large permeance are always demanded for a variety of industrial separation processes. In addition to the small gas pairs commonly tested in research papers, including CO2/CH4, CO2/N2, O2/ N2, H2/CH4, and H2/N2, new membrane materials fabricated via solvent-less vapor-phase approaches are expected to tackle more challenging and impactful separation problems, such as the separation of alkenes from alkanes, C6–C8 arenes from each other, and other realistic chemical mixtures [1]. Given that many emerging porous materials such as MOCNs, MOFs, COFs, and other covalent networks have been reported with appropriate pore sizes and affinity for specific chemical pairs, research efforts in this field should be directed to the development of novel solvent-less deposition methods for these advanced membrane materials.
In addition to exploring common membrane applications, research efforts are encouraged to explore novel applications in order to take full advantage of these advanced fabrication processes. Coupling membranes with catalysts could potentially achieve highly selective formation of chemical products while simplifying the traditional unit operations required for reaction conversion plus separation [73]. The wide variety of materials that can be deposited inside membrane pores via the abovementioned processes offers great opportunities for designing and synthesizing new membrane reactors. Applications of membranes beyond separations and filtrations also include use in biomedical devices such as artificial lungs [8] and biosensors [74], and in energystorage devices such as fuel cells [20] and Li–air batteries [75]. Their solvent-less feature and their compatibility with devicefabrication procedures in glove boxes and cleanrooms make these vacuum deposition processes promising for integrating active membranes into device structures.
The scale of manufacturing is also key to mass production. Fortunately, many large-scale roll-to-roll reactors have been developed and commercialized for solvent-less vapor-phase processes such as iCVD, ALD, and MLD (Fig. 7). For example, Fig. 7(a) and (b) [76,77] illustrate an industry-scale roll-to-roll ALD reactor with a spatially distributed precursor dose and inert gas purge outlets. These outlets enable continuous ALD deposition on the web surface as it moves along the process drum. Similar spatial design has also been reported in an effort to scale up the MLD process (Fig. 7(c)) [78]. Roll-to-roll iCVD reactors (Fig. 7(d)) [27] have been scaled up for 0.5 m wide textile and other soft materials, enabling continuous surface modification in high throughput. The design and scale-up of these vacuum reactors were motivated by the need for functional coatings and passivation layers on flexible substrates such as textiles and polymer films for soft electronics. By adapting a roll-to-roll reactor design, scaled-up reactors capable of handling large-area membrane substrates and forming high-performance membrane materials could significantly boost the advances in membrane production and make a real impact on the chemical industry.
《Fig. 7》

Fig. 7. (a, b) Spatial ALD in a commercial roll-to-roll ALD reactor for coatings on flexible substrates [76,77]; (c) a lab-scale spatial MLD reactor for large-area flexible substrates [78]; (d) a large-scale roll-to-roll iCVD reactor capable of surface modification for textiles and membranes [27]. TMC: trimesoyl chloride; mPD: m-phenylenediamine.
《Acknowledgements》
Acknowledgements
Junjie Zhao acknowledges the start-up funding supported by Zhejiang University, the research grant from the State Key Laboratory of Chemical Engineering (SKL-ChE-19T04), and the funding support from the Institute of Zhejiang University–Quzhou (IZQ2019-KJ-011). Junjie Zhao also acknowledges the funding from the National Natural Science Foundation of China (21908194 and 21938011).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Junjie Zhao and Karen K. Gleason declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




