《1. Introduction》
1. Introduction
About three million people around the globe suffer from traumatic spinal cord injury (SCI), and approximately 250 000–500 00 0 new cases occur per year [1–3]. SCI causes life-long disability, with motor and sensory neuronal deficits. These injuries not only decrease the life quality of patients, but also bring considerable social and economic burdens [4,5]. Great efforts have been made to explore efficient therapies for SCI. However, due to the complexity of SCI, current treatments have a poor prognosis with modest functional recovery [6,7]. Thus, gaining a better understanding of the cellular and molecular mechanisms underlying SCI will pave the way for developing new strategies to enhance neural regrowth and plasticity [8].
In brief, the pathophysiological process of SCI contains three consecutive phases: primary trauma, secondary damage and, eventually, the chronic injury phase [9]. Physical trauma initiates the mechanical disruption of the spinal cord and results in injuries to local neurons and oligodendrocytes. The blood vessels and the blood–spinal cord barrier (BSCB) in the lesion area undergo breakdown. These events trigger a multifactorial secondary injury cascade, which can last for weeks. During this stage, immune cells infiltrate the injury sites and release inflammatory cytokines. The inflammatory response leads to additional neuronal and glial cell death [10]. Finally, in the chronic phase, the reactive astrocytes, microglia/macrophages, and extracellular matrix molecules form intensive glial scars and subsequently prevent axon regrowth [9].
The injury-induced inhibitory microenvironment and the lack of intrinsic regenerating ability in the lesion hinder successful axon regeneration after SCI [11]. Researchers have conducted numerous studies with the aim of improving neuroregeneration and neural repair for SCI rehabilitation. These treatments include nonpharmacological therapies, pharmacological therapy, gene therapy, cell-based therapy, and biomaterials [12–15]. Neurotrophic factors, such as neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF), can modulate neuronal survival, axonal growth, synaptic plasticity, and neurotransmission [16,17]. The repair effects of neurotrophic factors on SCI depend on neurotrophic factor types, administration mode, location, and time [18,19]. For example, NT-3 expression supports the spouting and axonal plasticity of the corticospinal tract (CST) [20,21], alters synaptic transmission to motoneurons (MNs) [22], modifies local lumbar neural circuitry [23,24], and facilitates the proliferation and differentiation of neural stem cells (NSCs) after SCI [25,26]. However, some research groups have showed that continued spinal cord degeneration might limit the regeneration effect of NT-3 at 12 weeks after SCI [27]. Cell-based therapy has also been applied in SCI with various cell types, including embryonic stem cells (ESCs), NSCs, mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), olfactory ensheathing cells (OECs), Schwann cells, and so on. Cell therapy combined with biological factors or biomaterials can substitute for lost cells, provide neurotrophic factors, and modulate the lesion microenvironment in order to facilitate axon regeneration after SCI [7,28–32]. In addition, neuromodulation techniques (e.g., noninvasive magnetic stimulation or electrical stimulation), which affect neural network activity, have been adopted to promote neuroregeneration and neural repair after SCI [33].
In general, these therapies follow three main directions: ① diminishing the repulsive barriers that prevent axon sprouting to provide a permissive microenvironment; ② reconstructing the disrupted neural circuits to promote functional recovery; and ③ providing spinal cord-like tissue grafts to support and guide axon regeneration (Fig. 1). In this review, we focus on these three aspects to give a brief introduction of recent achievements that have been made in SCI therapy. We also propose several important future directions for the effective treatment of SCI.
《Fig. 1》
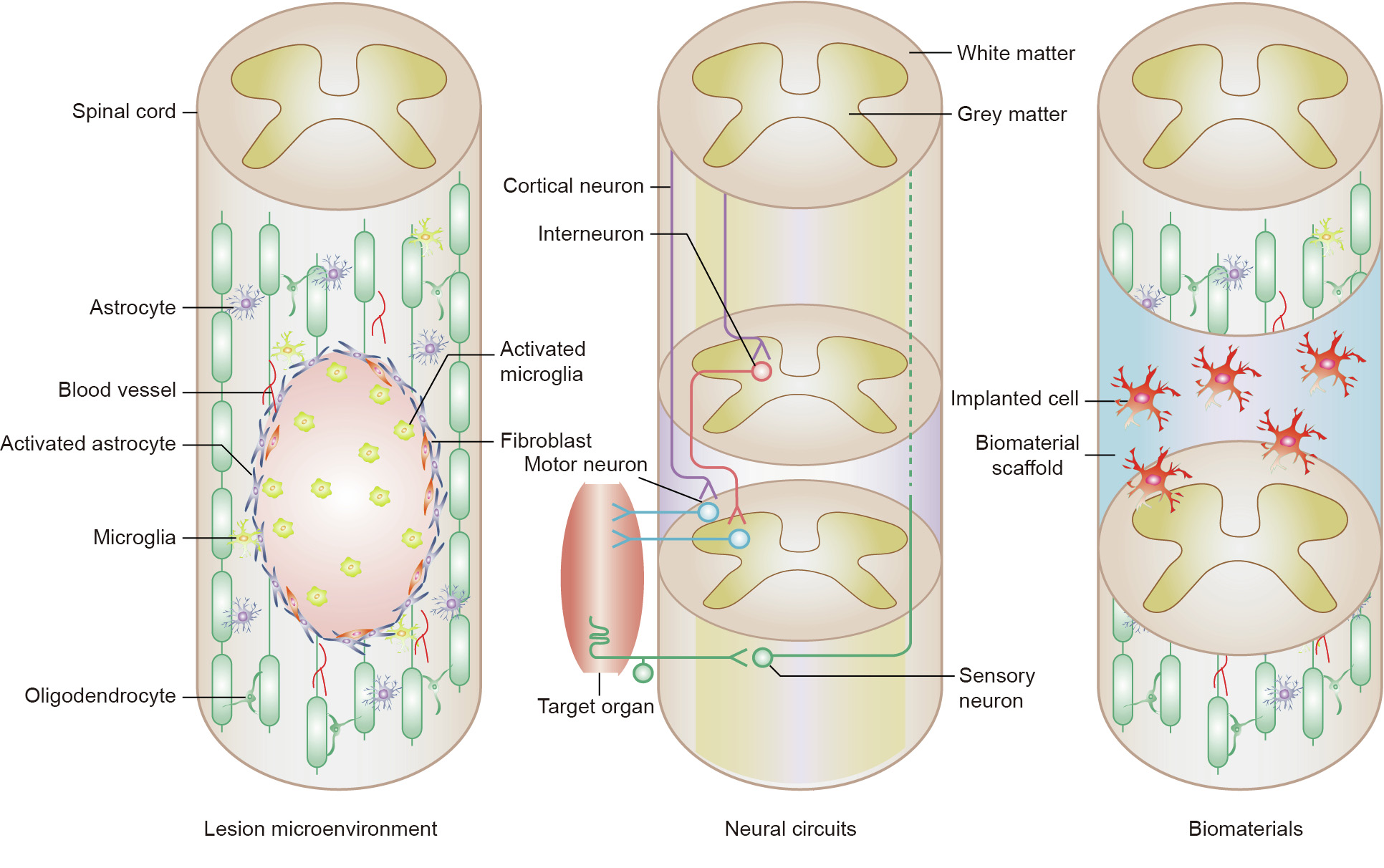
Fig. 1. A schematic diagram of current therapeutic treatments for SCI.
《2. The lesion microenvironment》
2. The lesion microenvironment
Unlike the peripheral nervous system (PNS), the central nervous system (CNS) has limited regenerative ability after an injury, which may be due to the passive microenvironment in the lesion area [11]. Neurons, glial cells, axons, myelin, blood vessels, the cell matrix, and neurotransmitters constitute the nerve microenvironment, which is regulated by various nutrient factors and cytokines [33]. After SCI, extensive inflammation, supportive substrates deficiency, inhibitory growth components, and glial scar formation hinder axon regrowth [34]. Previous studies have shown that spinal cord axons exhibit a transient regenerative ability immediately after injury when bridged to peripheral nerve segments[35]. This result raises the possibility of facilitating SCI repair and functional improvement by providing a hospitable environment. Researchers have made numerous attempts to ameliorate the regeneration-inhibitory microenvironment for SCI treatment, such as by targeting glial and inflammatory responses [36,37] and promoting remyelination [38,39].
The process of SCI is complicated and involves multiple cell types (i.e., neurons, glia cells, immune cells, and so on), cellular responses, and biological activities [8,11,40,41]. It is a critical challenge to elucidate the lesion microenvironment after SCI. The growing number of high-throughput sequencing technologies, such as RNA sequencing (RNA-seq) and single-cell RNA sequencing (scRNA-seq), provide powerful tools for SCI investigation [42,43]. Experts have performed a number of transcriptome analyses to uncover the complex environment of SCI [44–46]. For example, Yu et al. [47] conducted RNA-seq to identify significant genes and biological processes in the rostral and caudal spinal cord segments until 28 days after a rat hemisection SCI. This research detected the lesion environment at the multicellular level, examined the interactions among the multiple systems involved, and provided a comprehensive analysis of the complicated events that follow SCI [48]. Since the spinal cord has its maximal intrinsic growth capacity during embryogenesis [49], Yang et al. also analyzed rat spinal cords from the embryonic stage to adulthood using RNA-seq. They obtained the landscape of message RNAs (mRNAs), microRNAs (miRNAs), long non-coding RNAs (lncRNAs), small RNAs, and alternative splicing patterns during spinal cord development. This study provides a valuable genetic basis for the spinal cord and assists in improving technologies for spinal cord-related tissue engineering [50].
Recently, researchers have characterized the cellular heterogeneity in complex tissues and identified molecular markers for rare cell populations using scRNA-seq [51]. The complex spinal cord environment during development and after injury has also been uncovered using scRNA-seq technology [52–56]. These studies confirm the cellular heterogeneity in the rodent spinal cord and provide a preliminary sketch of the interactions between cells in the injury site. For example, by means of scRNA-seq, Li et al. [57] identified a subset of microglia that are transiently activated in the neonatal stage in a crush SCI model. The neonatal microglia secrete fibronectin to form the extracellular matrix and express certain molecules to resolve inflammation, thereby facilitating scar-free healing [57]. This research, which focused on microglia heterogeneity in the lesion area after a crushed SCI in neonatal and adult mice, proposed the possibility of improving healing and axon regrowth by modulating the injury environment. It was also a successful attempt to combine scRNA-seq with neurological validation, thus providing a new strategy for further investigation.
《3. Neural circuits》
3. Neural circuits
Neuron assemblies located in the spinal cord receive activating inputs from the brain and convey the rhythms of locomotion to MNs and muscles, thereby generating precise locomotor movements in vertebrates [58]. SCI disrupts the neural circuits with dendritic withdrawal or atrophy below the lesion, contributing to impaired locomotor movements and sensory feedback [59]. Functional recovery is one of the criteria and challenges for SCI therapy [60]. It is important to promote axonal regeneration, reconnect neural circuits, and enhance neuronal plasticity for spinal cord repair.
As the direct cortical output to the spinal cord, corticospinal neurons (CSNs) in the cerebral cortex are crucial in controlling spinal motor activities [61]. By means of a retrograde labeling system, Wang et al. [62] identified region-specific CSNs, which have distinct spinal projections and encode different movement modules. This research illustrates the organization between CSNs and their axons in the spinal cord, facilitating the understanding of neuronal circuits in goal-directed motor skills. Originating from CSNs in the cortex, the CST transmits cortical commands to the spinal cord. Traumatic brain or spinal cord injuries, which disrupt CST axons, result in motor function deficits [63,64]. Osteopontin (OPN)/insulin-like growth factor 1 (IGF1) treatment promotes CST axon regrowth and improves precision performance after spinal cord hemisection [65]. This research presents evidence that the activation of the intrinsic growth ability of CSNs can result in functional recovery after SCI. Aside from neurotrophins and growth factors, regulation of the energy metabolism is another promising strategy to facilitate axon regeneration after injury [66]. Han et al. [66] revealed that mitochondrial dysfunction induced by injury contributes to CNS axonal regenerative failure. Deletion of syntaphilin (Snph), a mitochondria protein anchor, rescued injury-induced mitochondrial depolarization. Furthermore, Snph–/– mice had enhanced CST regeneration, functional synapse formation, and motor function recovery after a T8 complete spinal transection.
Reorganization of the residual propriospinal relay connections or plasticity modulation of MNs can also remodel motor circuits and promote locomotor recovery after SCI [67–69]. Retrograde transportation of NT-3 to the lumbar MNs was found to significantly remodel lumber neural circuitry and synaptic connectivity in a T10 contusive SCI model in mice, facilitating behavioral and electrophysiological recoveries [70]. Further investigation revealed that the spared descending propriospinal pathway led to this NT-3- enhanced recovery. In addition, NT-3 reorganizes the propriospinal-MN circuit by promoting dendritic regrowth [59].
Spinal interneurons are essential for neuroplasticity [71,72]. Chen et al. [73] identified a small molecule, CLP290, which is an agonist of the neuron-specific K+ –Cl– co-transporter (KCC2) for SCI repair. KCC2 activation reactivates dormant spared circuitry through spinal inhibitory interneurons, hindering the integration of descending inputs into relay circuits after the injury. Administration of CLP290 can restore stepping ability by altering the disturbed spinal circuits into a functional state after spinal cord hemisection in mice. This research provides insights toward rescuing motor functions by regulating inhibitory interneuron excitability.
NSCs are self-renewing and multipotent cells that can differentiate into neurons, astrocytes, and oligodendrocytes [74]. Transplanted NSCs may also reconnect neural circuits. Lu et al. [75] reported that NSCs implanted with fibrin matrices containing growth factor cocktails after rat T3 complete transection could differentiate into mature neurons. These NSC-derived spinal cord neurons extend axons into the host spinal cord and form projections from the sites of injury into the host spinal cord with synapse formation. In addition, corticospinal axons were found to extensively regenerate into neural progenitor cell (NPC) grafts after rat T3 complete transection SCI. The regenerating corticospinal axons formed a functional synapse with grafted neurons and improved skilled forelimb function [76].
《4. Biomaterial scaffolds》
4. Biomaterial scaffolds
During the past decade, the application of natural or synthetic biomaterials for SCI treatment has increased. These biomaterials serve as physical scaffolds and provide structural support for axon regrowth, guiding the newly generating axons into the lesion area. In addition, the biomaterials act as a transporter for drugs or cells to modulate the lesion microenvironment, facilitating SCI repair [77–79].
As described earlier, neurotrophic factors such as NT-3 are important in the treatment of SCI. However, the lack of efficient delivery approaches limits the clinical applications of neurotrophic factors. Li et al. [25] and Yang et al. [26] designed a chitosan biodegradable carrier for the slow release of NT-3 over 14 weeks. They then applied this NT-3-chitosan tube to bridge a 5 mm gap in the completely transected spinal cord of rats. As a result, endogenous NSCs in the injured spinal cord were activated, differentiated into neurons, and formed functional neural networks, promoting sensory and motor behavioral recovery [80]. Transcriptome analyses indicated that this NT-3/chitosan tube can enhance neurogenesis, promote angiogenesis, and reduce inflammation, thereby providing a favorable regeneration microenvironment [45]. Furthermore, in an adult rhesus monkey T8 spinal cord hemisection injury model, Rao et al. [81] used this biomaterial carrier and found robust axonal regeneration, which provides a solid foundation for the potential therapeutic application of this NT-3- chitosan carrier.
Lin et al. [82] developed a linear-ordered collagen scaffold (NeuroRegen), which showed good cell compatibility and guided proper nerve outgrowth. They then used NeuroRegen scaffolds bound with multiple functional molecules, such as BDNF, in different SCI models and obtained improved functional recovery [83–85]. In addition, NSCs have been implanted in NeuroRegen scaffolds for SCI repair. To enhance the neuronal differentiation of NSCs in a permissive microenvironment, Li et al. [86] loaded paclitaxel (PTX)-encapsulated liposomes into the collagen microchannel scaffold to release PTX in a sustained manner. The results showed that this NSC-loaded collagen scaffold provided an instructive microenvironment for the neuronal differentiation of NSCs, axon regeneration, and locomotion recovery in a rat T8 complete transection SCI model. In a clinical context, NeuroRegen scaffolds combined with human umbilical cord MSCs were implanted into patients with chronic complete SCI, and showed primary efficacy outcomes during a year of follow-up without adverse events [87].
As described previously, SCI damages the ascending and descending nerve fiber tracts in the lesion, resulting in partial or total neural circuit disruption. Thus, it is of great importance to remold the neural circuits by promoting axon regeneration across injured sites, reconstructing synaptic connections, and bridging axotomized neural fibers [88]. Three-dimensional (3D) culturing can construct functional tissue or organoids for SCI repair by combining stem cells, biomaterials, and neurotrophic factors [14]. Lai et al. [89] proposed a novel stem cell-based tissue engineering therapeutic strategy named ‘‘tissue engineering neuronal relay” for SCI. By implanting a gelatin sponge scaffold in the spinal cord lesion with NT-3-modified Schwann cells and neurotrophic receptor tyrosine kinase 3 (TrkC)-modified NSCs, the researchers promoted the construction of a tissue engineering neural network and reestablished an anatomical neuronal relay after T10 spinal cord transection in rats. Mechanically, the NT-3/TrkC interaction activates the phosphatidylinositol 3-kinase (PI3K)/Akt serine/ threonine kinase 1 (AKT)/mammalian target of rapamycin (mTOR) pathway, stimulating the synaptogenesis of NSC-derived neurons. Moreover, to simulate the structure of the spinal cord, Lai et al. [90] developed a novel spinal-cord-like tissue (SCLT) in vitro by means of a modular assembly of white-matter-like tissue (WMLT) and gray-matter-like tissue (GMLT) from NSCs. This SCLT can function synergistically to rebuild the neural pathway and promote functional recovery after rat T10 transection SCI. In addition, SCLT might serve as an in vitro platform for investigating spinal cord pharmacology and development in the future.
《5. Future perspectives》
5. Future perspectives
《5.1. miRNA-based therapy》
5.1. miRNA-based therapy
Numerous studies have reported on the dysregulation of miRNAs after SCI [91–93]. miRNAs are involved in multiple processes, such as the astrocytic response, inflammation, and demyelination [94,95]. In most research, certain miRNAs are usually injected directly [96–98] or transfected into transplanted cells [99,100] for SCI treatment. Genetic-modified animal models are also used for this purpose [101,102]. However, many obstacles exist in miRNA-based therapy. For one thing, the mechanisms underlying the effects of miRNAs on SCI are still unclear. Current approaches for miRNA-based treatment usually overexpress or silence miRNAs without cell specificity. Since the spinal cord is a complex tissue organized with multiple cell types, it is difficult to identify the exact effects of miRNAs without focusing on certain cell types. Biomaterial scaffold systems can provide insights for miRNA-based therapy. Attempts to design new biomaterial nanoparticles, which combine cell-recognized polypeptides, will realize the delivery of miRNAs to specific cell types in the lesion area after SCI. This method will also help to elucidate the mechanisms underlying miRNAs for SCI. The pathogenesis of SCI is a spatiotemporal process that can last for months. Thus, biomaterials are beneficial in the sustained release of miRNAs in order to maintain appropriate local effective concentrations in lesions over a long period, thereby facilitating regeneration and functional recovery after SCI.
《5.2. Blood vessel intervention for SCI》
5.2. Blood vessel intervention for SCI
The vascular system transports oxygen and nutrients, takes away metabolic waste, and provides a supportive microenvironment for the nervous system [40]. SCI disrupts the blood vessels in the lesion area and increases vascular permeability, which further accelerates the inflammation response and tissue damage. Moreover, the injury-triggered endogenous angiogenesis is insufficient and usually malfunctions [103,104]. Thus, obtaining a further understanding of the vascular alterations in the injury lesion and developing new strategies to reduce blood vessel loss, compromise BSCB disruption, and promote effective vessel formation will be a promising therapy for SCI [105]. Recently, the role of vascularization after SCI has received an increasing amount of attention. Research has shown that ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX)/lysine demethylase 6A (KDM6A) deletion can increase endothelial cell tube formation in vitro and promote angiogenesis after T10 contusion SCI in mice, resulting in functional recovery [106].
《5.3. Combination of multiple treatments》
5.3. Combination of multiple treatments
Although numerous therapies have been shown to prevent further damage after SCI and to promote axon regeneration in animal models, the same therapies have no relevant efficacies when applied clinically [7]. One possible reason for this situation is that SCI is a multifaceted, concomitant, and consecutive pathological process, which requires comprehensive treatments. Thus, rather than current therapeutic strategies aimed at one or two pathophysiologies, an integrated therapy composed of multiple treatments might facilitate SCI repair [9]. For example, Anderson et al. [107] developed a combined therapeutic method that reactivates neuron growth capacity, induces growth-supportive substrates, and chemoattracts propriospinal axons. Biomaterials are also applied in this method for spatially and temporally controlled release of factors. As a result, mice and rats have been shown to achieve robust axon regrowth and enhanced functional recovery of remodeling circuits after a completely crushed SCI [107]. Current therapies for SCI are based on promoting axon regeneration [79], elevating interneuron excitability [108], diminishing inflammation response [109], or increasing revascularization [78,110]. In the future, we will be able to combine these therapies with cell implantation [7,15], factor administration, and biomaterial scaffolds. Our research group will also aim to identify the mechanisms and effects of critical regulatory factors (e.g., OPN, IGF1, BDNF, ciliary neurotrophic factor (CNTF), NT-3, or miRNAs) on different propriospinal pathways and reconnect brain–spinal cord neurite circuits. Modified biomaterials with stem cells, cytokines, drugs, or miRNAs will be implanted into the injury area to improve the lesion microenvironment and support axon regeneration after SCI. Our research group will also conduct additional rehabilitative training and physical stimulation to facilitate the outcome of SCI repair.
《6. Conclusion》
6. Conclusion
Research in the field of SCI therapy is still a long way from identifying a therapy that leads to significant recovery after SCI. Although more work is needed in the future, the previous efforts offer great hope for axonal regrowth and functional recovery after SCI, based on sound basic and clinical neuroscience research [111]. We hope that our present review of current therapeutic SCI treatments will facilitate the development of efficient therapies to obtain sustained nerve regeneration and functional recovery after SCI (Fig. 2).
《Fig. 2》
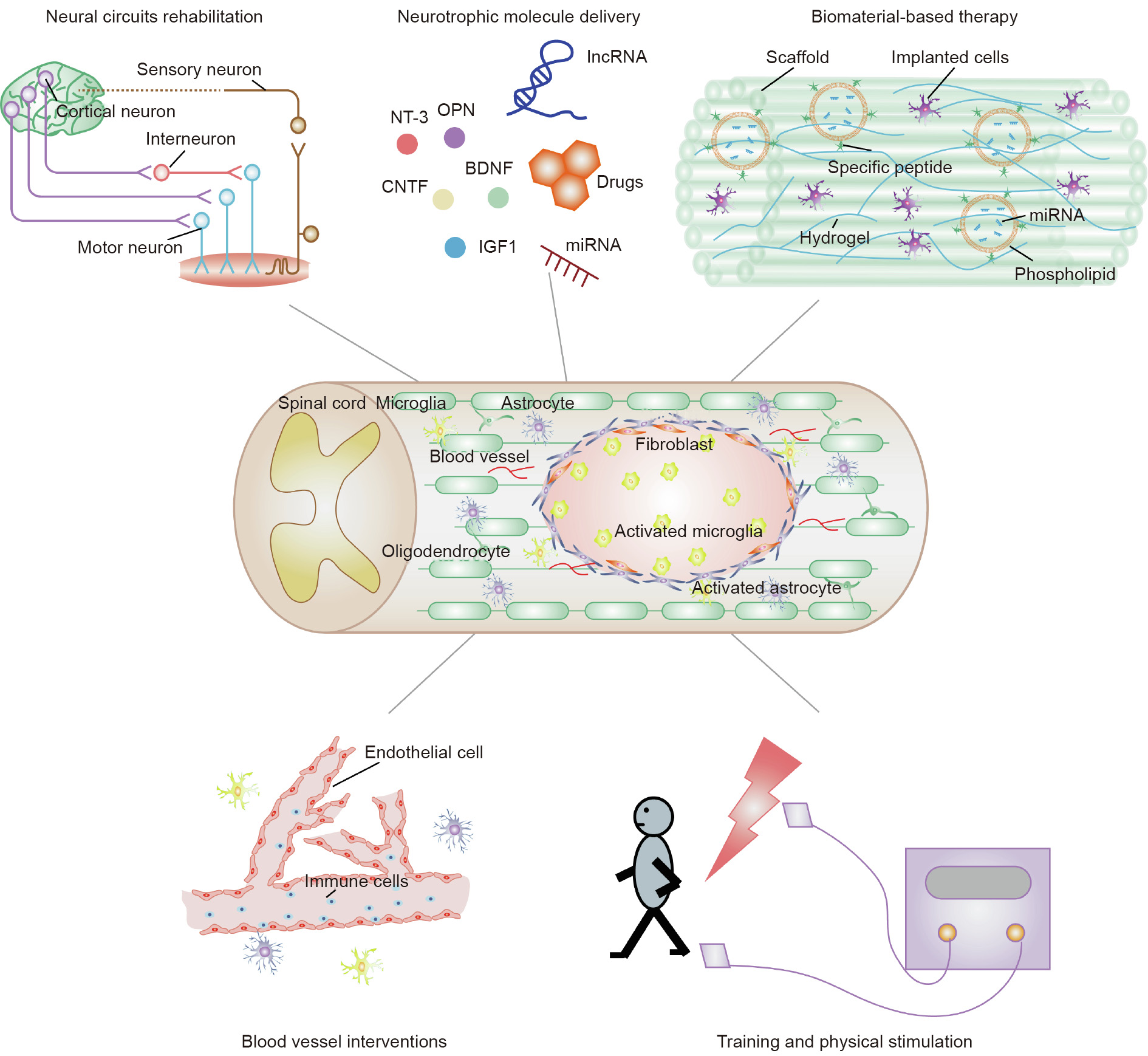
Fig. 2. A schematic diagram of potential combined therapeutic treatments for SCI in the future.
《Acknowledgments》
Acknowledgments
This work was supported by the National Major Project of Research and Development (2017YFA0104701 and 2020YFA0113600), and Jiangsu Provincial Key Medical Center and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Chun Yao, Xin Tang, Yuqi Cao, Xuhua Wang, and Bin Yu declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




