《1. Introduction》
1. Introduction
Supramolecular chemistry, which is based on non-covalent interactions, has attracted a considerable amount of attention in recent years [1–4]. Robust supramolecular systems, including complexes and assemblies constructed by electrostatic interactions, host–guest interactions, van der Waals forces, hydrogen bonds, π–π interactions, and hydrophobic–hydrophilic interactions, have provided a range of potential applications in fields such as selfhealing materials, biosensors, and drug nanocarriers [5–7]. Among various non-covalent interactions, host–guest molecular recognition has exhibited fascinating characteristics in the introduction of different host–guest pairs. In general, a host–guest pair includes a macrocyclic host unit as the receptor and a guest unit as the ligand. The receptor and ligand interact with each other via noncovalent bonding, similar to the relationship between a ‘‘lock” and ‘‘key” [5]. Large host units with a hydrophobic or hydrophilic cavity can recognize guest units specifically, allowing the direct embedment of guest units such as organic compounds, macromolecules, metal ions, and even nanoparticles (NPs) [8,9]. There are many advantages in using macrocycle-based host–guest interactions. The host or guest molecules can be tailored with specific, targeted functional groups. Moreover, the highly selective, robust, and dynamic host–guest interaction can be exploited to fabricate hierarchical structures that can assemble and disassemble reversibly with external stimuli, including light, redox, and chemical stimuli [5,8]. To date, various synthetic host units, such as crown ethers, cucurbit[n]uril, cyclodextrins, pillar[n]arenes, and calixarenes, have been used as molecular receptors for the preparation of supramolecular objects, enabling many fascinating applications in intelligent self-assembling structures, supramolecular polymerization, and molecular machines/switches [10–16].
A liquid–liquid interface, such as an oil–water interface, provides an attractive platform for the assembly of various materials and the construction of interfacial assemblies [17]. By assembling different building blocks at the interface, the interface can be endowed with unique properties, allowing the fabrication of interfacial systems with novel functionalities that can be used in encapsulation, reactive liquid systems, and delivery vehicles [18–23]. Numerous studies have been devoted to the construction of supramolecular interfaces via electrostatic interactions or hydrogen bonding, and considerable advances have been achieved [24– 30]. On the other hand, by taking advantage of host–guest chemistry, a versatile strategy to construct dynamic interfacial systems with multiple stimuli-responsiveness can be provided.
In recent years, colloidal particles and polymers grafted with host or guest units have been exploited to form supramolecular systems at the oil–water interface, leading to the successful preparation of interfacial films, microcapsules, and structured liquids, which display potential applications in many areas including material engineering and life sciences [31–33]. Here, we briefly summarize the current development in supramolecular interfacial systems constructed by host–guest molecular recognition, including the preparation, characteristics, and applications of supramolecular colloidal interfaces, supramolecular polymeric interfaces, and supramolecular colloidal jammed interfaces that have recently emerged.
《2. Supramolecular colloidal interfaces》
2. Supramolecular colloidal interfaces
Ramsden [34] and Pickering [35] were the first to identify and describe Pickering emulsions. Generally, for a typical oil–water Pickering emulsion system, colloidal particles tend to migrate to the interface, forming either water-in-oil (w/o) or oil-in-water (o/w) emulsions, in order to minimize the interfacial energy of immiscible liquids [36]. According to the theoretical model established by Pieranski [37], with a single particle at the oil–water interface, the reduced interfacial energy, 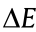 , can be described by the following formula:
, can be described by the following formula:

where r is the effective radius of the particle, and 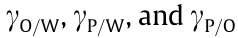 represent the interfacial tensions that arise from the oil–water interface, particle–water interface, and particle–oil interface, respectively. Eq. (1) shows that
represent the interfacial tensions that arise from the oil–water interface, particle–water interface, and particle–oil interface, respectively. Eq. (1) shows that 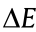 is highly correlated to the particle size. The interfacial energy decreases less for small particles than for large particles. This effect is more significant when using NPs, where
is highly correlated to the particle size. The interfacial energy decreases less for small particles than for large particles. This effect is more significant when using NPs, where 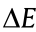 is comparable to thermal energy. As a result, thermal fluctuations can easily weaken the confinement of NPs at the interface and lead to the eventual detachment of NPs from the interface [38].
is comparable to thermal energy. As a result, thermal fluctuations can easily weaken the confinement of NPs at the interface and lead to the eventual detachment of NPs from the interface [38].
To realize the stable assembly of NPs at the oil–water interface, as well as the generation of macroscopic assemblies, it is both crucial and challenging to suppress the thermally activated desorption of NPs. The crosslinking of interfacial NPs by covalent bonds, such as ring-opening metathesis polymerization, ‘‘click” chemistry, and coordination chemistry, provides a feasible strategy to achieve this purpose [39–43]. Given the dynamic nature of assemblies at the interface, the non-covalent-interaction-mediated interfacial assembly of NPs based on host–guest interactions can be used as an alternative strategy that provides a promising pathway to generate novel functional materials.
In earlier work, Wang et al. [31] reported the fabrication of macroscopic NP monolayers at the interface using β-cyclodextrins (β-CDs) or their derivatives dissolved in water and CoPt3 NPs capped with 1-adamantylcarboxylic acid dispersed in oil. Furthermore, by introducing functional groups onto β-CDs, macroscopic heterogeneous NP multilayers were generated in a stepwise manner. In comparison with two-dimensional (2D) films, much more attention has been focused on the construction of dynamic three-dimensional (3D) colloidal microcapsules, which have broad application potential in biomedicine, cargo release, and microreactors [44–46]. In this section, we will introduce two approaches for fabricating robust colloidal microcapsules using host–guest interactions at the oil–water interface.
《2.1. Microcapsules fabricated by classical emulsion templates》
2.1. Microcapsules fabricated by classical emulsion templates
With Pickering emulsions as templates, Patra et al. [47] reported the fabrication of stimuli-responsive colloidal microcapsules via the recognition of β-CD and adamantane (ADA) at the toluene–water interface. Robust and stable microcapsules were generated by vigorously shaking an aqueous solution of β-CD-functionalized gold (Au) NPs and a toluene solution of ADA-functionalized Au NPs, forming a crosslinked NP layer as the shell of microcapsules at the interface (Figs. 1(a) and (b)). Unlike microcapsules fabricated using covalent crosslinking, the host– guest interaction endows the microcapsules with size tunability, which can be triggered by introducing the external competing guest ligand adamantane tetraethylene glycol (ADA-TEG-OH) to the system. As shown in Fig. 1(c), with the addition of ADA-TEGOH, partial disassembly of the interface is observed, resulting in the coalescence of small microcapsules and the eventual formation of larger microcapsules
《Fig. 1》
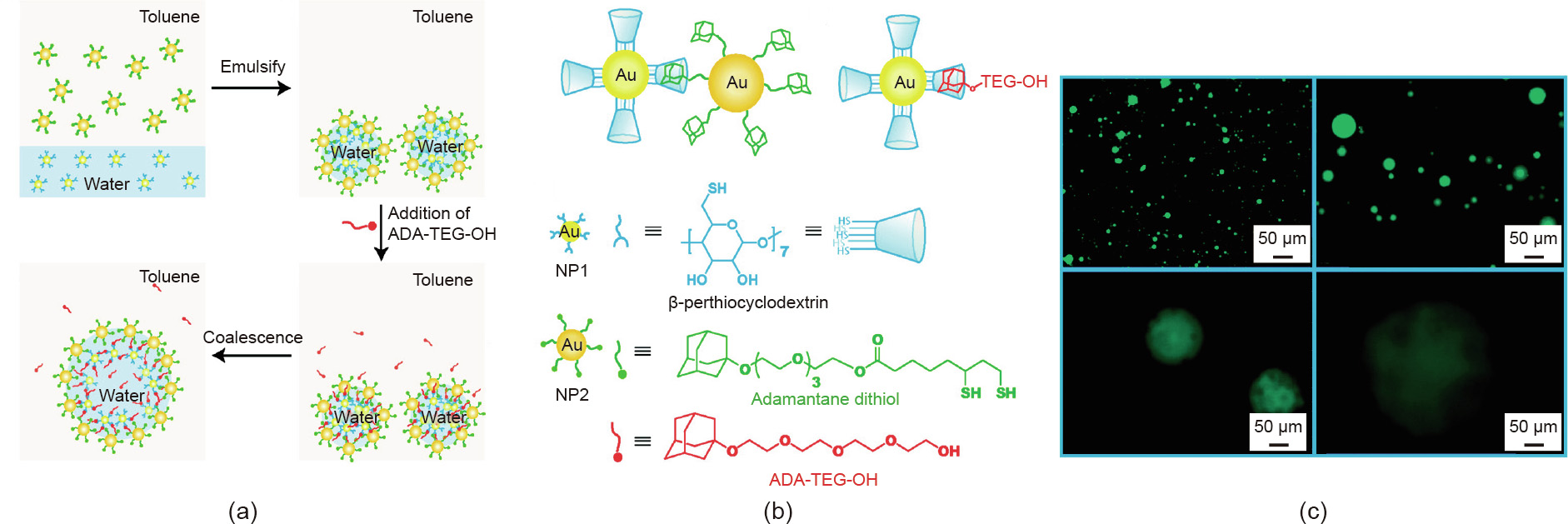
Fig. 1. (a) Schematics of the formation and size tunability of the microcapsules fabricated using classical emulsion templates. (b) NP structures modified with different ligands and the host–guest interactions in the system. (c) Disassembly and coalescence of microcapsules after adding the competing guest ADA-TEG-OH. Reproduced from Ref. [47] with permission of American Chemical Society, © 2009.
In addition to cyclodextrins, cucurbit[n]urils (CB[n], n = 5–8, 10), a large family of macrocyclic molecules, are popular as hosts and have attracted a great deal of attention. CB[n] are macrocyclic oligomers of glycoluril with a hydrophobic cavity, and the portals are surrounded by carbonyl groups. Taking advantage of the discrimination originating from different portal and cavity sizes, specific host–guest complexation can be achieved between CB[n] and different guests [1,9]. Meethal et al. [38] reported a method to generate a colloidal film and control-released microcapsules by using cucurbit[7]uril (CB[7])-mediated host–guest interactions at the chloroform–water interface. In their study, aminohexylterminated Au NPs (Hex-AuNPs) dispersed in chloroform and complementary CB[7] dissolved in the aqueous phase interacted at the interface, significantly enhancing the interfacial binding energy of the NPs and forming stable microcapsules, which could be used to encapsulate cargo (Fig. 2(a)). In addition, due to the cationic Hex-AuNPs at the interface, macromolecules with negative charge could be immobilized on the surface of the microcapsule. Fig. 2(b) shows dual cargo loading in which the fluorescein-conjugated bovine serum albumin (BSA) proteins are selectively absorbed onto the shell of the microcapsule, while the hydrophobic Nile red are encapsulated inside. Microcapsules with a dynamic nature can be achieved by introducing a competing ADA guest that has a higher affinity with CB[7] than Hex-AuNPs. Using hydrophilic doxorubicin as a model cargo, the microcapsule structure is disturbed by the addition of ADA, triggering the release of doxorubicin (Figs. 2(c)–(e)).
《Fig. 2》

Fig. 2. (a) Schematics of the preparation of microcapsules using interfacial molecular recognition between CB[7] and Hex-AuNPs. (b) Dual cargo loading of the microcapsules. (c–e) Disassembly of microcapsules and release of cargo after adding the competing guest ADA. Reproduced from Ref. [38] with permission of American Chemical Society, © 2018.
Using specific host–guest chemistries, the mechanical strength of NP films assembled at the microdroplet surface can also be effectively controlled. Jeong et al. [48] reported the fabrication of buckled microparticles using three different guest linkers dissolved in chloroform and β-CD-functionalized Au NPs dispersed in water (Fig. 3). Guest linkers in oil, taking AB-Hex-AB as an example, crosslink the interfacial NPs via the host–guest interaction, producing stable microcapsules with a robust NP membrane shell (Fig. 3(d)). When the oil phase is replaced with a mixture solution of the linker, dicyclopentadiene, and catalyst, the dicyclopentadiene in the inner phase is polymerized, generating stable microparticles with measurable buckled surfaces (Fig. 3(c)). The surface buckling of microparticles is the result of the contraction of the oil phase, which is caused by the removal of chloroform and the reduced volume of the polymer relative to its monomers (Fig. 3(e)). Finally, the researchers demonstrated that the characteristic lengths of periodic buckling, which can be used to estimate the elastic modulus by means of numerical simulations and experimental observation, are related to the binding affinities between the host NPs and the guest linkers, making manipulation of the mechanical strength of NP films possible.
《Fig. 3》
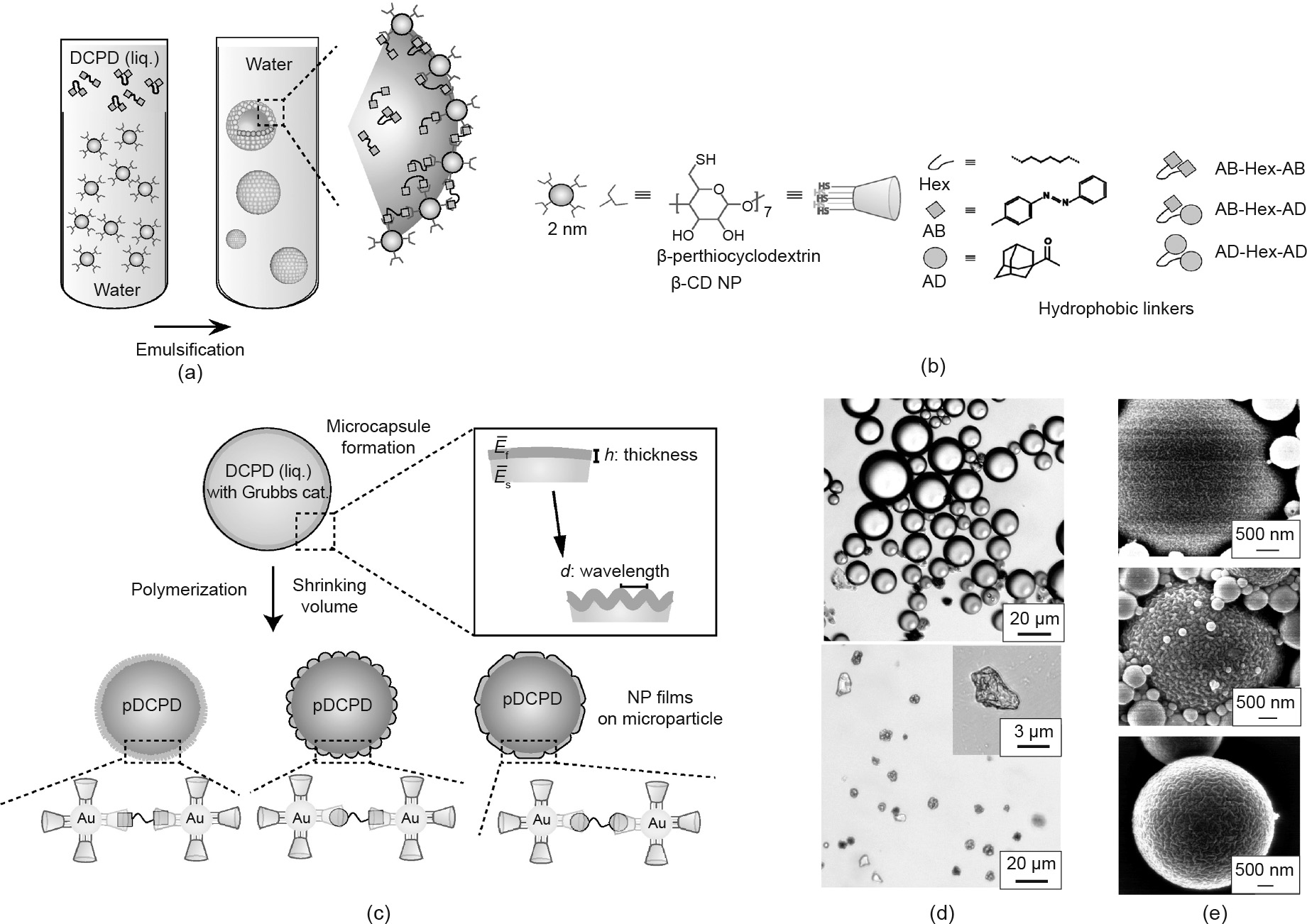
Fig. 3. (a) Schematics of the formation of microcapsules. (b) Structures of β-CD-functionalized Au NPs and different guest linkers. (c) Fabrication of microparticles with buckled surfaces by polymerizing the inner phase. (d) Optical images of the microcapsules before (top) and after (bottom) drying in chloroform. (e) Scanning electron microscopy (SEM) images of microparticles with specific bulking patterns when using different guest linkers (top: AB-Hex-AB; middle: AB-Hex-AD; bottom: AD-Hex-AD). AB: azobenzene; AD: 1-adamantyl methyl ketone; DCPD: dicyclopentadiene; pDCPD: poly(dicyclopentadiene); 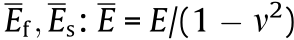 , E is the elastic modulus, v is the Poisson’s ratio, and the subscripts f and s represent film and substrate, respectively; liq.: liquid; cat.: catalyst. Reproduced from Ref. [48] with permission of WILEY-VCH Verlag GmbH & Co. KGaA, © 2014.
, E is the elastic modulus, v is the Poisson’s ratio, and the subscripts f and s represent film and substrate, respectively; liq.: liquid; cat.: catalyst. Reproduced from Ref. [48] with permission of WILEY-VCH Verlag GmbH & Co. KGaA, © 2014.
《2.2. Microcapsules fabricated by microfluidic devices》
2.2. Microcapsules fabricated by microfluidic devices
In general, colloidal microcapsules made by classical emulsification approaches tend to have a fairly broad size range distribution [49]. To decrease the size distribution, many investigators have begun to use microfluidics to generate microcapsules [50–52]. Zhang et al. [32] reported a one-step method to generate hollow and uniform microcapsules using host–guest chemistry and a four-channel microfluidic device (Fig. 4). In their study, microcapsules were produced when the oil phase sheared off the water phase, which contained three materials: CB[8], methyl viologen (MV)-functionalized Au NPs, and naphthol-functionalized polymers (Fig. 4(a)). Au NPs tend to segregate to the water–oil interface, directing host–guest recognition with the complementary polymers and resulting in the formation of the microcapsule membrane. The monodispersed microcapsules can be easily separated after the water droplets evaporate, and maintain a spherical shape in the subsequent rehydration process (Figs. 4(e) and (f)). During the formation of the microcapsules, the highyield encapsulation of different cargos can be easily achieved (Figs. 4(b)–(d)). Also, due to the dynamic nature of the host–guest interactions, the supramolecular interface can be disrupted by external stimuli, and the on-demand release of cargos can be triggered.
《Fig. 4》
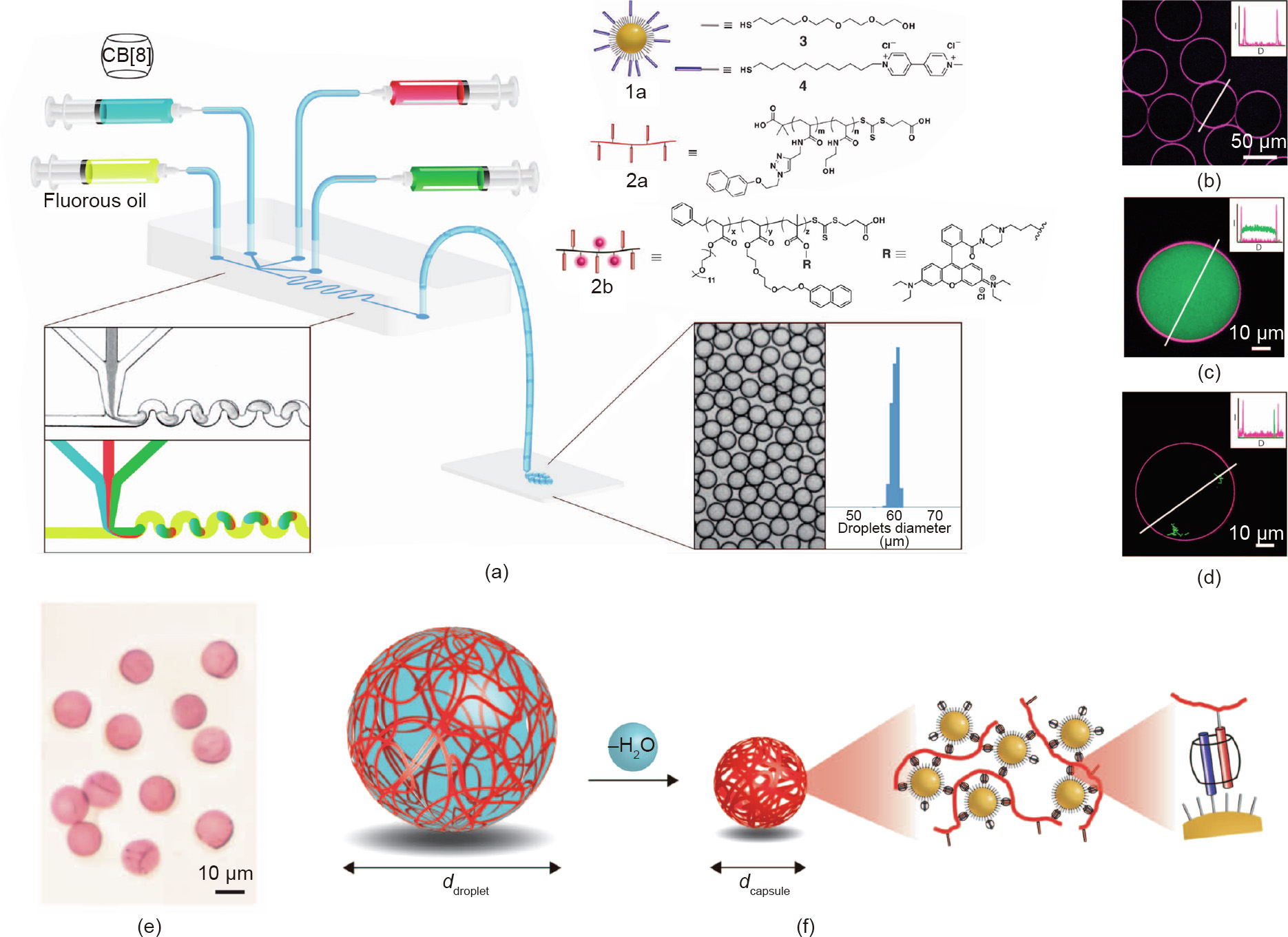
Fig. 4. (a) Schematics of the formation of microdroplets using microfluidic devices. (b) Laser scanning confocal microscope images of empty microcapsules with 2b assembled at the interface. Laser scanning confocal microscope images of (c) microcapsules with fluorescein isothiocyanate labeled dextran (FITC-dextran) or (d) Escherichia coli cells encapsulated inside. (e) Optical images of microcapsules. (f) The dehydration process for microcapsules. UVA: ultraviolet A. Reproduced from Ref. [32] with permission of American Association for the Advancement of Science, © 2012.
Subsequently, the same group [53] extended this method to a CB [8]-mediated supramolecular system, in which MV-functionalized NPs were reversibly crosslinked via a naphthol-functionalized polyacrylamide linker at the oil–water interface to produce stimuli-responsive microcapsules. Water-soluble cargo could be encapsulated during the formation of microcapsules, which could be triggered to release by adding a competitive guest to disrupt the supramolecular shell. The researchers also prepared dual-responsive supramolecular colloidal microcapsules in a similar way [54]. In that study, the shell of the microcapsules was a CB[8]-mediated complex consisting of thermo-responsive particles functionalized with MV (MCP) and photoresponsive azobenzene (Azo)-functionalized poly(vinyl alcohol) (AP) (Fig. 5(a)). Poly (N-isopropylacrylamide) (PNIPAM), which is responsive to temperature, was used to form the shell of the particles. Above the lower critical solution temperature (LCST) of PNIPAM, the shell of the particles collapsed with increasing temperature, introducing larger gaps between particles and leading to the release of the encapsulated cargo (Fig. 5(b)). Moreover, AP imparted the microcapsules with photoresponsiveness. Upon exposure to ultraviolet (UV) light, the photoisomerization of Azo units caused the dissociation of supramolecular complexes at the interface, and a light-triggered release of the cargo was achieved (Fig. 5(c)). This bottom-up assembly approach for fabricating microcapsules makes it possible to obtain a wide range of uniform and intelligent microencapsulation materials.
《Fig. 5》
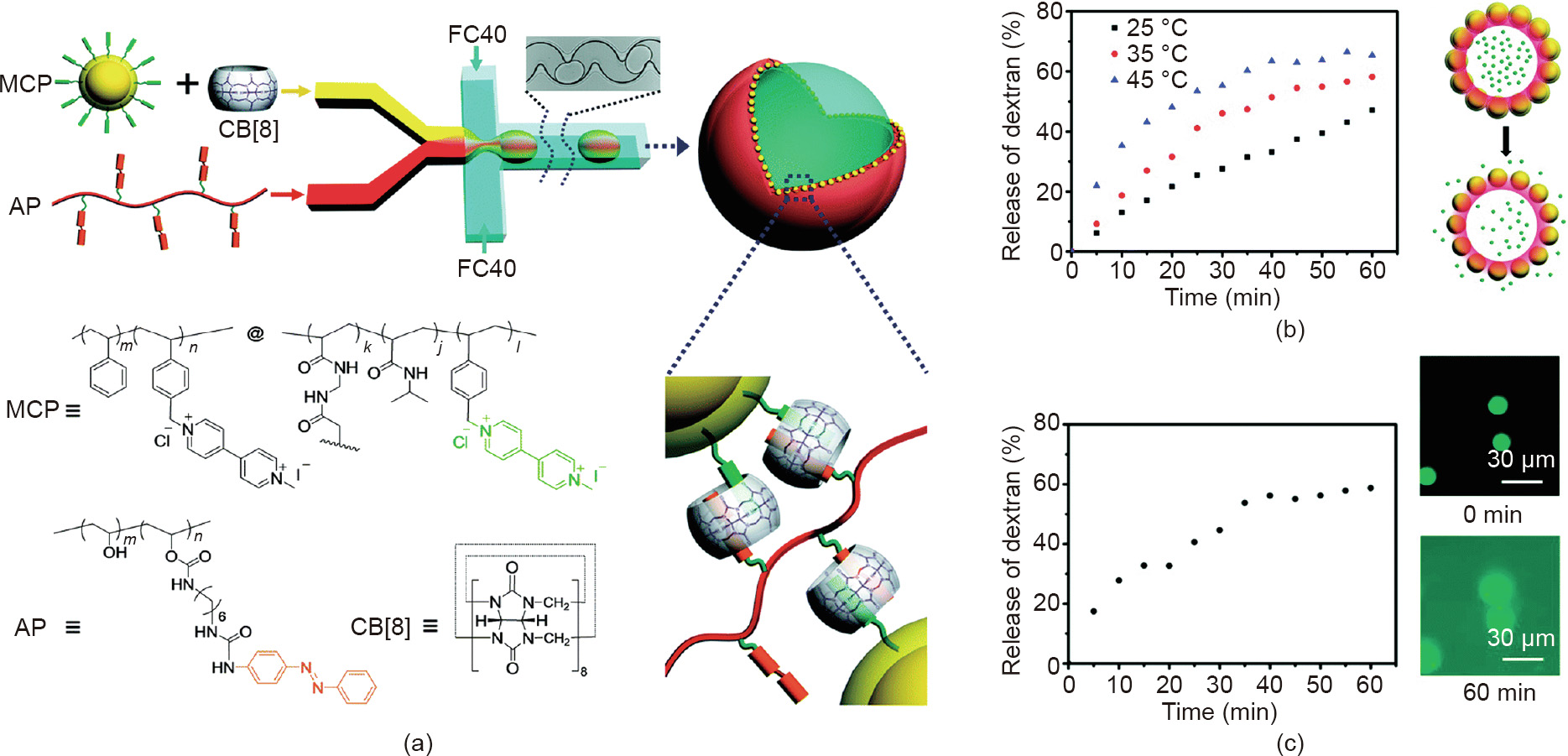
Fig. 5. (a) Schematics of the generation of CB[8]-mediated supramolecular microcapsules and structures of MCP, AP, and CB[8]. (b) Thermal-controlled cargo release. (c) Photo-controlled cargo release. FC40: flouriner FC-40 (3M, USA). Reproduced from Ref. [54] with permission of the Royal Society of Chemistry, ©2016.
《3. Supramolecular polymeric interface》
3. Supramolecular polymeric interface
The unique properties of polymers and the rapid progress that has been achieved with them have promoted the emergence of hollow structures formed by polymer-only materials. Layer-bylayer (LbL) self-assembly provides a successful strategy to construct polymeric microcapsules with multilayers utilizing particles as templates, driven by electrostatic interaction, hydrogen bonding, base-pair interactions, or host–guest interactions [55–60]. However, the typical LbL method is usually procedurally complex and time consuming, as polymers is sequentially deposited onto a sacrificial template. Zheng et al. [61] reported the interfacial self-assembly of two copolymers functionalized with different guest groups and the generation of multilayer supramolecular microcapsules by taking advantage of host–guest interactions mediated by CB[8] and microfluidic devices (Fig. 6). In their study, P1(MV-containing copolymer) and CB[8] were dissolved in the water phase, while P2 (naphthol-containing copolymer) was dissolved in chloroform, forming the ternary supramolecular complex at the interface, and leading to the production of microcapsules within a microfluidic device (Figs. 6(a) and (b)). Microcapsules can be generated using either chloroform-in-water or water-inchloroform droplets. Fluorescein-labeled P1 (green fluorescence) and Rhodamine B-labeled P2 (red fluorescence) assemble at the interface of the microdroplet to form the double layer of microcapsule shell (Fig. 6(d)). In addition, when replacing P2 with highly branched H1 during the formation of microcapsules, the microcapsules produced can be used to encapsulate hydrophilic small molecules due to the unique 3D and dendritic nanoscale structure of H1 (Fig. 6(c)). Under UV irradiation, the multilayer microcapsule skin is disassembled by the photoisomerization of Azo units, triggering the release of small molecule cargo. Owing to the diversity of synthetic polymer materials, the properties and functions such as shell thickness, permeability, and the restriction of molecular weight for cargos can be well controlled. More importantly, the double-layer microcapsules provide a powerful way to quantitatively understand the mechanism and kinetics of the molecular recognition of prefabricated polymers at the interface, which is of great significance in the chemical or biological fields.
《Fig. 6》
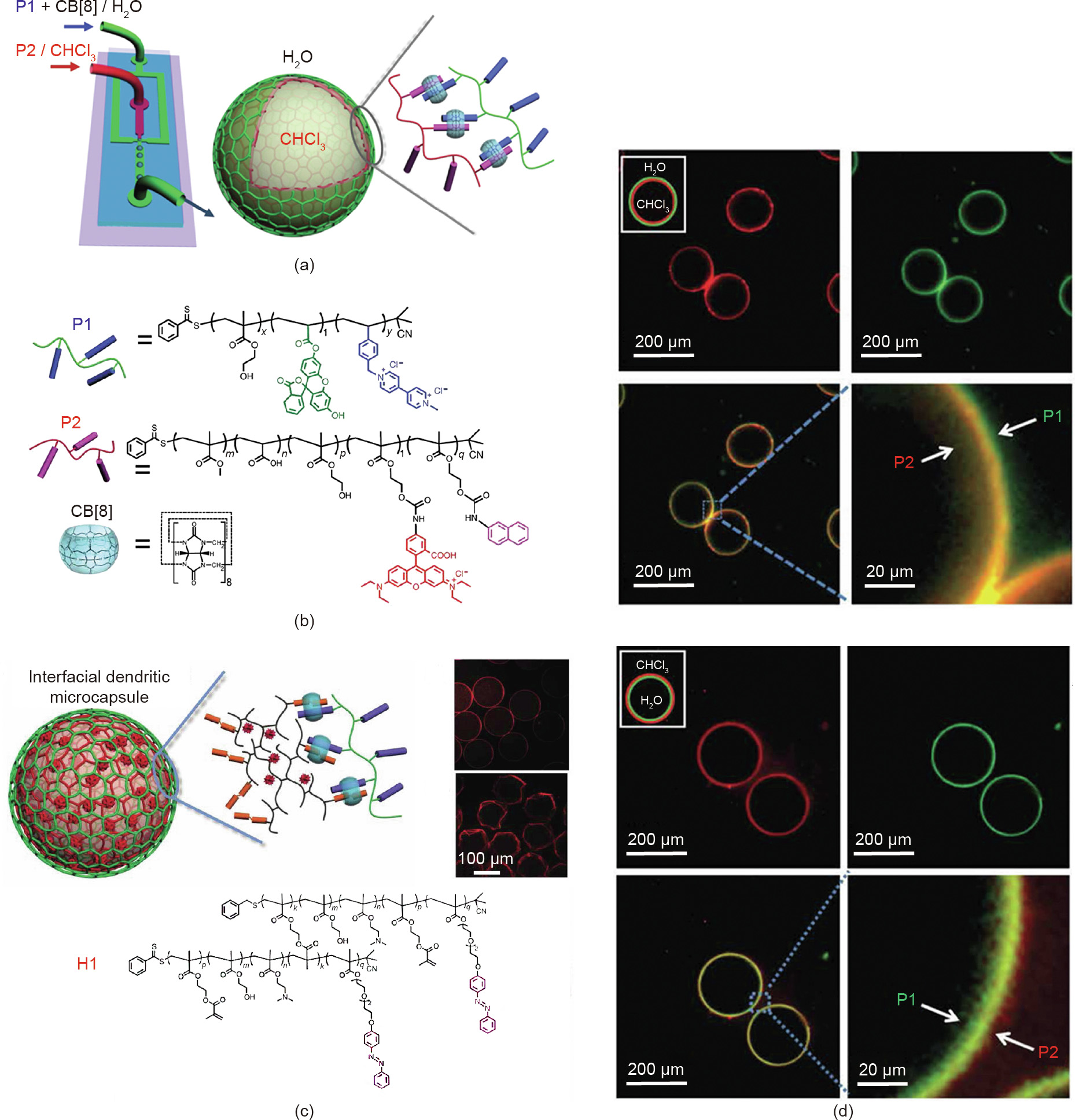
Fig. 6. (a) Schematics of microcapsule generation. (b) Structures of the P1, P2, and CB[8]. (c) Schematics of microcapsules prepared using dendritic copolymer H1 as one of the interfacial components. (d) Laser scanning confocal microscope images of the monodisperse microdroplets generated using either chloroform-in-water or water-in-chloroform droplets. Reproduced from Ref. [61] with permission of Springer Nature, © 2014.
Parker et al. [62] reported another strategy to construct the supramolecular polymeric shell of microcapsules, in which the microcapsule-forming materials are directly segregated to the oil–water interface by electrostatic interactions. In their study, aqueous microdroplets were fabricated using a microfluidic device. The continuous oil phase contained a charged surfactant and the dispersed aqueous phase consisted of two complementary charged copolymers functionalized with guests and CB[8] (Figs. 7(a) and (b)). The low concentration of the copolymer solution prevents gelation of the aqueous phase after adding CB[8]. Charged surfactants assemble at the oil–water interface first, making the interface positively charged or negatively charged. Then, copolymers with complementary charges are attracted to the interface due to electrostatic interactions, forming robust interfacial host–guest complexes that lead to the generation of supramolecular microcapsules (Fig. 7(c)). With only anionic copolymer 1A(-) dissolved in the aqueous phase, as the concentration of the positively charged surfactant K(+) increases, more 1A(-) segregates to the interface. However, when using a negatively charged surfactant K(-) , 1A(-) tends to be uniformly dissolved in the droplet, demonstrating that the interfacial assembly of the copolymer is driven by electrostatic interactions (Figs. 7(d) and (e)). With CB[8], 1A(-) , and 1B(-) dissolved in the aqueous phase, as the concentration of the positively charged surfactant K(+) increases, a morphology variation from microparticles to robust microcapsules can be achieved during the evaporation of microdroplets (Fig. 7(e)). Moreover, with a mixture of competing copolymers dissolved in the aqueous phase, more complex architectures can be obtained. As shown in Fig. 7(f), with 1A(-) , 1B(-) , 2A(+), 2B(+), and CB[8] dissolved in water, only homogenous microparticles can be obtained with a neutral surfactant dissolved in the oil. With K(+) dissolved in the oil, microcapsules with a 1A(-) –CB[8]–1B(-) shell and a 2A(+)–CB[8]–2B(+) hydrogel core can be generated. Microcapsules with an inverted structure can also be produced by replacing K(+) with K(-) .
《Fig. 7》

Fig. 7. (a) Optical image of the generation of microdroplets using a microfluidic device. (b) Schematics of the prepared microcapsules. (c) Optical image of microcapsules after drying. (d) Structures of functionalized copolymers and charged surfactants. (e) Laser scanning confocal microscope images (top) of aqueous microdroplets containing 1A(-) with the increasing concentration of K(+); optical images (bottom) of evaporated aqueous microdroplets containing 1A(-) , 1B(-) , and CB[8] with the increasing concentration of K(+). (f) Distribution of functional polymers with different fluorescence in microdroplets (left), with the aqueous phase containing 1A(-) , 1B(-) , 2A(+), 2B(+), and CB[8] and the oil phase dissolving different surfactants; resultant microdroplet morphology during evaporation (right). Reproduced from Ref. [62].
Using a similar strategy, Yu et al. [63] reported the generation of polymeric microcapsules with UV-controlled permeability by means of host–guest and electrostatic interactions, and cargo release was triggered from the microcapsules when a competitive ADA guest was added (Figs. 8(a)–(d)). In general, the permeability of an interfacial shell after microcapsule generation is unchangeable. However, in this study, the researchers overcame this obstacle by regulating the covalent crosslinking degree of the guests inside the CB[8]. Upon exposure to UV light, two anthracene groups (guests) embedded in the CB[8] transformed to a [4 + 4]- photo-dimer, providing a strategy to manipulate the permeability of the microcapsule shell. As shown in Figs. 8(e) and (f), the release rate of cargo can be significantly reduced during longer UV irradiation.
《Fig. 8》

Fig. 8. (a) Schematics of the generation of microcapsules via microfluidic devices and the structure of the supramolecular polymeric shell at the interface (anthracenefunctionalized hydroxyethyl cellulose (Ant-HEC)). (b) Optical images of dried microcapsules with cargo encapsulated inside. (c) Optical and (d) fluorescent images of rehydrated microcapsules in ADA solution. (e) Schematics of the disassembly of supramolecular assembly upon the addition of ADA and the [4 + 4]-photo-dimerization of anthracene groups in the cavity of CB[8]. (f) Release rate of cargo at different UV irradiation times. Reproduced from Ref. [63] with permission of the Royal Society of Chemistry, © 2015.
Charged polymer micelles and supramolecular hyperbranchedlike polymers (SHPs) were also used to locate materials at the interface of a microdroplet directed by electrostatic interactions, forming a non-covalently crosslinked interfacial layer via CB[8]- mediated host–guest interaction [64,65]. Yu et al. [64] fabricated novel hierarchical microcapsules made by polymer micelles selfassembled from linear amphiphilic block copolymers (Fig. 9(a)). For the prepared microcapsules, the ability of dual cargo loading is quite attractive, and can be achieved in two steps. The first step is the fabrication of micelles in water, where the hydrophobic cargo (Nile red, red fluorescence) is loaded within the hydrophobic core of the micelles. The second step is the assembly of microcapsules, where a water-soluble cargo (fluorescein isothiocyanate labeled dextran (FITC-dextran), green fluorescence) is encapsulated into the microcapsules (Figs. 9(b)–(d)). This strategy allows the microcapsules to load hydrophobic cargo in the shell and encapsulate hydrophilic cargo in the core simultaneously, and release the cargos synergistically under external multi-stimuli.
《Fig. 9》
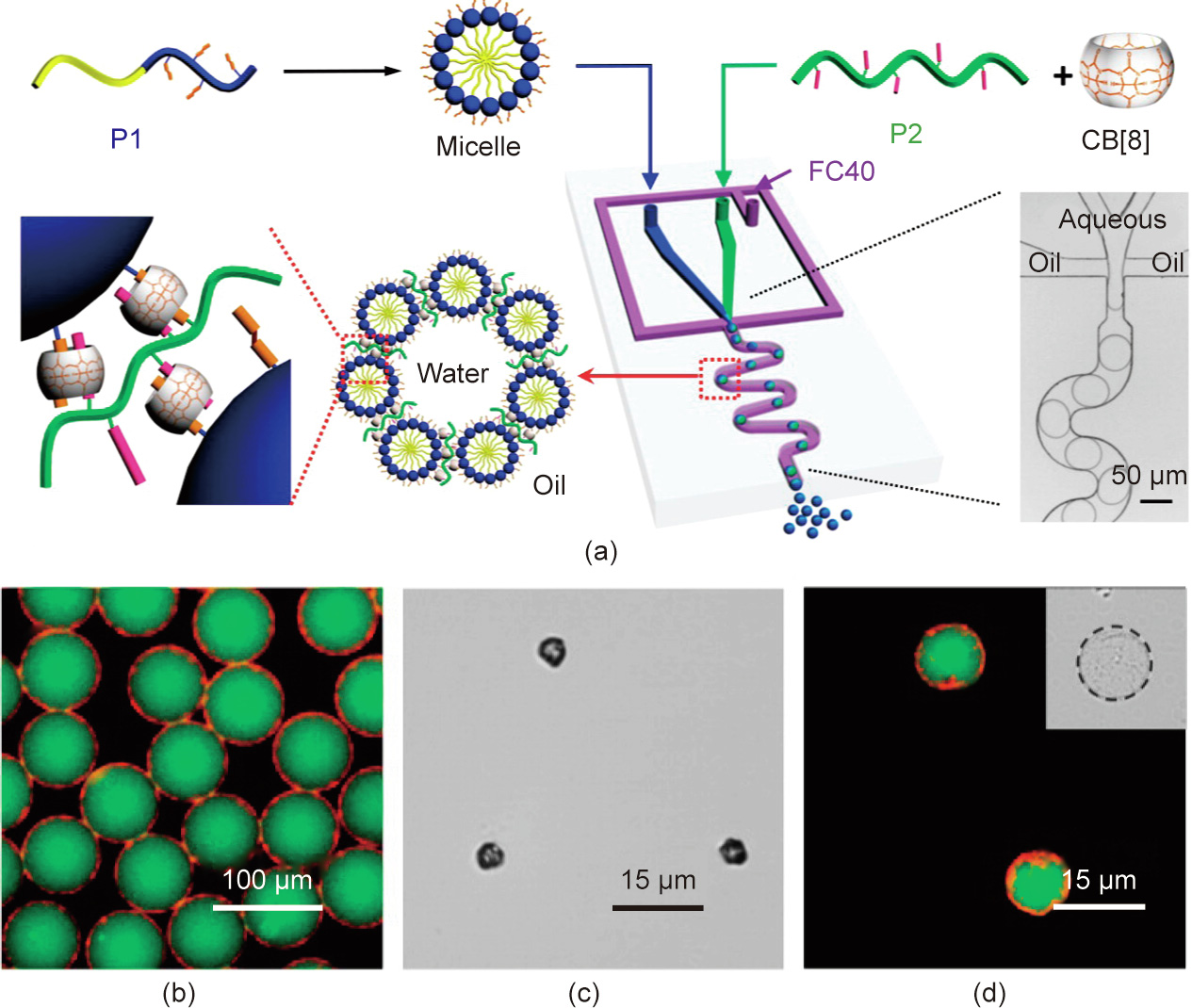
Fig. 9. (a) Schematics of supramolecular microcapsules with hierarchical structure. (b) Fluorescent image of hierarchical microcapsules with hydrophobic cargo (Nile red) loaded in the shell and water-soluble cargo (FITC-dextran) encapsulated in the core. (c) Optical image of dried microcapsules and (d) fluorescent image of rehydrated microcapsules. Reproduced from Ref. [64].
Groombridge et al. [65] reported the preparation of aqueous interfacial gels based on SHPs. The cationic SHP is prepared from small molecules by supramolecular polymerization, which is dual responsive, exhibiting dynamic properties upon exposure to UV light or the addition of competitive guests. Also, no supramolecular precipitation can be observed at low concentrations of SHP. Using microfluidic devices, interfacial gels of SHP can be generated in the presence of an anionic surfactant dissolved in the oil (Figs. 10(a)–(d)). Measurements investigated using a pendant drop demonstrated that the interfacial system undergoes a gel transformation within a very short time (~2 s) (Fig. 10(e)). The buckled interface recovers to a smooth shape within a minute due to the self-healing property of the supramolecular interfacial gel. In addition, buckling is not observed after adding ADA as a competitive guest to disassemble the supramolecular polymers. This work presented a method to synthesize stimuli-responsive SHPs and construct a dynamic interfacial gel that can be used as a barrier to suppress the coalescence of microdroplets. Furthermore, Salmon et al. [66] confirmed that phase transformation (gelation) occurred under compression, leading to the buckling at the interface. During evaporation, the density and thickness of the interfacial film increase until the critical density is reached.
《Fig. 10》
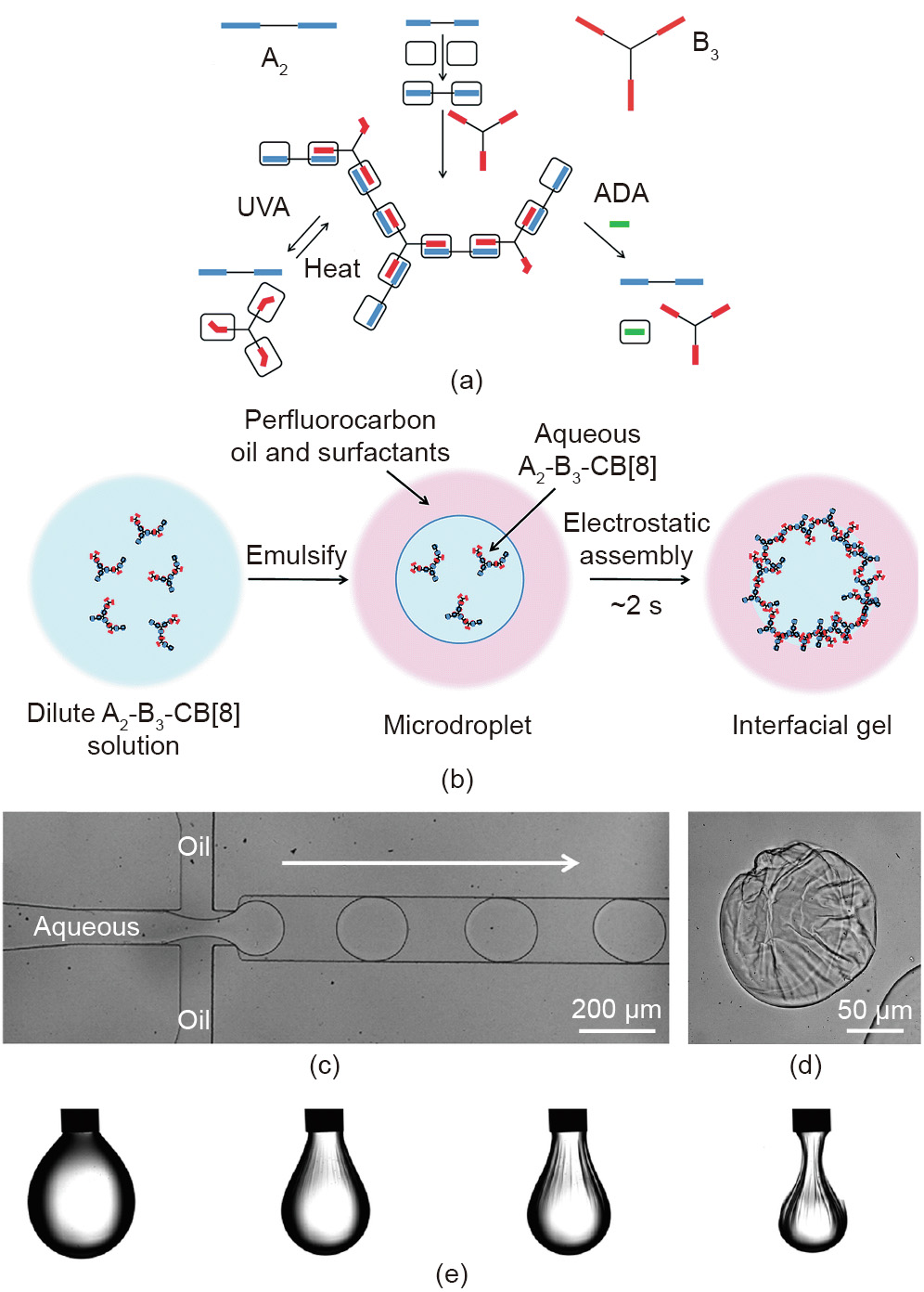
Fig. 10. (a) Schematics of the preparation of dual-responsive SHP. (b) Schematics of the assembly process from dilute A2–B3–CB[8] solution to an interfacial supramolecular gel, directed by electrostatic interaction. (c) Schematics of the generation of monodisperse microdroplets via microfluidic devices. (d) Optical image of the dried microdroplets. (e) Morphology evolution of the pendent droplet under compression. UVA: ultraviolet A. Reproduced from Ref. [65] with permission of the Royal Society of Chemistry, © 2017.
Engineered patterned surfaces provide an attractive pathway for self-assembly, due to characteristics such as high specificity, controlled affinity, and reversibility [67,68]. Recently, Zhang et al. [69] presented a method to generate patterned microcapsule arrays with a supramolecular shell, which can be used for delivery systems and sensors. In this study, a positively charged supramolecular complex consisting of CB[8]-threaded highlybranched polyrotaxanes (HBP-CB[8]) and hydroxyethyl cellulose with naphthyl moieties (HEC-Np) was formed initially by host– guest interaction in the sessile droplet. When a layer of oil containing a complementary charged surfactant covered the surface of the microdroplet, the complex was drawn to the oil–water interface, driven by electrostatic interactions. A robust hydrogel shell formed at the interface, which could be used to encapsulate cargo (Figs. 11(a)–(c)). Taking the linear analogue poly(N-hydroxylethyl acrylamide-co-methyl viologen-styrene) as a control, it was found that microcapsule patterns made by highly branched HBP-CB[8] had a better sustained release effect under the external stimulus (Fig. 11(d)). Moreover, when Au NPs were loaded, the prepared microcapsule substrates could be used for surface-enhanced Raman spectroscopy sensing. The microcapsule arrays fabricated by this simple self-assembly can be responsive to multiple stimuli including competitive guests, light, and temperature.
《Fig. 11》
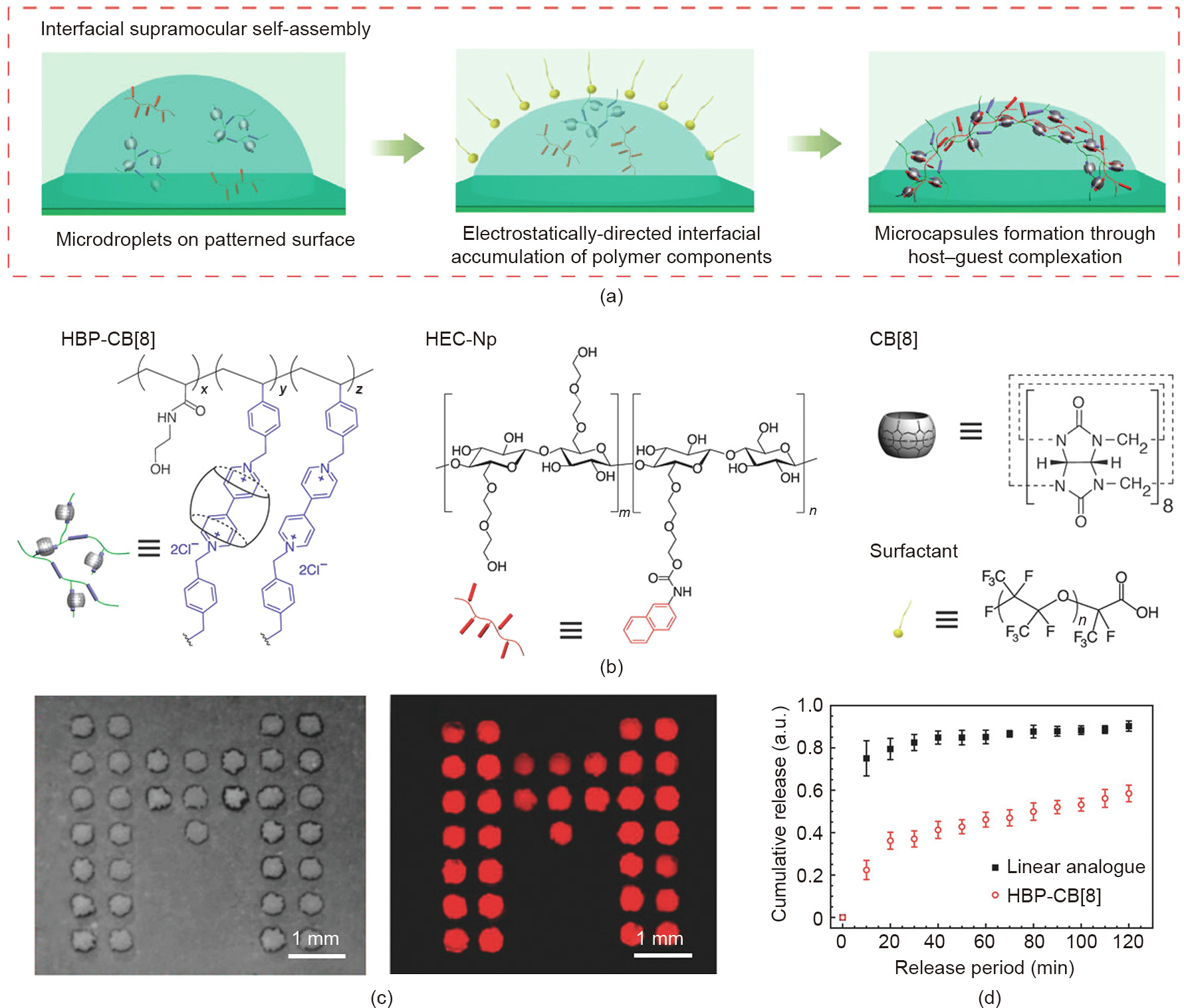
Fig. 11. (a) Schematics of the generation of patterned microcapsule arrays. (b) Structures of HBP-CB[8], HEC-Np, CB[8], and anionic surfactant. (c) Optical and fluorescent images of cargo-loaded microcapsules in a bright field. (d) The cargo release profiles of microcapsule arrays. Reproduced from Ref. [69].
《4. Supramolecular jammed colloidal interface》
4. Supramolecular jammed colloidal interface
Interfacial jamming, which is caused by the increased density of colloidal particles at the liquid–liquid interface, generates crowded interfacial assemblies with a loss of mobility [26]. In the jammed state, NPs are densely aggregated at the interface, transforming the interface monolayer from ‘‘liquid-like” to ‘‘solid-like,” accompanied by increased mechanical properties [70–74]. Using the interfacial jamming of nanoparticle surfactants (NPSs), which are formed via cooperative assembly between NPs and polymer ligands at the oil–water interface, a strategy to structure liquids was reported [24,75,76]. Tremendous applications of structured liquids in encapsulation, delivery systems, and all-liquid chemical reactors have been explored in recent years [77–81]. At present, however, electrostatic interaction has been the dominant force used for the formation and assembly of NPSs. As a result, only pH-, ionic strength-, and temperature-responsiveness can be achieved.
Recently, Sun et al. [33] reported a new type of photoresponsive NPSs based on host–guest chemistry at the oil–water interface and realized the construction of photoresponsive structured liquids. In this study, α-CD-functionalized Au NPs are dispersed in water and Azo-terminated polystyrene (Azo-PS) or Azo-terminated poly(L-lactide) (Azo-PLLA) are dissolved in oil, forming NPSs at the interface (Fig. 12(a)). In comparison with the system using Azo-PS as the ligand, the Azo-PLLA-based NPSs are more interfacially active due to the hydrogen bonding interaction between poly(L-lactide) (PLLA) and water/α-CD. The hydrogen bonding locates NPs and ligands at the interface, increasing the probability of collision between host and guest units, which leads to an enhanced binding energy of NPs at the interface (Fig. 12(b)). With photoresponsive NPSs at the interface, the jamming and unjamming of NPSs can be reversibly controlled using light as the trigger, which can be demonstrated by the morphology change of the pendant drop with jammed NPSs at the interface. As shown in Figs. 12(c) and (d), under visible light, no morphology changes of the wrinkled droplet are observed. Under UV irradiation, the wrinkles on the droplets disappear, and the droplet shape returns to spherical (an unjammed state of NPSs), which can be re-jammed under visible light. Photoresponsiveness of structured liquids can also be achieved using a droplet with a more complex shape (Fig. 12(e)).
《Fig. 12》
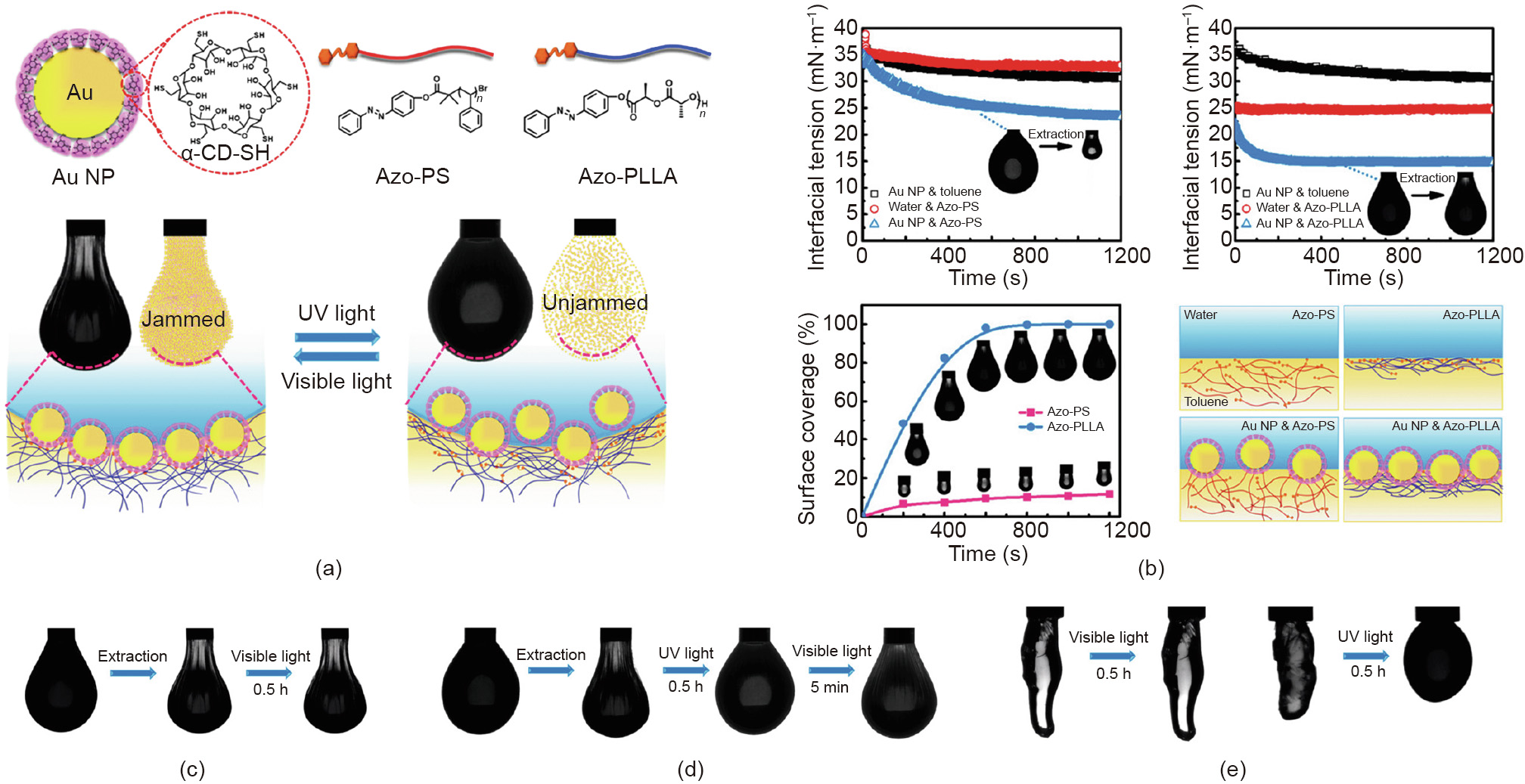
Fig. 12. (a) Schematics of the formation of photoresponsive NPSs. (b) Assembly kinetics of photoresponsive NPSs using either Azo-PS or Azo-PLLA as the ligand. (c, d) Morphology evolution of the pendent droplet with jammed NPSs at the interface under different light (visible light or UV light). (e) Morphology evolution of the highly deformed microdroplet under visible light or UV light. α-CD-SH: thiolated α-cyclodextrin. Reproduced from Ref. [33] with permission of American Chemical Society, ©2020.
《5. Conclusion and outlook》
5. Conclusion and outlook
In this review, we summarized recent developments in host–guest chemistry at liquid–liquid interfaces, provided a brief overview of the self-assembly strategy of various supramolecular interfaces, including colloidal and polymeric interfaces, described the unique properties of these interfaces, and emphasized applications in encapsulation and cargo release. Host–guest molecular recognition provides a powerful approach for the construction of a dynamic interface, allowing the resultant assemblies to be structurally manipulated in size and shape; thus, it opens up a pathway to construct smart supramolecular systems with interfacial multiresponsiveness.
In comparison with numerous studies on supramolecular systems related to host–guest chemistry, studies focused on liquid– liquid interfaces are much sparser, leaving a large workspace that can be exploited. Fabricating supramolecular interfacial systems with well-defined structures, permeability, and mechanical strength is still challenging. The size of the microcapsules fabricated using either Pickering emulsions or the microfluidic technique is usually large, which limits their efficiency when used for cargo delivery. Future studies need to focus on producing microcapsules with exceptionally small sizes, down to the nanoscale. In addition to oil–water systems, host–guest molecular recognition can be extended to aqueous two-phase systems (ATPSs), which show promising applications in areas including biology, cosmetics, and food. By using the interfacial jamming of colloidal particles and host–guest chemistries, it should be possible to produce structured all-liquid systems by means of 3D printing or all-liquid molding, which can be used to prepare complex microfluidics and biphasic reaction systems. Solving these issues will be beneficial for the production of next-generation dynamic soft materials with novel functions.
《Acknowledgements》
Acknowledgements
This work was supported by National Natural Science Foundation of China (51903011). Thomas P. Russell was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division under Contract No. DE-AC02-05-CH11231 within the Adaptive Interfacial Assemblies Towards Structuring Liquids program (KCTR16).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Beibei Wang, Hao Chen, Tan Liu, Shaowei Shi, and Thomas P. Russell declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




