《1. Introduction》
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common liver diseases worldwide. The current global incidence is 20%–33%, but this number is rapidly increasing [1,2]. NAFLD is commonly defined by a person having hepatic fat infiltration > 5% of hepatocytes on liver biopsy specimens, without excessive alcohol intake (less than 20 and 30 g·d–1 for women and men, respectively) and no evidence of viral, autoimmune, or drug-induced liver disease [3]. NAFLD refers to a spectrum of liver disorders, ranging from simple fat infiltration (steatosis) to non-alcoholic steatohepatitis (NASH) [4].
NAFLD initiates with excessive lipid accumulation in the liver. Potential sources of absorbed lipids include fatty acids (FAs) released by peripheral adipose tissue, lipids synthesized by de novo lipogenesis in the liver, and dietary FAs [5]. Lipids are mainly eliminated through FA oxidation and triacylglycerol secretion as lipoprotein particles [6]. Normally, the amount of lipid influx into the liver equals the hepatic lipid disposal. When the balance is disturbed, NAFLD occurs. Subsequently, steatosis combined with multiple other factors, including inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress, leads to hepatocyte injury. Without immediate and effective treatment, NAFLD can eventually progress into fibrosis, cirrhosis, and even hepatocellular carcinoma, which is the second most common type of cancer to cause death worldwide [7]. However, there is currently no definitive medical treatment for steatosis, other than lifestyle modifications such as diet interventions, regular exercise, and weight loss [8]. Therefore, it is urgent to find effective therapies for ameliorating NAFLD.
The gut and liver communicate via tight bidirectional links through the biliary tract and portal circulation that are referred to as the gut–liver axis [9,10]. In addition to oxidative stress [11], insulin resistance [12], and inflammation [13], which are considered to be major contributors in the pathogenesis of NAFLD, the gut microbiota play a critical role [14]. Under normal physiological conditions, the gut microbiota maintain intestinal homeostasis by facilitating nutrient digestion, metabolism, immune modulation, and barrier protection; this combination of functions has earned the gut microbiota the moniker of ‘‘a forgotten organ” [15]. Dysbiosis of the gut microbiota can lead to a series of problems, including overgrowth of detrimental bacteria, increased gut barrier permeability, bacterial translocation, and the flow of metabolites to the liver, thus initiating and aggravating NAFLD [16].
Emerging research is showing that manipulation of the gut microbiota could be a viable therapeutic strategy for the treatment of NAFLD [17]. In particular, probiotics have already demonstrated their potential for restoring homeostasis and ameliorating gut microbiota-related diseases [18]. This review focuses on reviewing the therapeutic efficacy of probiotics in treating NAFLD by modulation of the gut microbiota. In addition to the traditional probiotics Bifidobacterium and Lactobacillus, the newly reported nextgeneration probiotics (NGPs) will also be discussed.
《2. The role of the gut microbiota in NAFLD pathogenesis》
2. The role of the gut microbiota in NAFLD pathogenesis
The most dominant commensal organisms of the gut microbiota belong to the phyla Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. The structure and composition of the gut microbiota may be influenced by various factors, such as age, diet, health, or disease. The microbiota can aggravate NAFLD through various mechanisms, including by altering the energy harvesting capacity from the diet, influencing de nova lipogenesis and shortchain FAs (SCFAs) synthesis pathway, altering choline and bile acid metabolic signaling pathways, increasing intestinal permeability and inflammation, and producing endogenous ethanol in the gut [19] (Fig. 1).
《Fig. 1》
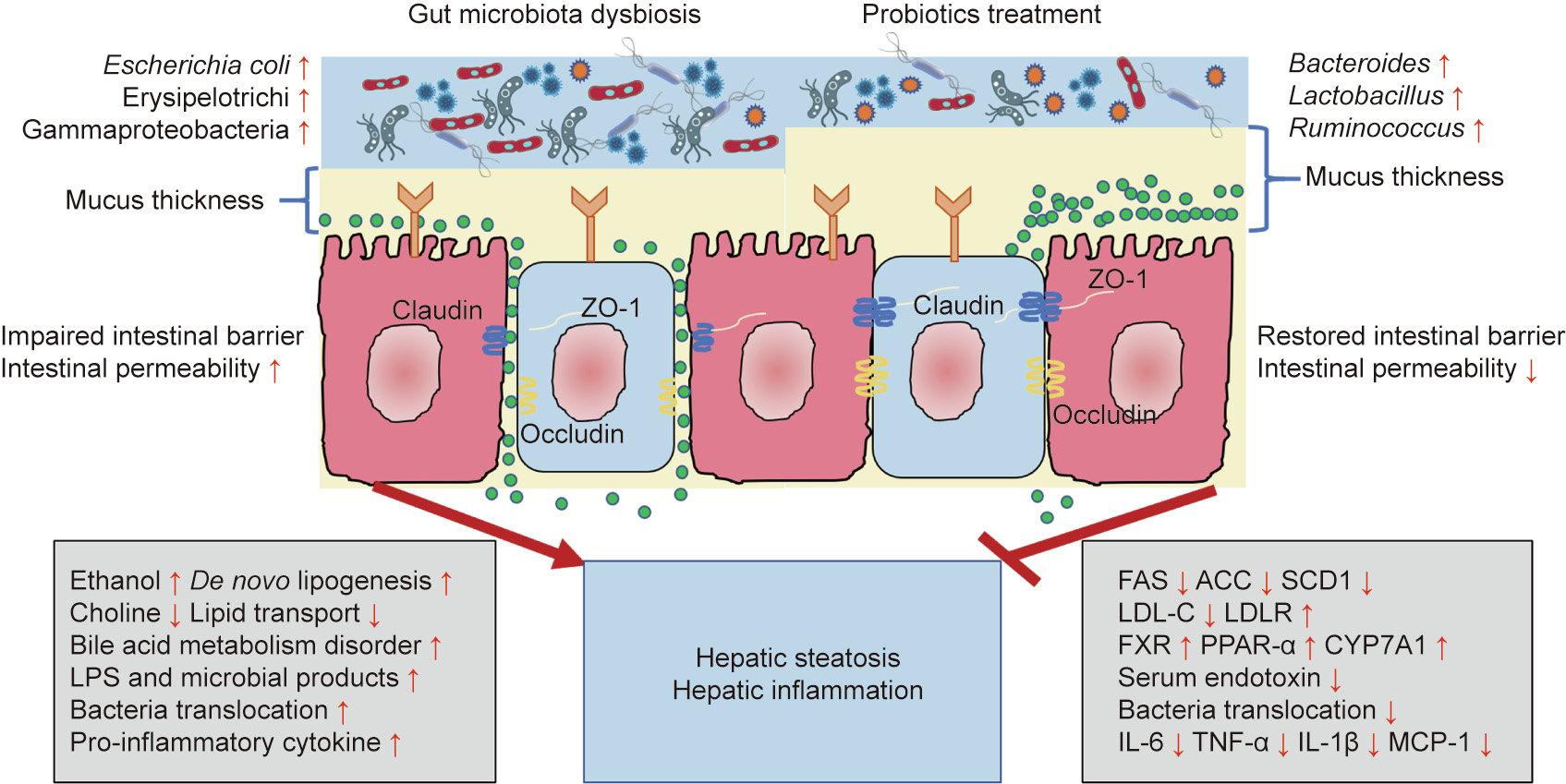
Fig. 1. Pathophysiological mechanisms of NAFLD development and the anti-NAFLD function of probiotics. LPS: lipopolysaccharide; ZO-1: zonula occluden-1; FAS: fatty acid synthetase; ACC: acetyl-coenzyme A carboxylase; SCD1: stearoyl coenzyme A desaturase 1; LDL-C: low-density lipoprotein cholesterol; LDLR: low-density lipoprotein receptor; FXR: farnesoid X receptor; PPAR: peroxisome proliferator-activated receptor; CYP7A1: cholesterol 7α-hydroxylase; IL: interleukin; TNF: tumor necrosis factor; MCP: monocyte chemotactic protein.
《2.1. Alteration of the energy harvesting capacity》
2.1. Alteration of the energy harvesting capacity
NAFLD is commonly associated with obesity. The critical role of the gut microbiota in harvesting energy from dietary food has been demonstrated in animal studies; compared with wild-type mice, germ-free mice are less prone to develop obesity when consuming a high fat diet (HFD) [20]. However, after germ-free mice were transplanted with microbiota from wild-type mice, body fat mass and insulin resistance dramatically increased [21]. Therefore, gut microbiota may contribute to harvesting additional energy from food, leading to the development of NAFLD.
《2.2. De novo lipogenesis and SCFAs》
2.2. De novo lipogenesis and SCFAs
De novo lipogenesis is a complex and highly regulated lipid metabolic pathway that is usually activated in liver and adipose tissue. Under normal conditions, de novo lipogenesis converts excessive carbohydrates into free FAs, which will be esterified into storage TGs that can subsequently provide energy via β-oxidation [22]. SCFAs, including acetate, propionate, and butyrate, are mainly generated by gut microbiota fermentation. Acetate is a substrate for cholesterol synthesis, propionate is a gluconeogenic substrate in the liver, and butyrate is used as an energy substrate for colonocytes [23]. In addition to stimulating de novo lipogenesis in the liver, SCFAs are ligands of the G-protein-coupled receptors (GPRs) GPR43 and GPR41, which are also called free FA receptor 2 and 3, respectively. The activation of GPR43 by SCFAs inhibits lipolysis and adipocyte differentiation, which ultimately leads to the accumulation of adipose tissue in HFD mice [24].
《2.3. Alterations of choline and bile acid metabolism》
2.3. Alterations of choline and bile acid metabolism
Choline plays an important role in lipid metabolism, especially for very low density lipoprotein production and hepatic lipid transfer. Choline facilitates lipid transport in hepatocytes, thus preventing abnormal lipid accumulation in the liver [25]. Therefore, a choline-deficient diet is usually used to set up hepatic steatosis experimental models. Indeed, the content of dietary choline influences the composition and abundance of Gammaproteobacteria and Erysipelotrichi, which are associated with the development of NAFLD [26]. Moreover, the gut microbiota is capable of converting choline into trimethylamine oxide, which causes liver inflammation and damage, thus exacerbating NAFLD [27].
Bile acids are synthesized from cholesterol in hepatocytes, which are metabolized by the gut microbiota. In the gastrointestinal tract, primary bile acids are capable of facilitating the digestion and absorption of fat-soluble food, maintaining the intestinal barrier, and preventing bacterial translocation [28,29]. In addition, bile acids activate farnesoid X receptor (FXR) and G-protein-coupled bile acid receptor 1 (TGR5) and, through these endogenous receptors, can regulate lipid and glucose metabolism and energy expenditure [30].
《2.4. Increased intestinal permeability and inflammation》
2.4. Increased intestinal permeability and inflammation
The gut and the liver communicate through the gut–liver axis, which consists of the gut, the liver, and the intestinal barrier. Tight junctions, composed of zonula occluden-1 (ZO-1), occludin, and claudin, link the intestinal epithelial cells and maintain intestinal barrier integrity [31]. Both intestinal barrier malfunction and dysbiosis of the gut microbiota play important roles in the pathophysiology of liver diseases [32]. Indeed, higher intestinal permeability has been observed in children with steatohepatitis compared with children with steatosis, indicating that the level of intestinal permeability is also dependent on the different stages of NAFLD [33].
Small intestinal bacterial overgrowth and inflammation are main causes of increased intestinal permeability in NAFLD. The definition of small intestinal bacterial overgrowth is a bacterial concentration equal to or in excess of 105 colony forming units (CFU)·mL-1 in proximal jejunal aspirates, for which the normal concentration is approximately 104 CFU·mL-1 or lower [34]. Small intestinal bacterial overgrowth can also induce bacterial translocation from the gut to the portal circulation, which leads to liver damage [35]. Lipopolysaccharide (LPS) is a major component of the membrane of gram-negative bacteria and is recognized by Toll-like receptor (TLR) 4. LPS stimulates macrophages and monocytes to produce pro-inflammatory and pro-fibrogenic cytokines (e.g., tumor necrosis factor (TNF)-α and interleukin (IL)-8, which also trigger hepatic recruitment of neutrophils and monocytes, possibly resulting in hepatic injury and systemic inflammation [36].
《2.5. Production of endogenous ethanol》
2.5. Production of endogenous ethanol
Although, by definition, people diagnosed with NAFLD do not consume excessive amounts of ethanol, elevated serum ethanol concentrations have been described in children and adolescents with NASH who did not have frequent access to alcoholic beverages or food containing alcohol. An observed increase in the abundance of alcohol-producing bacteria (e.g., Escherichia coli) was hypothesized to be the source of the excess ethanol [37]. Intestinal microbiota-derived ethanol contributes to steatosis by increasing hepatic lipogenesis via sterol regulatory element-binding protein-1 (SREBP-1) activation [38]. Moreover, ethanol impairs the intestinal barrier, resulting in increased bacterial translocation and hepatic inflammation [39].
《3. Probiotics and probiotic functionality》
3. Probiotics and probiotic functionality
Probiotics are defined by the Food and Agriculture Organization (FAO) of the United Nations and World Health Organization (WHO) as ‘‘live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [40]. The most commonly used probiotics, also called traditional probiotics, belong to the Bifidobacterium and Lactobacillus genera. With the development of improved cultivation methods, next-generation sequencing technologies, and bioinformatic methods, a novel group of probiotics called ‘‘NGPs” is emerging, including species such as Akkermansia (A.) muciniphila [41,42], Faecalibacterium (F.) prausnitzii [43], and Bacteroides fragilis [44]. Since most probiotics consist of commensal bacteria from the gastrointestinal tract, no extra supplements are generally required. However, decreased levels of Bifidobacterium have been detected both in rodent NAFLD models and among NASH patients [37,45]. The effects of probiotics on the human body include gut microbiota modification, improvement of the gut barrier function, increased competitive adherence to the mucosa and epithelium, and regulation of the gut-associated lymphoid immune system [46] (Fig. 1). Numerous studies have demonstrated the beneficial effect of probiotics in animals and patients with NAFLD (Table 1 [47–57] and Table 2 [58–71]).
《Table 1》
Table 1 Traditional probiotics shown in animal experiments to ameliorate NAFLD.

LGG: L. rhamnosus GG; ALT: alanine aminotransferase; AST: aspartate aminotransferase; PGC1α: PPAR-γ coactivator 1α; CPT: carnitine palmitoyltransferase; TG: triglyceride; TGF: transforming growth factor; TC: total cholesterol.
《Table 2》
Table 2 Traditional probiotics used in clinical therapy of patients with NAFLD.

S.: Streptococcus; B.: Bifidobacterium; P.: Pediococcus; IHF: intrahepatic fat; IFN: interferon; BMI: body mass index; HOMA-IR: homeostasis model assessment of insulin resistance; VSL#3: generic name of Bifidobacterium, Lactobacillus, and Streptococcus; GLP: glucagon-like peptide; aGLP: activated GLP.
《4. The role of traditional probiotics in NAFLD treatment》
4. The role of traditional probiotics in NAFLD treatment
《4.1. In vivo studies》
4.1. In vivo studies
The reduction of hepatic triglycerides (TGs) has been regarded as one route to slow the progress of NAFLD [72]. Several in vivo studies have shown evidence of the efficacy of probiotic treatment of fatty liver via a reduction of fat content. NAFLD animal models can be based on HFD, genetic modification, or a combination of more than one intervention, which have been reviewed previously [73]. For example, one study on rats with NAFLD induced through HFD showed that the levels of fat accumulation decreased and liver function was restored after treatment with Lactobacillus (L.) plantarum NCU116 for five weeks [47]. The results indicated that the possible mechanisms included upregulation of lipolysis and FA oxidation-related gene expression, and downregulation of lipogenesis [47]. Another study showed that co-administration of L. rhamnosus GG (LGG) and L. plantarum WCFS1 to rat models reduced the serum levels of TG, total cholesterol (TC), free FAs, and fat deposition in the liver via upregulation of cholesterol 7α-hydroxylase (CYP7A1), a enzyme involved in the regulation of liver cholesterol metabolism [48]. Another study showed that administration of LGG alone can also result in decreased hepatic fat content and upregulation of CYP7A1 [49]. In addition, supplementation of heat-killed L. reuteri GMNL-263 (Lr263) to hamsters on an HFD has been shown to improve lipid and cholesterol metabolic function, which could potentially improve the health of the liver [50].
Inflammation is another main cause of NAFLD exacerbation. Therefore, reduction of the pro-inflammatory response in the liver is important for NAFLD treatment. A L. johnsonii BS15-based formulation has been developed to protect HFD mice with NAFLD from hepatic steatosis and hepatocyte apoptosis via lowering of the intestinal permeability, modification of the gut flora, reduction in the level of serum LPS, and downregulation of inflammatory cytokines, such as TNF-α, in the liver [51]. Another study revealed that administration with L. paracasei N1115 alleviated HFDinduced hepatic steatosis and the release of TNF-α, and slowed the progression of cirrhosis [52]. In another study, treatment with L. paracasei was demonstrated to significantly reduce levels of pro-inflammatory cytokines TNF-α and monocyte chemotactic protein-1 (MCP-1) through inhibition of the pro-inflammatory M1 Kupffer cell response and activation of the anti-inflammatory M2 response [53]. Moreover, intervention with Saccharomyces boulardii can improve hepatic steatosis and reduce the expression of TNF-α in NAFLD rats induced by HFD [54]. In addition, treatment with the multi-probiotic Symbiter, which contains 14 probiotic bacterial genera, has been shown to result in significantly reduced IL-1 levels, whereas the IL-10 levels were restored [55].
Probiotics can also ameliorate NAFLD through the production of SCFAs. Intestinal metabolite SCFAs, including acetate, propionate, and butyrate, are key regulators in lipid metabolism and intestinal immunity [74]. Butyrate and propionate are considered to be antiobesogenic, while acetate is obesogenic. Butyrate plays an important role in increasing eubiosis and decreasing intestinal permeability, which may prevent the release of endotoxins [18]. Coadministration of Lactobacillus and Bifidobacterium has been shown to significantly increase the levels of fecal butyrate and lead to downregulation of GPR109a, inhibiting systemic adiposity and inflammation [56]. GPR109a, an SCFA receptor, is expressed in adipocytes, hepatocytes, and colon cells, and is mainly activated by butyrate [75]. Another study revealed that a compound probiotic composed of six strains of Lactobacillus and three strains of Bifidobacterium led to upregulation of another SCFA receptor, GPR43, which can inhibit lipid deposition and chronic metabolic inflammation [57].
《4.2. Clinical trials》
4.2. Clinical trials
A number of randomized clinical trials (RCTs) have been conducted to evaluate the therapeutic effect of probiotics on NAFLD. The efficacy of probiotics is highly dependent on the constituent strains and other supplementations. The results from an RCT performed with 30 patients showed a significant decrease in the activity of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) after oral administration of L. acidophilus for one month and, for some patients, relief from dyspepsia was observed [58]. Levels of ALT and AST are indicators of inflammation and hepatocellular injury in patients with fatty liver. However, most of the RCTs for the treatment of NAFLD used formulations containing two or more strains in order to obtain a synergistic effect. Aller et al. [59] conducted an RCT to evaluate the effect of a probiotic composed of L. bulgaricus and S. thermophilus on 28 patients with NAFLD. The results showed reduced levels of ALT and AST after treatment. Another RCT revealed that consumption of a probiotic yogurt containing L. acidophilus La5 and Bifidobacterium (B.) lactis Bb12 lowered the levels of hepatic enzymes, the TC in serum, and the levels of low-density lipoprotein cholesterol (LDL-C) [60]. In a recent study, 68 obese patients with NAFLD were randomized for treatment with probiotics or a placebo for 12 weeks. The probiotics mixture included six bacterial species: L. acidophilus, L. rhamnosus, L. paracasei, Pediococcus (P.) pentosaceus, B. lactis, and B. breve. The results showed that the administration of probiotics can reduce body weight, total body fat, intrahepatic fat (IHF), and levels of TG, but not levels of ALT, AST, glucose, insulin, and LPS [61]. Another RCT conducted among 72 patients with type 2 diabetes (T2D) and NAFLD showed that treatment with the multiprobiotic Symbiter for 30 d significantly reduced proinflammatory cytokines in plasma, especially IL-6, IL-8, TNF-α, IL1β, and interferon (IFN)-γ [62]. Another RCT conducted on 42 patients with NAFLD indicated that multi-strain probiotic supplement significantly decreased levels of TNF-α and IL-6 [63]. However, no significant differences in any measured parameters were observed among patients with liver cirrhosis after the administration of probiotics [64]. Another RCT conducted in patients with liver cirrhosis showed that the administration of LGG decreased levels of endotoxin and TNF-α, with reduced numbers of Enterobacteriaceae and increased numbers of Clostridiales Incertae Sedis XIV [65].
Prebiotics and synbiotics are two other supplementations that are gaining attention due to their potential beneficial effect of alleviating liver damage. A prebiotic is defined as ‘‘a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health” [76], whereas synbiotics are probiotics combined with food ingredients or dietary supplements (i.e., prebiotics). When 50 patients diagnosed with NASH were administered a synbiotic composed of L. reuteri and inulin, a reduction in the grade of hepatic steatosis was observed, together with a reduction of metabolic parameters associated with NASH, including body weight, body mass index (BMI), and uric acid serum levels [66]. Another RCT conducted in patients with NASH evaluated the effects of B. longum with fructo-oligosaccharides and lifestyle modification versus lifestyle modification alone for 24 weeks. The results showed that probiotics with fructo-oligosaccharides and lifestyle modification significantly reduced TNF-α, serum AST levels, serum endotoxin, steatosis, and the NASH activity index compared with lifestyle modification alone [67]. The combined effects of probiotics with lifestyle modification or drugs have also been evaluated. Behrouz et al. [68] investigated the effect of probiotics in 89 patients with NAFLD. These probiotics consisted of L. casei, L. rhamnosus, L. acidophilus, B. longum, and B. breve. Compared with lifestyle interventions alone, probiotics and prebiotics supplementation along with lifestyle intervention was confirmed to have a more favorable impact on glycemic parameters and leptin levels. Furthermore, probiotics have been used in combination with drugs to improve the therapeutic effect. Shavakhi et al. [69] found that probiotics in combination with metformin improved liver ALT levels better than metformin alone in patients with NASH.
There has also been some progress regarding RCTs evaluating the beneficial effects of probiotics on children with NAFLD. One RCT conducted among 64 obese children with NAFLD showed that intervention by the supplementation of probiotic capsules containing L. acidophilus, L. rhamnosus, B. lactis, and B. bifidum decreased levels of ALT, AST, cholesterol, and TG [70]. Another RCT conducted in children with NAFLD showed that administration with VSL#3 (generic name of Bifidobacterium, Lactobacillus, and Streptococcus) decreased BMI and increased glucagon-like peptide-1 (GLP-1) and activated GLP-1 (aGLP-1) levels, thus alleviating NAFLD [71]. In summary, although the beneficial effects of probiotics for NAFLD treatment have been demonstrated in RCTs, the underlying mechanisms are not yet fully understood.
《5. The potential benefits of NGPs for NAFLD treatment》
5. The potential benefits of NGPs for NAFLD treatment
Aside from traditional probiotics, NGPs are also gaining attention as possible therapeutic tools for treating NAFLD [77]. The bacteria used in NGPs include A. muciniphila [41,42], F. prausnitzii [43], Bacteroides uniformis [78], Bacteroides xylanisolvens [79], Bacteroides fragilis [44], Eubacterium hallii [80], Propionibacterium [81], and members of Clostridia clusters IV, XIVa and XVIII [82], some of which have been shown to have ameliorative effects on NAFLD.
《5.1. A. muciniphila》
5.1. A. muciniphila
The intestinal mucus consists of an inner layer devoid of bacteria and an outer layer colonized by commensal bacteria, the major components of which are amino acids and oligosaccharides favored by some bacteria, including A. muciniphila [41]. A. muciniphila is a mucin-degrading microbe, and is one of the single most abundant species in the human intestinal microbiota, accounting for 0.5%– 5.0% of the total number of bacteria [83]. Although the species has no history of being used in the food and pharmaceutical industry, it has been confirmed that there is an inverse correlation between the abundance of A. muciniphila in the intestine and metabolic diseases such as obesity and NAFLD [84–86]. A significant decrease in A. muciniphila abundance has been observed in mice with NAFLD compared with wild-type mice, and interventions resulting in an increased abundance of A. muciniphila improved metabolic parameters [86]. Administration of prebiotics (e.g., oligofructose) can restore A. muciniphila abundance and ameliorate related disorders [87,88]. A. muciniphila treatment did not significantly induce changes in the gut microbiota composition of dietinduced obese mice, but it reversed HFD-induced metabolic disorders, including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance, indicating its potential use for the prevention or treatment of obesity and associated metabolic disorders [87]. It has also been observed that A. muciniphila and its main metabolites, that is, propionate and acetate, affect the expression of genes involved in host lipid metabolism and epigenetic activation or silencing of gene expression [89]. In addition, an unexpected advantage of using A. muciniphila as probiotics is that when compared with live cells, the administration of pasteurized A. muciniphila has been shown to have a similarly beneficial impact on diet-induced metabolic disorders in mice, due to the presence of numerous highly abundant proteins on the A. muciniphila outer membrane [90,91].
《5.2. Bacteroides spp.》
5.2. Bacteroides spp.
The genus Bacteroides belongs to the phylum Bacteroidetes and consists of the most predominant species in the human intestinal tract of the Bacteroidales order [92]. Highly enriched in the colonic mucus layer, Bacteroides are obligate anaerobes, and most are resistant to 20% bile salt [93]. One of the most wellacknowledged characteristics of Bacteroides spp. is their robust polysaccharide degradation systems. Consumption of a wide spectrum of targeted polysaccharides, especially non-digested dietary fibers, enables the production of considerable amounts of SCFAs, mostly in the form of acetate and propionate [94]. The absorbed SCFAs can induce lipogenesis, which is associated with NAFLD initiation [95]. Interestingly, Bacteroides uniformis CECT 7771 has already been confirmed by Gauffin Cano et al. [78] to be capable of reducing body weight and alleviating steatosis by downregulating cholesterol and TG concentrations in both liver and serum in HFD-fed mice. Further studies are required to explore the potential of Bacteroides spp. for use as a probiotic for NAFLD treatment.
《5.3. F. prausnitzii》
5.3. F. prausnitzii
F. prausnitzii is a commensal gut bacterium that makes up approximately 4% of the main luminal microbiota [96]. This species is considered to have great potential as a probiotic used to prevent the progress of NAFLD because it is a butyrate producer, and the absence of butyrate is linked with Crohn’s disease [97]. According to a recent study, elevated levels of F. prausnitzii, A. muciniphila, and Peptostreptococcus anaerobius were observed in the gut microbiota of obese persons with diabetes after weight loss [98]. Moreover, a recent study showed that a hepatic fat content of > 5% was associated with low F. prausnitzii abundance and increased adipose tissue inflammation, independent of weight [99]. Although fecal F. prausnitzii abundance has been shown to not differ significantly (P = 0.082) among obese and non-obese persons, differences between men and women have been observed, indicating that stratification may be important for future studies on the species [100]. The diversity of F. prausnitzii may also play a role in inflammatory processes in the host: One study showed that phylotypes of F. prausnitzii differed between obese persons with and without type 2 diabetes, which could lead to differences in insulin resistance due to epigenetic regulation, mitochondrial β-oxidation, glucose sensitivity, and adiposity [101,102]. Elucidating the differences in function between different phylotypes of F. prausnitzii could be key in designing probiotic formulations based on the species. Treatment with F. prausnitzii ATCC 27766 has been shown to improve hepatic health and decrease adipose tissue inflammation in mice, indicating that the presence of the species may be linked to enhanced mitochondrial respiration, modified gut microbiota composition, and improved intestinal integrity [103]. A study employing an ex vivo experimental model based on mouse ileal organoids compared the effect of A. muciniphila and F. prausnitzii on the transcription of genes involved in cellular metabolism and growth, and found that in comparison with A. muciniphila, F. prausnitzii affected the transcription of considerably fewer genes [89].
《5.4. Roseburia》
5.4. Roseburia
The genus Roseburia belongs to the Clostridia cluster XIVa of the phylum Firmicutes, and consists of five species: Roseburia (R.) intestinalis, R. hominis, R. inulinivorans, R. faecis, and R. cecicola [104]. Just like F. prausnitzii, Roseburia spp. can produce SCFAs, especially butyrate, by breaking down indigestible carbohydrates [105]. Studies have indicated that SCFAs have anti-inflammatory properties mediated through the GPR, as well as a cascade of apoptosis and innate immunity-related processes. Butyrate is the main source of energy for colonic epithelial cells and inhibits the expression of pro-inflammatory cytokines in the mucosa by inhibiting nuclear factor-κB (NF-κB) activation [106]. Since inflammation is one of the main causes of progression from fatty liver to steatohepatitis, as mentioned above, it can be inferred that probiotics consisting of Roseburia spp. are promising in preventing the progress of NAFLD. Indeed, one study has shown that supplementation with chitin gclucan fibers significantly decreased weight gain in HFD mice and increased the abundance of Roseburia spp. [107]. However, further studies are still needed to verify its efficacy in NAFLD therapy.
《6. Conclusions》
6. Conclusions
Due to the absence of approved medications, innovate therapeutic strategies are urgently needed for preventing the development and progression of NAFLD. Gut microbiota have been shown to be an important factor in NAFLD pathogenesis. Modulation of the gut microbiota through the administration of traditional, commercial probiotics has been reported in many studies, including in vivo studies and clinical trials, and the efficacy of probiotics as a therapy for NAFLD has been demonstrated. Regulating lipid metabolism, reducing fat accumulation in the liver, restoring gut microbiota homeostasis, repairing the intestinal barrier, and relieving inflammation are possible mechanisms of amelioration of NAFLD due to probiotics treatment. Although probiotics have traditionally consisted of strains of Lactobacillus or Bifidobacterium, recent studies suggest that formulations should not be limited to strains of these genera. NGPs (e.g., A. muciniphila) have shown great therapeutic potential for the treatment of NAFLD. Nevertheless, further studies are still needed to provide sufficient evidence of therapeutic efficacy prior to clinical application of probiotics, and more studies are required to find novel probiotics that are superior to traditional probiotics.
《Acknowledgements》
Acknowledgements
This study was supported by grants from the National Key Research and Development Program of China (2018YFC2000500), and the National Natural Science Foundation of China (81790631 and 81330011).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Mingfei Yao, Lingling Qv, Yanmeng Lu, Baohong Wang, Björn Berglund, and Lanjuan Li declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




