《1. Introduction》
1. Introduction
The last three decades have witnessed the emergence and progress of regenerative medicine. Tissue engineering is one of the most effective strategies for regenerative medicine. The term ‘‘tissue engineering” was coined as a new discipline in the 1980s by Professor Yuan-Cheng Fung, a Chinese-American scientist and a pioneer of biomechanics. In 1985, he submitted a proposal to the National Science Foundation (NSF) of the United States for establishing an engineering research center entitled ‘‘Center for the Engineering of Living Tissues” and coined the term tissue engineering [1]. Since then, scientists from various subjects have gradually realized the importance of the concept of ‘‘tissue engineering.” In 1993, Professor Robert Langer at Massachusetts Institute of Technology and Professor Joseph Vacanti at Harvard Medical School jointly published an article in the journal Science, marking the formal introduction of the subject tissue engineering [2]. According to the latest consensus conference on the Definitions of Biomaterials for the Twenty-First Century, organized by the International Union of Societies for Biomaterials Science and Engineering in 2018, tissue engineering means ‘‘the use of cells, biomaterials, and suitable molecular or physical factors, alone or in combination, to repair or replace tissue to improve clinical outcomes.” Moreover, regenerative medicine means ‘‘therapies that treat disease, congenital conditions, and injury by the regeneration of functional tissue or organ structures” [3].
For tissue engineering with materials and cells, or in situ tissue regeneration with implantation of scaffolds without external cells, one of the core scientific issues is the cell microenvironment. For instance, bone marrow stromal cells or mesenchymal stem cells (MSCs) differentiate into chondrocytes after the construction of MSCs, and the porous poly(lactide-co-glycolide) (PLGA) scaffolds are implanted into the normal joint cavity. In contrast, subcutaneous implantation led to only a scar-like tissue far away from the in vivo site of chondrogenesis [4].
The cell microenvironment includes not only the microenvironment of the organ or tissue itself, but also the microenvironment affected by the implanted materials. Modern biomaterials are not merely bioinert materials; instead, bioactivities have been recognized as an increasingly important property of biomaterials. According to the latest consensus definition, a biomaterial is ‘‘a material designed to take a form that can direct, through interactions with living systems, the course of any therapeutic or diagnostic procedure” [3]. Therefore, the cell microenvironment in tissue engineering and regenerative medicine is closely related to the basic science of ‘‘cell-material interactions” [5–13]. The present review focuses on the introduction and investigation of biomaterial-related cell microenvironments.
The cell microenvironment influences the adhesion, migration, differentiation, proliferation, and communication of cells on the surface of or inside the extracellular matrix (ECM) or biomaterials. In most cases, an ideal biomaterial for tissue engineering and regenerative medicine is aimed at mimicking the corresponding ECM, affording an appropriate microenvironment for cells. Therefore, the study of the cell microenvironment can guide the design of a new generation of tissue engineering materials.
Herein, we share our understanding of some key aspects of the biomaterial-related microenvironment of cells, as shown schematically in Fig. 1. In the following sections, we will introduce the following six aspects: ① chemical composition of materials, ② material dimensions and architecture, ③ material-controlled cell geometry, ④ effects of material charges on cells, ⑤ matrix stiffness and biomechanical microenvironment, and ⑥ surface modification of materials.
《Fig. 1》
Fig. 1. Key aspects of the material-related cell microenvironment.
《2. Chemical composition of materials》
2. Chemical composition of materials
Biomaterials used in tissue engineering include polymers, inorganic materials, metals, bioderived materials, and composite materials consisting of the aforementioned materials. In addition, many bioactive substances can facilitate tissue repair and regeneration. The classification of the main tissue engineering biomaterials is presented in Table 1 [14–105].
《Table 1》
Table 1 Chemical composition of biomaterials for tissue engineering and regenerative medicine.

The human body is made up of cells and ECM. After removing all cells from a tissue, the remaining ECM represents an ideal porous scaffold for tissue regeneration, providing not only the appearance of tissue but also an excellent microenvironment for cells. However, due to the limitations of the acellular matrix, biomaterials composed of polymer, inorganic, metal, and composite materials provide the ‘‘biomimetic” requirements. In general, metal and inorganic materials are more suitable for hard tissue repair owing to their high strength, while some polymer materials such as hydrogels are suitable for soft tissue regeneration. Some peptides and bioactive molecules play important roles in cell support and tissue regeneration. All biomaterials used for tissue engineering should be biocompatible, biodegradable, and feasible for fabrication.
As this paper is focused on tissue engineering materials, some important but non-degradable materials are not listed.
《3. Material dimensions and architecture》
3. Material dimensions and architecture
The materials for tissue engineering and tissue regeneration are usually in the form of three-dimensional (3D) porous scaffolds to support the adhesion, migration, and growth of cells. Porous scaffolds can be fabricated by foaming [106,107], electrostatic spinning [108–110], freeze-drying [111], and compression molding combined with particulate leaching [112,113]. Our group has developed a ‘‘room-temperature’’ injection molding approach combined with particulate leaching (RTIM/PL) to fabricate 3D porous scaffolds composed of biodegradable polyesters for tissue engineering [114], some of which are shown in Fig. 2(a). In recent years, 3D printing and additive manufacturing have emerged as an ideal way to customize scaffolds with different external shapes and internal pore structures [115]. Typical cell images of scaffold filaments with different structures are shown in Fig. 2(b) [116]. Pore structures of different scales (macro/micro/nano) affect tissue repair and regeneration [117].
《Fig. 2》

Fig. 2. (a) Global views of tubular and ear-shaped PLGA porous scaffolds fabricated by the RTIM/PL. (b) Fluorescence micrographs of MSCs after seven days of culture on the 3D-printed PLA scaffolds with tetragonal, hexagonal, and wheel-like scaffold structures from left to right. (c) Schematic diagram of trimodal macro/micro/nano-porous scaffolds loaded with rhBMP-2 for accelerated bone regeneration. (a) Reproduced from Ref. [114] with permission of Elsevier Ltd., © 2005; (b) reproduced from Ref. [116] with permission of IOP Publishing, Ltd., © 2019; (c) reproduced from Ref. [117] with permission of Elsevier Ltd., © 2015.
Preparation of tissue engineering porous scaffolds involves two major aspects: obtaining interconnected porous structures and controlling the appropriate scaffold shape and size. The pore size and porosity are regarded as important geometric features of tissue-engineering scaffolds [118,119]. For instance, pore sizes of 100–200 μm in the cartilage layer and 300–450 μm in the subchondral bone generate better results for osteochondral repair. Pore sizes that are too small hinder the transport of nutrients, while excessively large pore sizes lead to cell leakage.
High porosity and interpore connectivity are also key to tissue formation. High porosity promotes the formation of bone tissue within the scaffold. Kruyt et al. [120] studied the osteogenesis effect of a hydroxyapatite (HAp) containing composite material with porosities of 60% and 70% following implantation of goat bone marrow stromal stem cells. As a result, they found that 70% porosity achieved more new bone formation; this was attributed to the larger surface area of high-porosity materials, resulting in better adsorption of bone-inducing factors and ion exchange.
Only the interconnecting pore structure is conducive to the growth of new tissue and the support of the tissue interface [121]. Lu et al. [122] found that cartilaginous or osteoid tissues were formed when the pores were connected at 20–50 μm, and mineralized bone could be formed when the pores were >50 μm. Pore nonunion prevented new bone and blood vessels from interconnection, leading to unsatisfactory bone repair.
Based on the percolation theory, Ding et al. [123] reported that tissue engineering scaffolds with spherical pores might exhibit better internal connectivity than those with cubic pores, which is conducive to the inward growth of cells and tissues. In 2001, Ma and Choi [124] proposed a strategy for preparing spherical paraffin pore-forming agents, resulting in the successful preparation of poly(lactic acid) (PLA) porous scaffolds with spherical pores and interconnected internal pore network structures. Ding et al. [123,125,126] developed a water-soluble porogen by sugar–gluing salt microparticles and prepared PLGA porous scaffolds with spherical shapes by combining room-temperature molding and particle leaching. Compared with to the traditional polymer scaffolds with cubical macropores prepared by large sodium chloride particles, the modified scaffolds with spherical macropores exhibited better inner pore connectivity and less pore-forming agent residue. In addition, good cellular adhesion and osteogenic differentiation were observed in the pore walls of sugar-glued sodium chloride internal pores, which may arise from the distribution of many tiny sodium chloride particles on the surface of the pore–foaming agent and, consequently, the formation of a rough inner surface in the pore walls of the resultant scaffold.
Hierarchical pore structures perform different functions in tissue repair and regeneration in the cell microenvironment, as shown in Fig. 2(c) [117]. A macroporous structure is beneficial for the exchange of nutrients, while a microporous structure can be loaded with bioactive factors [127]. The channel structure and orientation arrangement of the scaffolds induce directional recruitment of cells and ultimately affect endochondral ossification [128,129].
In addition to porous scaffolds, injectable hydrogels, with a high water content and similarity to natural ECM, provide an alternative way to load seed cells in tissue engineering. Injectable hydrogels also have a significant advantage in that they can fit the shape of the defect site, which is particularly useful in the case of an irregularly shaped damaged tissue site to be repaired or regenerated. Injectable hydrogels can also replace conventional surgery with minimally invasive injections. In recent years, various injectable hydrogels have been developed, including chemically crosslinked hydrogels [130–133] and physically crosslinked hydrogels [134– 155], which can be applied in tissue engineering, drug release, and other medical applications [156–177].
From the perspective of tissue engineering, seeded cells are closely surrounded by hydrogel networks. The corresponding cell microenvironment in a hydrogel is different from that on the internal surface of a porous scaffold. Such a material may also influence cell behavior and can usually better reflect the 3D characteristics of cells [178–180].
《4. Material-controlled cell geometry》
4. Material-controlled cell geometry
The shape of a cell can be influenced by the appropriate patterning of the material to which the cell adheres. Ingber et al. [181] conceived the geometric control of single cells using surface patterning for the first time. Their patterns were composed of adhesive microislands on a non-fouling background. Subsequently, a series of patterned materials have been developed for cell studies, particularly for cell adhesion [182–186].
Further development revealed that even the differentiation of a single cell could be significantly regulated by cell geometry. However, the patterning technique for studies of cell differentiation is more difficult than that of cell adhesion because it is difficult to maintain the cell shape for a long time. Indeed, most of the nonadhesive background could not maintain non-fouling for several days in a culture medium with full serum.
Ding’s group [187–190] developed a persistent micropattern with potent cell adhesion contrast, which enabled extensive investigation of stem cell differentiation on micropatterned surfaces. Micropatterns with arginine-glycine-aspartic acid (RGD) peptides on poly(ethylene glycol) (PEG) hydrogels were fabricated by combining photolithography techniques, macromonomer techniques, and transfer lithography, among others. Single MSCs from rats could be well localized on RGD microislands on this strong and persistent non–fouling background by keeping the different shapes of the adhesive microislands of appropriate sizes for single–cell adhesion. The cell shapes were found to influence the differentiation of MSCs, as shown in Fig. 3 [191].
《Fig. 3》
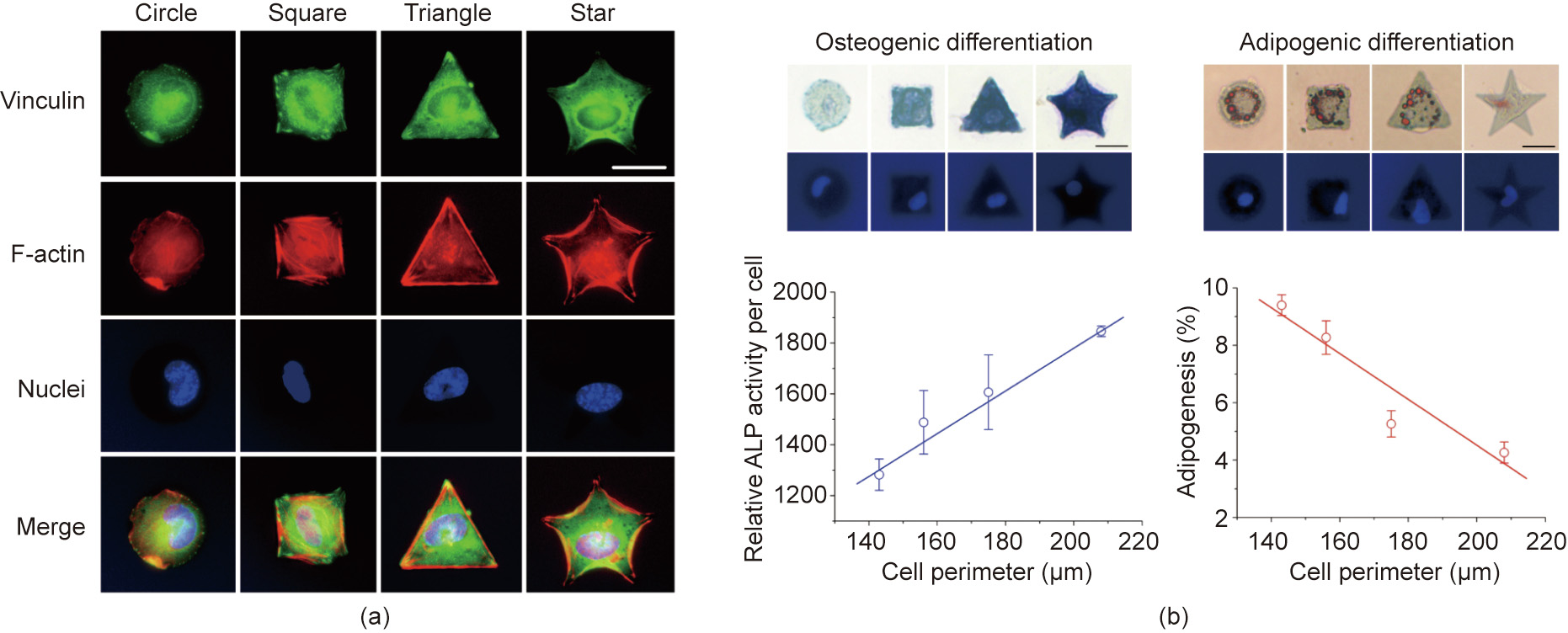
Fig. 3. (a) Fluorescent micrographs of single cells on RGD microislands of the indicated different shapes, and (b) the effects on the differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. ALP: alkaline phosphatase. Scale bar: 25 μm. Reproduced from Ref. [191] with permission of Elsevier Ltd., © 2011.
Using the unique micropatterning technique, it was illustrated that cell size (spreading) and cell–cell contact were two underlying factors behind cell density in terms of regulating stem cell differentiation [192]. Circular-shaped cells favor adipogenesis, whereas osteogenesis is positively related to cell tension [193]. Besides differentiation, the morphology, migration, and transcription of cells are also influenced by materials with different surface architectures [194].
With the assistance of the surface patterning technique, it has been revealed that besides cell spreading size, the extent of cellcell contact, cell fate, and other cellular behaviors can be well controlled and significantly regulated by material cues [195–203].
《5. Effects of material charges on cells》
5. Effects of material charges on cells
Functional groups, as crucial chemical cues, are known to affect cell behavior. It has been reported that hydroxyl (–OH) and amino (–NH2) terminated surfaces are more beneficial for osteogenesis than carboxyl (–COOH) and methyl (–CH3) terminated surfaces [204]. Curran et al. [205] revealed varied differentiation of MSCs on cell culture glass modified with –CH3, –NH2, hydrosulfuryl (–SH), –OH, and –COOH groups. As for functional groups incorporated into PEG hydrogels, the phosphate-functionalized gel promoted osteogenesis of MSCs, while the tert-butyl-functionalized gel promoted adipogenesis [206].
Proteins from cell culture media can be adsorbed on material surfaces. According to measurements of quartz crystal microbalance with dissipation, there are fewer adsorbed proteins on the neutral –CH3 and –OH surfaces than on the charged –COOH and –NH2 surfaces. Based on a study of chemical biology, Ding’s group [207] reveals that functional groups regulate stem cell differentiation by tuning protein adsorption, which affects nonspecific cell adhesion and cell spreading (Fig. 4).
《Fig. 4》
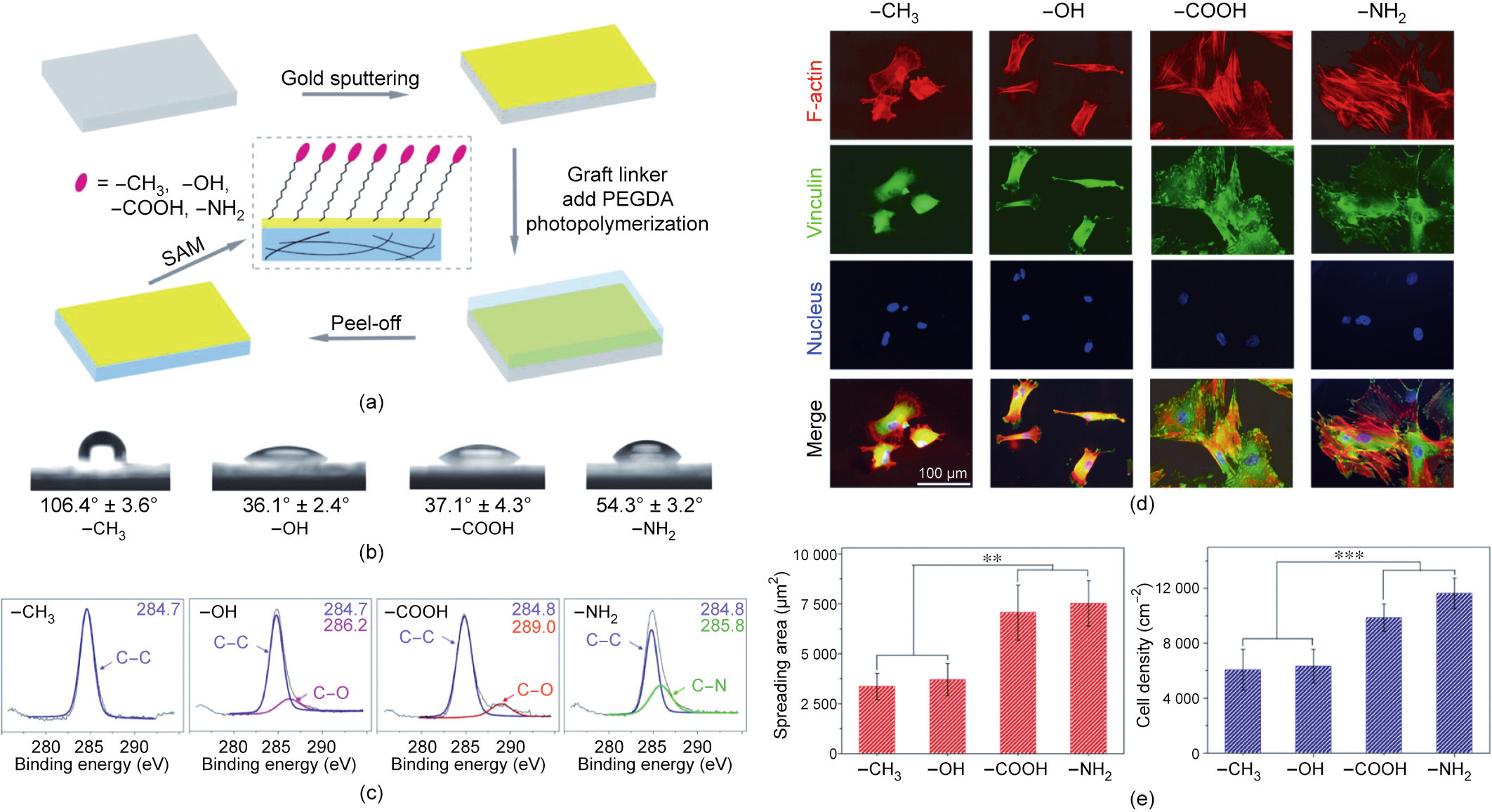
Fig. 4. (a) Fabrication of PEG-network-based gold surfaces self-assembled with four types of functional groups to examine the effect of functional groups on MSCs. The terminal functional groups are –CH3, –OH, –COOH, and –NH2. SAM: self-assembly monolayer; PEGDA: poly(ethylene glycol) diacrylate. (b) Measurement of water contact angles on gold surfaces modified with functional groups. (c) X-ray photoelectron spectroscopy (XPS) detection of C1s on gold surfaces self-assembled with the four functional groups. (d) Fluorescence micrographs of cells with F-actin, vinculin, and nuclei stained after the MSCs underwent chondrogenic induction for nine days on surfaces with the indicated different functional groups. (e) Statistics of the spreading area and cell density on surfaces with different functional groups after nine days of chondrogenic induction of MSCs (n = 4). The data are shown as mean ± standard deviation and treated via one-way ANOVA analysis. The significant differences are indicated as ** for 0.001 < p < 0.01, *** for p < 0.001. Reproduced from Ref. [207] with permission of American Chemical Society, © 2017.
《6. Matrix stiffness and biomechanical microenvironment》
6. Matrix stiffness and biomechanical microenvironment
Matrix stiffness provides a crucial physical cue for stem cell differentiation. Discher et al. [208] reported for the first time that the stiffness of the base materials could significantly affect the differentiation of MSCs. As shown in Fig. 5 [208], the lineage specification is directed by the matrix stiffness. Following culture, MSCs underwent neural differentiation on the soft substrate, myogenic differentiation on the medium-stiffness substrate, and osteogenic differentiation on the hard surface [208]. Similar experimental results were later reported in other studies, including that by Schaffer et al. [209].
《Fig. 5》
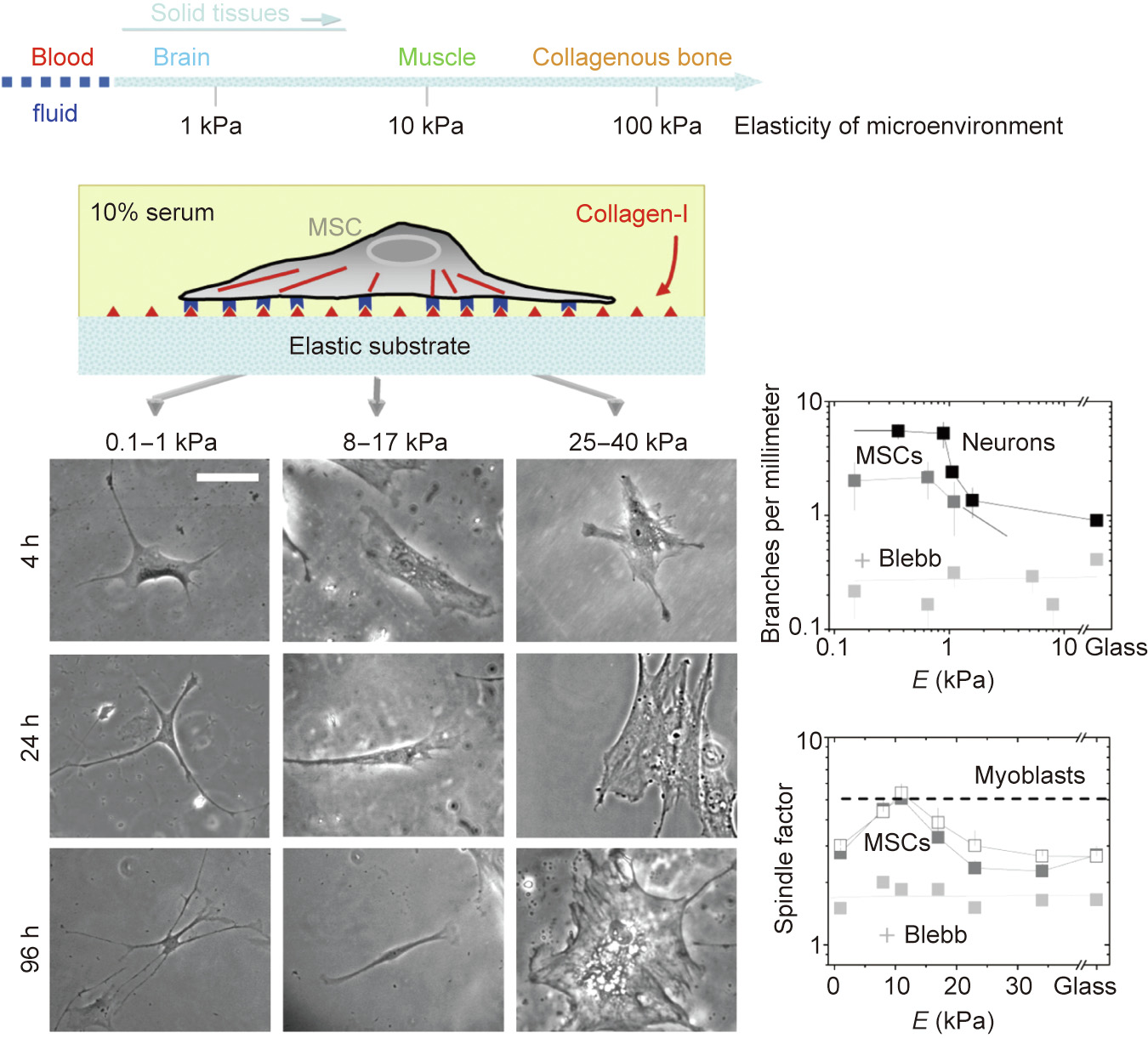
Fig. 5. Matrix elasticity directs stem cell lineage specification. + Blebb means addition of blebbistatin, a selective cell-permeable inhibitor of non-muscle myosin II ATPases, in order to destroy cytoskeleton to some extents, and thus block branching, elongation, and spreading of MSCs in this study. Reproduced from Ref. [208] with permission of Cell Press, © 2006.
Although the stiffness effect could be regarded as a milestone in the history of the material-related cell microenvironment [208,210], it was once challenged by other groups [211]. When Discher’s group [208,210] changed the moduli of poly(acrylamide) (PAAm) and then performed surface modification using biomacro-molecules to enhance cell adhesion, they attempted to maintain the same amount of surface grafting and adsorption of proteins. However, other groups have argued that this is impossible given the sensitivity of the cells and instead declared that it might be the underlying surface chemistry and not matrix stiffness that led to the difference in cell responses [211]. This argument was later refuted by Engler et al. [212]. In both experiments, the matrix stiffness and surface chemistry were coupled; therefore, it is necessary to develop a model material system that can decouple matrix stiffness and surface chemistry in a strict manner.
Ding et al. [213] developed a unique surface nanopatterning technique for cell-adhesive RGD arrays on non-fouling PEG hydrogels by transfer lithography. The nanopatterns were originally generated via block copolymer micellar nanolithography and were well controlled. If the same nanopattern is transferred to different PEG hydrogels crosslinked with different macromonomer lengths and/or concentrations, it is possible to obtain materials with the same surface chemistry and tuned matrix stiffness. Under such strict conditions, different cell spreading areas and extents of stem cell differentiation were observed and their deterministic experiments provided the most solid evidence of the fascinating stiffness effect related to the biomaterial-related cell microenvironment. Furthermore, an ‘‘abnormal” extent of adipogenesis was observed with the matrix stiffness, although the osteogenesis was normal. Ultimately, Ding et al. [214] interpreted these interesting behaviors using cell–cell contact and cell–matrix contact unified under the matrix stiffness effect. Strong mechanical feedback from a stiff hydrogel leads to increased activation of F–actin complexes and stronger cell tension. The corresponding inside–outside–inside sensing results in increased osteogenesis (Figs. 6(a) and (b))[213]. Cells usually adopt a more spread morphology on stiff hydrogels. Vinculins were observed to be densely distributed around the cell periphery on the stiff hydrogels, and betteraligned actin filaments were observed on the stiff hydrogels, which suggested higher cell tension, as shown in Fig. 6(c) [214]. It is interesting that Chen et al. [215] used microarrays of varied heights to adjust the stiffness of the base materials. They found that adipogenic differentiation favored soft micropillars, whereas osteogenic differentiation favored hard micropillars.
The stiffness of a biomaterial affects cell differentiation and tissue regeneration. For example, some metals exceed the hardness of authentic bone and thus form stress shielding [216], which hinders the normal process of osteogenesis and osteoclastogenesis, while soft scaffolds may not be able to bear the mechanical requirements of the tissue. Matrix stiffness can also influence nervous tissue. Moreover, an increase in immunological responses has been reported after the implantation of rigid materials in the central nervous system [217,218]. Therefore, matching the tissue hardness and material stiffness must be taken into consideration in tissue engineering. It is worth noting that even the in vivo stiffness of the spinal cord after implanting a hydrogel can be semiquantified by ultrasound elastography as a non-invasive, clinically established tool in a canine model [218]. Many comprehensive investigations of matrix stiffness and biomechanical microenvironments are expected in the near future.
《Fig. 6》
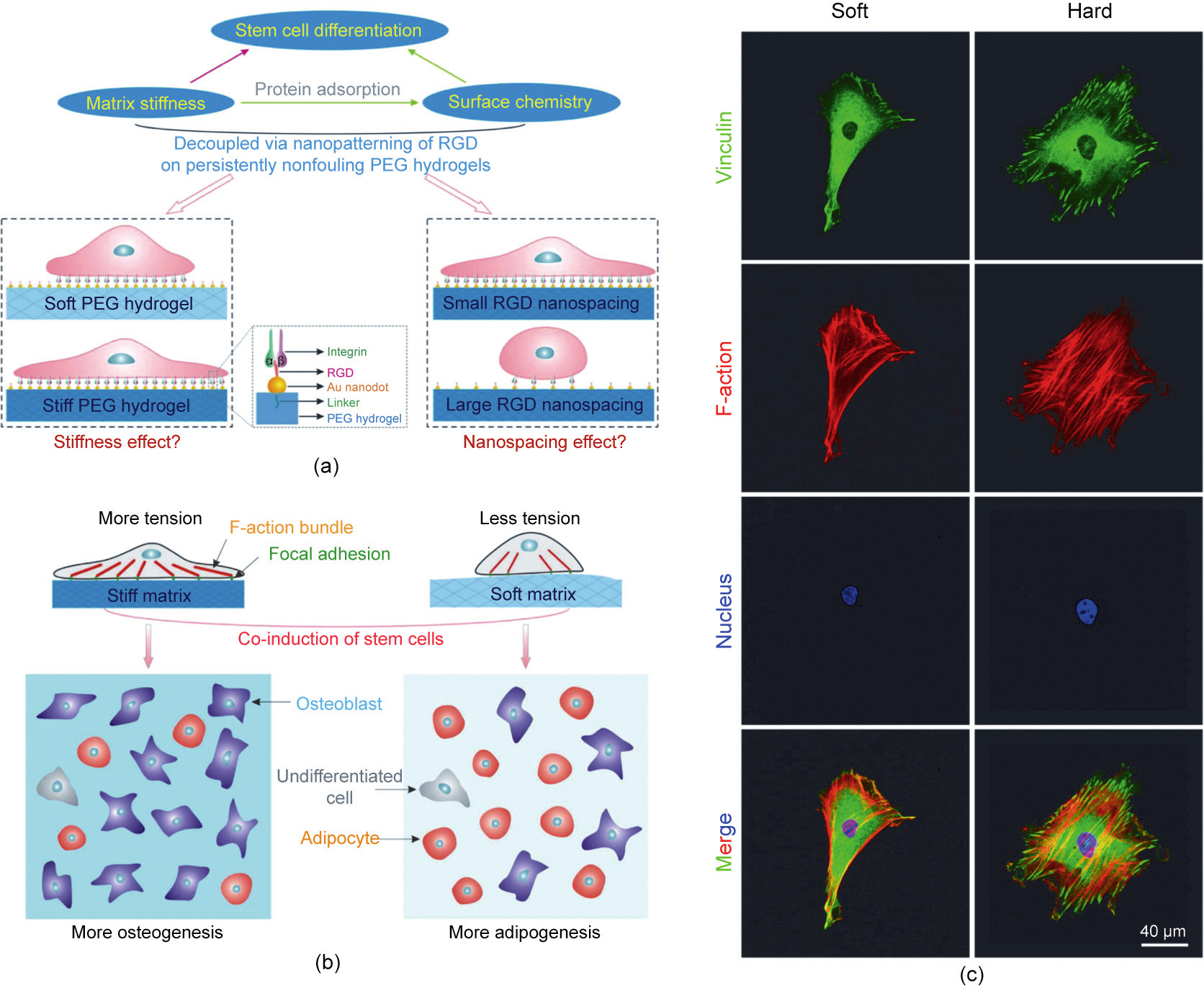
Fig. 6. (a) Schematic illustration of the investigation into the effects of matrix stiffness and organization of cell-adhesive ligands on the adhesion and differentiation of stem cells. (b) Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. (c) Fluorescence micrographs of adherent cells on RGD nanopatterned PEG hydrogels of the indicated stiffnesses. Stem cells were allowed to adhere to the substrates for 24 h, followed by immunofluorescent staining to visualize vinculins (green), F-actins (red), and cell nuclei (blue). (a, b) Reproduced from Ref. [213] with permission of American Chemical Society, © 2015; (c) reproduced from Ref. [214] with permission of American Chemical Society, © 2016.
《7. Surface modification of materials》
7. Surface modification of materials
It is worth noting that the series of the strict experiments of Ding’s group based on their unique nanopatterning techniques and careful experimental design has supported not only the stiffness effect originally suggested by Discher et al. [208,210], but also the effect of surface chemistry by Watt et al. [211]. The RGD nanopatterning technique on PEG hydrogels can decouple the effects of matrix stiffness and surface chemistry, and can also be used to examine the surface chemistry simply by transferring nanopatterns of varied nanospacings to a hydrogel with the same matrix stiffness. Significant nanoscale cues to regulate cell behavior have been revealed by Ding et al. [219].
RGD nanospacing might be an inherent regulator of stem cell differentiation, which may be suitable to study the effect of surface modification and structure on cells. The biological focal adhesion is well formed only when the RGD nanospacing on an ECM or a material substrate is less than the critical value of approximately 70 nm, as shown in Fig. 7 [97,220]. Thus far, RGD modification has been found to influence cell adhesion, migration, proliferation, differentiation, and dedifferentiation [98,221–224].
《Fig. 7》

Fig. 7. (a) Effect of RGD nanospacing on the differentiation of stem cells. (b) Fabrication of RGD micro/nanopattern and corresponding study of stem cell differentiation. (a) Reproduced from Ref. [220] with permission of Elsevier Ltd., © 2013; (b) reproduced from Ref. [97] with permission of American Chemical Society, © 2015.
Surface modification of biomaterials is an efficient way to improve the biocompatibility and bioactivity of biomaterials without altering their bulk properties. This is far beyond RGD grafting. surface modification includes chemical modification [225–232] and physical modification [233–247], and methods of surface modification usually include immersion [248,249], coating [250], grafting [251], and plasma treatment [252]. For example, HAp is a natural component of bone and is often used to modify the surface of bone repair materials. HAp can promote the proliferation and metabolism of osteoblasts because of its presence in natural bone. As shown in Fig. 8 [35,53], the modified PLGA scaffolds with nanoscale HAp (nHA) showed a better regenerative effect than pure PLGA scaffolds. Physical modification is usually achieved by regulating the surface topological morphology or roughness of scaffolds, thereby controlling cell adhesion, proliferation, and differentiation. It has been confirmed that microscale-roughness scaffolds benefit the adhesion of bone marrow-derived stem cells and do not hamper the proliferation and osteogenic differentiation of the cells [253]. Kim et al. [254] revealed that 3D porous scaffolds with different and appropriate topological surfaces can stimulate both osteogenesis and chondrogenesis, and thus provide a design strategy for bilayered osteochondral repair.
《Fig. 8》

Fig. 8. (a) Field-emitting scanning electron microscopy (FE-SEM) images of the pore surfaces of PLGA scaffolds with HAp coating (upper); FE-SEM images of the pore surfaces of a virgin PLGA scaffold (lower). (b) Histological images of bone regeneration and nHA coated PLGA scaffolds showed better bone formation. (c) Schematic presentation of regeneration of articular cartilage and subchondral bone by PLGA porous scaffold. (a, b) Reproduced from Ref. [53] with permission of Dove Press, © 2013; (c) reproduced from Ref. [35] with permission of American Chemical Society, © 2018.
For tooth or bone tissue regenerative materials, surface modification is usually applied to improve bone integration. Regarding biosealing of an implant with soft tissues, the excellent versatile adhesion of polydopamine provides a good option for covalent binding of biomacromolecules to an even chemically inert surface to enhance the surface bioactivities [255,256]. Surface modification of vascular grafts using heparin or other bioactive molecules can efficiently enhance hemocompatibility and consequently prevent thrombosis in artificial vascular grafts [257–261]. Swartz et al. [262] developed an acellular tissue-engineered vessel on the basis of small intestinal submucosa that was functionalized sequentially with heparin and VEGF. Some bioactive ingredients can promote vascularization and osteogenesis. For instance, Qi et al. [263] reported that deferoxamine promoted bone regeneration with MSCs and vascularization in endothelial cells.
《8. Perspective》
8. Perspective
Tissue engineering and tissue regeneration have developed significantly in recent years. Fundamental research on the interactions between cells and materials has enriched the design of biomedical materials, and strategies for using bioactive factors to improve the compatibility and activity of biomaterials have also been proposed. Modular self-assembly and biological 3D printing offer unprecedented flexibility and potential in tissue engineering, and the design and manufacture of regenerative scaffolds. While much progress has been made, there are still some key issues that require attention, as schematically presented in Fig. 9.
《Fig. 9》

Fig. 9. Perspective key issues regarding tissue engineering and the cell microenvironment.
(1) More findings of nanoscale material cues to influence various cell behaviors. It has been recently revealed that cells can delicately sense and respond to external nanoscale features in intricate living systems. The nanoscale spatial arrangement of cell-adhesive ligands affords a new independent regulator of cell differentiation, which should be taken into consideration in biomaterial design for regenerative medicine. In the near future, we believe that researchers will establish additional nanoscale material cues that influence various cell behaviors.
(2) Combination of multiple spatial effects of tissue-engineered porous scaffolds. While various geometrical features of biomaterials that affect cells have been examined on macroscopic, mesoscopic, and microscopic levels, the structure of an organism is complex and gradient. To better mimic organisms that are hierarchical, it is imperative to prepare porous scaffolds with multi-scale responses.
(3) Complex microenvironments in vivo, such as the immune response after implantation of biomaterials. The foreign body immune reaction, composed of macrophages and foreign-body giant cells, is the end-stage inflammatory and wound healing responses following implantation of a medical device, prosthesis, or biomaterial [264–266]. Immune-mediated tissue regeneration driven by a biomaterial scaffold is emerging as an innovative regenerative strategy to repair damaged tissues. Manipulating the adaptive immune system using biomaterial engineering may support the development of therapies that promote both local and systemic pro-regenerative immune responses, ultimately stimulating tissue repair [267–270].
(4) Material degradation and the dynamic microenvironment. An ideal tissue engineering and regeneration material should be biodegradable, and considerable efforts have been made to tune the biodegradation of various materials [80,81,271–286]. Moreover, bioimaging techniques have promoted the detection of this biodegradation process [287–291].
The ECM is dynamic, and biomaterials implanted into a body are influenced by physical, chemical, and biological signals, and stress loads. To recapitulate the dynamic nature of the ECM, many reversible chemistries have been incorporated into biomaterials to regulate cell spreading, biochemical ligand presentation, and matrix mechanics [292]. Under the stimulation of such long-term and complex action, the scaffold material may have complex structural failures or molecular fractures; therefore, the biomaterials should be carefully designed.
In addition to degradation products [293–296], the degradation rate has recently been found to afford a dynamic cue to regulate the adhesion and differentiation of cells on well-designed hydrogels [297]. The process of material degradation is inevitably accompanied by changes in the crosstalk of the material with cells, thus creating a dynamic material microenvironment for cells. Cells under fast degradation might sense the impact of changes of the hydrogel, leading to cell activation along with the reconstruction of the cytoskeleton and a higher traction force. The dynamic microenvironment is becoming a research focus in tissue engineering and tissue regeneration.
(5) Synergistic effects of various microenvironmental cues. Cell microenvironments include comprehensive considerations related to both material and non-material factors [298,299]. The intricate relationship between these factors determines the biomaterialrelated cell microenvironment. In particular, more attention should be paid to synergism among different cues to adjust the cell microenvironment in the future.
(6) Development and application of in vitro quasi-tissue engineering techniques. The development of in vitro tissue engineering techniques, such as organ-on-a-chip or organoids, helps us to better understand the tissue regeneration process. Such in vitro construction devices and techniques can also be used for large-scale drug screening [300–304]. In addition, organoids, which use hydrogels or other materials to assist multiple cells in forming ordered cell clusters, is a promising trend in quasi-tissue engineering [305,306].
(7) Precise biomaterial manufacturing and individual medical treatment for in vivo tissue engineering. Along with the emergence of precision medicine and personalized medicine, the customization and design of scaffold structures has become increasingly important. 3D printing is a cutting-edge technique to build customized scaffolds [307–320], but some key technologies still need to be improved, particularly in terms of accuracy and reliability.
(8) Clinical transformation of biomaterials and tissue engineering. The long-term outcomes of engineered tissue and regenerated tissue remain inconclusive [321]. Moreover, it remains unclear what the roles and ultimate fate of the early transplanted cells are in in situ tissue regeneration. More attention should be paid to the standardization of biomaterials in clinical transformation. With the increase in industrialization demands, the standardization of batch manufacturing and testing has become prominent.
In summary, tissue engineering has paved the way to repair damaged tissues or build new tissues to treat a multitude of diseases and illnesses. In situ induction after implanting bioactive material scaffolds can also be used to regenerate tissues in vivo. Despite rapid progress with in vitro and small animal studies, progress toward realizing clinical and commercial endpoints has been slow. With the integration of multiple disciplines, especially material-related microenvironments, it is hoped that greater progress will be made in tissue engineering and tissue regeneration for the benefit of mankind. The future trend of the tissueengineered cell microenvironment might lie in the modular integration of bioactive materials and the gradual realization of precision medicine.
《Acknowledgments》
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (21961160721 and 52130302) and the National Key Research and Development Program of China (2016YFC1100300).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jingming Gao, Xiaoye Yu, Xinlei Wang, Yingning He, and Jiandong Ding declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




