《1 Introduction》
1 Introduction
Nowadays, white LEDs (w-LEDs) have become mainstream solid-state light sources owing to their low energy consumption, long life, compactness, fast response, high luminescence efficiency, and pollution-free properties. However, traditional w-LEDs exhibit problems of low color rendering index, high color temperature, and blue light hazard [1]. As the requirements for lighting quality increase, high quality and full-spectrum lighting has become the new global trend for “healthy and green lighting”. A full-spectrum white LED refers to the LED [2–4], which is close to the natural spectrum (as depicted in Fig. 1) with wide spectra (380–780 nm), good spectral continuity, excellent color rendering index, and strong color reduction ability to objects. Therefore, it is widely used in operating lights, camera lights, up-market stage, healthy lighting, plant growth lamps, etc. [5–7]
《Fig.1》
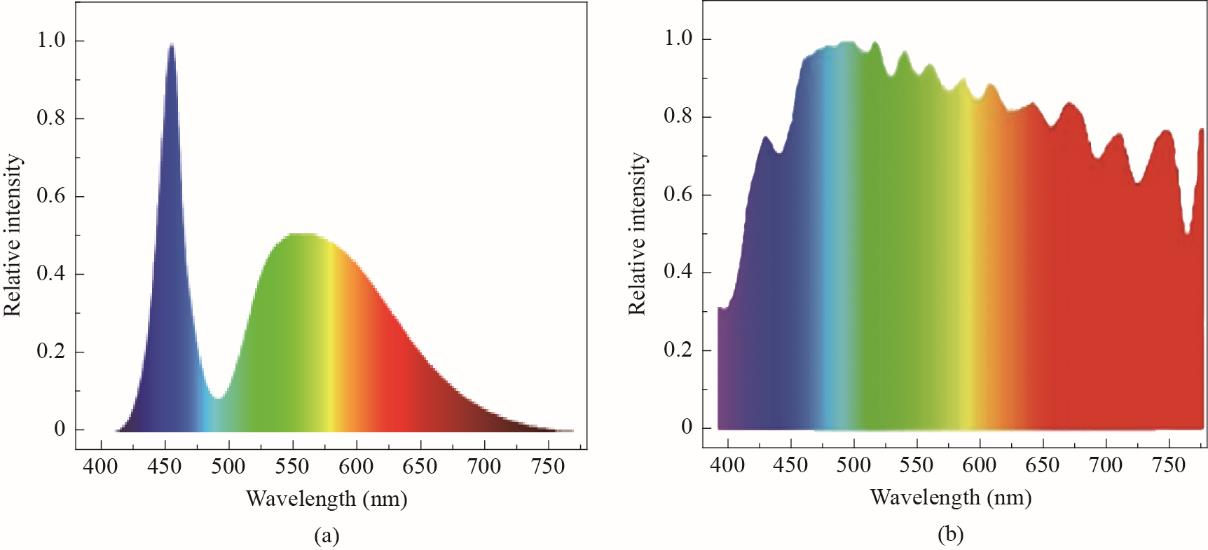
Fig.1. Conventional LED spectra (a) and solar visual spectra (b).
At present, there are two main ways to realize full-spectrum LED [8,9]. The first is multi-chip synthesis of white light composed of multiple blue\cyan\green\yellow\red chips. The multi-chip method has the advantage of high luminous intensity and color rendering. However, a “green gap” exists in the spectra because of different performance of chips, especially because the luminous efficiency of green and yellow are lower than that of other chips. In addition, the light attenuation of the chip is quite different, which leads to an unstable color temperature, complex design control in the circuit, high cost, and poor performance [10,11]. The other way to realize full spectra is single chip LED coated phosphors; it is to match a variety of color phosphors on violet/ near ultraviolet chips. Compared with multi-chip LEDs, the method of single chip LED coating phosphors has advantages of light color stability, uniformity, simple manufacturing technology, and low cost [12,13], thus becoming the mainstream method used in full-spectrum white LEDs.
In recent years, the performance of violet–near ultraviolet chips has greatly improved. The performance of full-spectrum white LEDs is mainly determined by phosphors. In this study, the research status of phosphors excited by violet and near ultraviolet light is analyzed systematically. The findings reveal that the current full-spectrum technology faces difficulties in the achievement of spectrum continuity. This is mainly due to the low luminous efficiency and poor stability of the existing luminescent materials. Therefore, future development of high-efficiency phosphors excited by violet and near ultraviolet light is crucial.
《2 Development status of phosphors for full-spectrum LED lighting》
2 Development status of phosphors for full-spectrum LED lighting
The International Commission on Illumination (Commission Internationale de l´Eclairage, CIE) presents the color rendering index according to the degree to which the light source depicts the real color of the object, and the color rendering indexes Ra of standard light sources are set to 100. For some specific scenarios, the Illuminating Engineering Society of North America has proposed indicators (color fidelity Rf and color saturation Rg, IEC TM-30-15) that can better evaluate the ability of light sources to reveal true colors. In addition to international standards, different standards have been established in each region of the industry [14,15]. The performance of full-spectrum LED products in the industry can attain Ra = 97, Rf = 96, and Rg ≈ 100 [16]. To obtain this unique continuous and uniform spectrum, phosphors of various colors must be matched together, including blue (400–480 nm), cyan (470–505 nm), yellow-green (515–535 nm), yellow (540–575 nm), red (600–780 nm) phosphors, and single matrix white phosphors (400–780 nm) excited by violet - near ultraviolet light.
《2.1 Blue phosphors excited by violet - near ultraviolet light》
2.1 Blue phosphors excited by violet - near ultraviolet light
The traditional blue phosphor is BaMgAl10O17: Eu2+ (BAM). Its excitation spectrum is located at 240–420 nm and the emission peak at 450 nm [17]. However, the full width at half-maximum (FWHM) and thermal stability of BAM are insufficient, which limits its application.
Researchers have studied silicate, chloride, phosphate, oxide, and other systems of blue phosphors. Among them, the raw materials of traditional silicate phosphors with high luminous efficiency are abundant in natural reserves and cheap. However, it exhibits poor thermal quenching behavior [18]. Chlorophosphate and oxide systems with high thermal stability are promising blue phosphors. The excitation spectra of chlorophosphate phosphor ranges from 210–420 nm, and its emission peak is located at 441 nm. The decrease in quantum efficiency at 150 °C relative to room temperature is comparable to that of the YAG phosphor [19]. The oxide system SrLu2O4: Ce3+ phosphor, with a rigid structure, exhibits excellent physical and chemical stability, and perfect moisture resistance. The strongest excitation peak of the SrLu2O4: Ce3+ phosphor is 405 nm, which is consistent with the violet chip. Its emission peak is located at 460 nm, with a FWHM of ~ 90nm. It has good thermal stability and the luminous intensity remains at 86% at 150 °C compared to that at room temperature [20].
《2.2 Cyan phosphors excited by violet - near ultraviolet light》
2.2 Cyan phosphors excited by violet - near ultraviolet light
Among cyan phosphors, chlorophosphate has been applied preliminarily. The Sr5(PO4)3Cl: Eu2+ phosphor reveals a redshift of the emission peak from blue light at 441 nm to cyan light at 470 nm by replacing Sr with Ba [21]. Mitsubishi Chemical Co., Ltd. (MCC) first introduced the commercial application of this phosphor. In recent years, the domestic Grirem Advanced Materials Co., Ltd. (GRIREM) also launched a series of chorophosphate phosphor products with good crystallization morphology and luminescent properties; its morphology is illustrated in Fig. 2.
《Fig.2》

Fig.2. SEM of commercial chlorophosphate phosphor particles.
There are many problems in other cyan phosphors, which are difficult to deal with in practical applications. For example, the oxynitride BaSi2O2N2: Eu2+ (with an emission peak of 495 nm and FWHM of 31 nm) displays high luminous efficiency but poor thermal quenching performance [22]. New Ba9Lu2Si6O24: Ce3+ silicate has high stability, and its quantum efficiency at 150 °C remains 92% relative to room temperature. However, its crystalline morphology is inadequate, which is not conducive to packaging applications [23]. The new carbonitride phosphor YScSi4N6C: Ce3+ has a relatively wide FWHM (89 nm). YScSi4N6C: Ce3+ cyan phosphor, LuAG: Ce3+ green phosphor, and CaAlSiN3: Eu2+ red phosphor are mixed together to emit uniformly warm white light under the excitation of ultraviolet light. The color rendering index Ra is 94.7, and the relevant color temperature is 4159 K. The spectrum can effectively fill the “spectrum chasm” of the current white LED [24]. It has a certain application prospects, but its luminous efficiency still needs to be improved.
《2.3 Yellow-green phosphors excited by violet - near ultraviolet light》
2.3 Yellow-green phosphors excited by violet - near ultraviolet light
At present, the main materials among the yellow-green phosphors are aluminate, oxynitride, silicate, phosphate, and oxyhalide. Commercial phosphors are aluminates that can be excited by violet light. They emit yellow-green light that peaks at 515 nm to 540 nm by changing the matrix content and luminescent center content. In recent years, with the independent development of high-temperature firing equipment and the continuous upgrading of preparation technology, the industrialized preparation of high-efficiency Ga-containing Y3Ga2.5Al2.5O12:Ce3+ and Lu-containing Lu3Al5O12:Ce3+ aluminate phosphors has been realized in China. Commercial phosphors have the advantages of spherical shape, high crystallinity, high luminous efficiency, and good stability. In the field of lighting, aluminate yellow-green phosphors have become mainstream phosphors for full-spectrum LED lighting. In addition, the new design of phosphors with garnet structure is a vital development in recent years. In the A3B2X3O12 structure, a new type of M2LnZr2Ga3O12:Ce3+ phosphor(M=Ca, Sr; Ln=La, Gd, Y, Lu) was developed by designing the A and B positions [25,26]. It emits yellow-green light with a peak at 515 nm under near-ultraviolet excitation. A new series of Ca3Zr2(Si,Ge)Ga2O12:Ce3+ phosphors with good thermal stability was obtained by replacing Ga with a tetravalent ion Si or Ge with a similar radius at the X-site to balance the charge.
Oxynitride β-SiAlON: Eu2+ phosphor has a wide excitation band (250–460 nm) and reveals emission peaks at 535 nm, as portrayed in Fig. 3. With excellent thermal stability, its emission intensity is 90% at 150 °C relative to room temperature [27]. However, the patent, technology, and market have been monopolized by foreign countries.
《Fig.3》
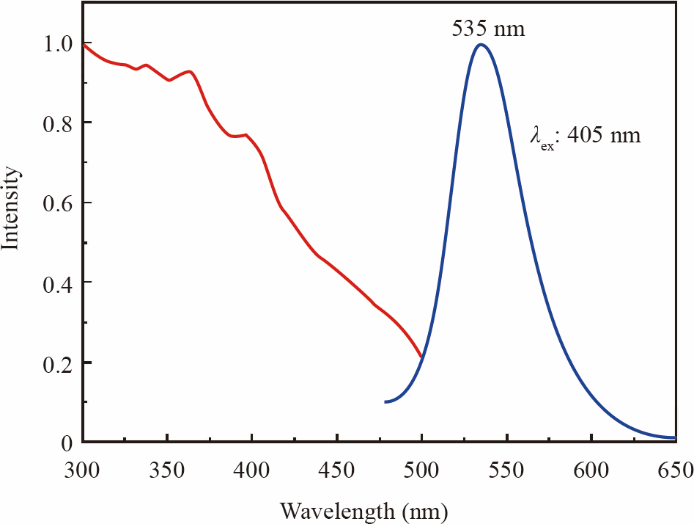
Fig.3. Photoluminescence excitation and emission spectra of the β-SiAlON: Eu2+ phosphor.
Researchers have also studied other systems, such as phosphate and oxyhalide systems; however, the stability is insufficient for the application needs.
In general, among yellow-green phosphors, aluminate phosphors have been used in practice. The stability of silicate and phosphate phosphors needs to be further improved. There are some problems in β-SiAlON: Eu2+ phosphors, such as narrow FWHM and lack of domestic preparation technology.
《2.4 Yellow phosphors excited by violet - near ultraviolet light》
2.4 Yellow phosphors excited by violet - near ultraviolet light
The Y3Al5O12:Ce3+ (YAG:Ce3+) aluminate with a garnet structure is the most widely used yellow phosphor. However, its excitation spectrum exhibits weak absorption and low conversion efficiency in the violet-near ultraviolet region, which makes it difficult to fulfill the full-spectrum LEDs [28]. In 2009, Seto et al. [29] first reported La3Si6N11:Ce3+ (LSN:Ce3+) phosphor, with a highly condensed network structure. The emission spectra can be decomposed into two bands centered at 543 nm and 585 nm. The sample is also packaged with a chip, and the wLED exhibits a high color rendering index and low color temperature. In addition, the thermal stability of LSN: Ce3+ is superior to that of YAG: Ce3+ . However, similar to YAG: Ce3+, its excitation spectra are at a low level at approximately 400 nm, which results in a low absorption rate to violet light. Furthermore, the silicate yellow phosphor has been extensively studied, but its stability is insufficient [30]. The Eu2+ -doped phosphate system has strong absorption in the violet region and can emit a wide spectrum of yellow light [31].
At present, the yellow phosphor excited by violet light has not been applied well, mainly because of the low absorption of violet light or near ultraviolet light of aluminates and nitrides, and the poor stability of silicates and phosphates.
《2.5 Red phosphors excited by violet - near ultraviolet light》
2.5 Red phosphors excited by violet - near ultraviolet light
Red phosphors play a pivotal role in the color rendering index and color temperature in full-spectrum LEDs. At present, the commercialized red phosphors are Eu2+ -activated nitrides (including (Ca, Sr) AlSiN3: Eu2+, (Ca, Sr)2Si5N8: Eu2+) [32–35], which can be excited by a wide spectrum of 200–600 nm and emit a broad spectrum of red light with a peak wavelength of 620–670 nm, as depicted in Fig.4. The luminous efficiency and stability of the nitride red phosphors are adequate for the requirements of full-spectrum LEDs.
In addition, a series of novel red phosphors have been developed in recent years, such as phosphate, silicate, and other systems. Eu3+ is typically chosen as the activator of these phosphors, which have narrow emission spectra and the disadvantages of weak emission intensity and low wavelength matching with the chip [36,37]. At the same time, the phosphors with other ions as activators have also been studied. For example, under the excitation of 406 nm violet light, the phosphors of CaMoO4: Sm3+ emit red light with a peak wavelength of 659 nm [38], but the luminous efficiency is relatively low. In view of the serious problem of far red light missing in the current conventional LED spectrum, the Cr-doped gallate garnet phosphor can effectively suppress far-red light in the spectra. Its excitation spectra are located at 360–500 nm, and the peak wavelength is approximately 710–780 nm [39,40]. However, because of the large Stokes displacement, the conversion of violet or near ultraviolet light is low, and the luminous efficiency is still not sufficient to meet the actual needs.
《Fig.4》
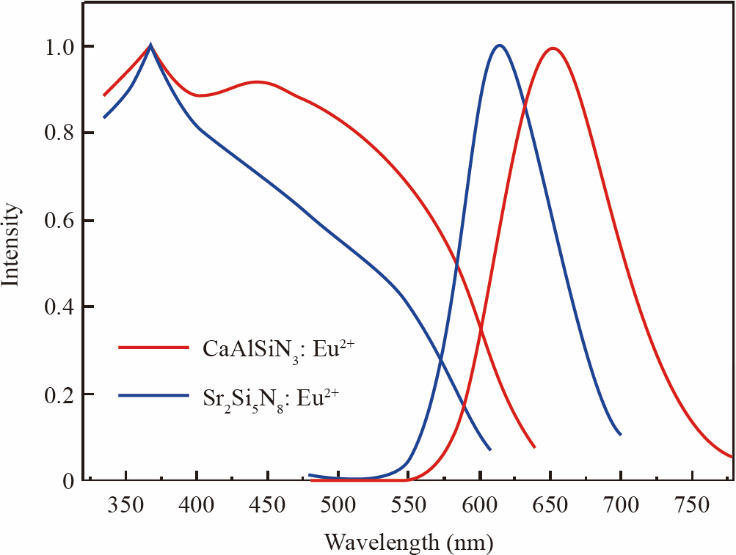
Fig.4. Excitation and emission spectra of the CaAlSiN3: Eu2+, Sr2Si5N8: Eu2+ phosphors.
《2.6 Single matrix white phosphors excited by violet - near ultraviolet light》
2.6 Single matrix white phosphors excited by violet - near ultraviolet light
There are two primary methods to study the single-matrix white phosphors. These are several (two or more) ion doping and single ion doping. At present, many kinds of ion doping systems are widely studied, mainly through the energy transfer between different activated ions to synthesize white light emission. For example, Ce3+ and Mn2+ codoped Ba2Ca(BO3)2 phosphors have two types of Ce3+ centers, emitting blue and green light, whereas the Mn2+ center emits red light. Therefore, white light emission can be generated under near-ultraviolet excitation through energy transfer from Ce3+ to Mn2+ [41]. There are also single ion-doped white phosphors, such as Ba9Y2Si6O24: Ce3+ phosphor under near-ultraviolet excitation; the emission spectrum covers from 430nm to 675 nm [42].
However, single matrix white phosphors generally face problems such as low quantum efficiency, insufficient red-light emission, and high color temperature, which make it difficult to obtain commercial applications in the market.
《3 Existing problems》
3 Existing problems
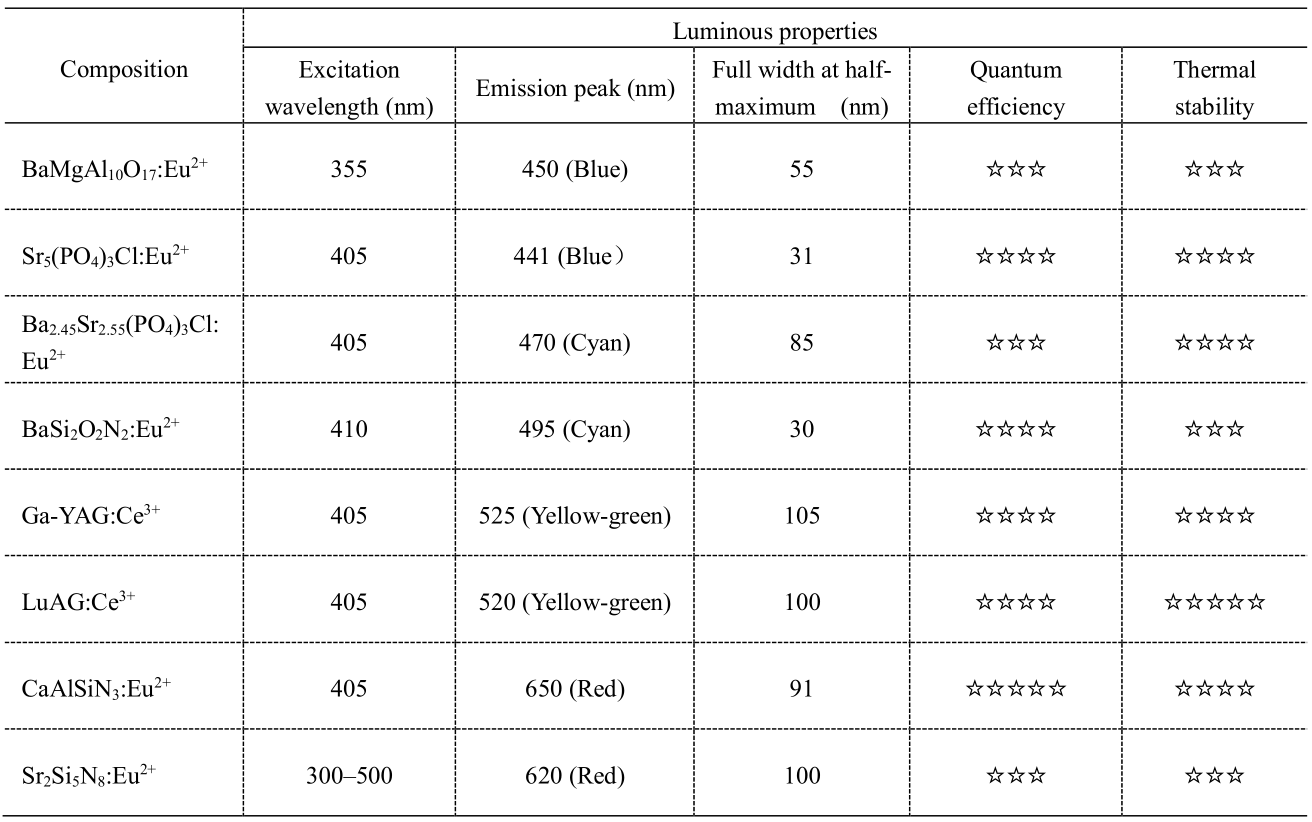
Recently, researchers have focused on phosphors that can be excited by violet/near-ultraviolet light. However, only a few systems have been preliminarily applied (Table 1).
BaMgAl10O17: Eu2+ and Sr5(PO4)3Cl: Eu2+ blue phosphors are preliminarily applied in full-spectrum lighting, and the latter has higher quantum efficiency. Chlorophosphate (Ba2.45Sr2.55(PO4)3Cl: Eu2+) and oxynitride (BaSi2O2N2:Eu2+) are predominantly used as cyan phosphors, among which the wider FWHM of chlorophosphate phosphors is conducive to the enhancement of spectral color rendering ability. The garnets are typically used as yellow-green phosphors. They can also emit strong yellow light. However, they are not suitable for full-spectrum illumination owing to their weak absorption in violet and near-ultraviolet light. The nitride phosphors are chiefly used as red phosphor for full-spectrum illumination.
Although there are certain bands of phosphors that can be applied in full-spectrum LEDs, at present, the continuity of the full-spectrum is poor, the saturation of color gamut is low, the color rendering index still requires further improvement, and there still exists a gap with the ideal solar spectrum. The lack of key phosphors is the key factor restricting the development of full-spectrum LEDs. The main problems are that the emission spectrum of blue phosphor available for full spectra is narrow, light efficiency and thermal stability are also low, short-wave red phosphor has mutual absorption in the blue-yellow region, and high-light-efficiency and long-wave red phosphor is deficient.
《Table 1》
Table 1. Phosphors for violet - near ultraviolet light LED chips.
《4 Key preparation technology》
4 Key preparation technology
Preparation technology is the key factor affecting the comprehensive properties of phosphors. At present, the preparation technology of phosphors includes a high-temperature solid-phase method, gas-phase deposition method, sol-gel method, precipitation method, and hydrothermal method [43–46].The high-temperature solid-phase method is the traditional method, which possesses the advantages of simple process, lower cost, easier industrialization, larger particle size, and higher luminous efficiency. This is the preferred preparation technology for full-spectrum LED phosphors.
Pressure, vacuum, temperature, and atmosphere are the key factors in determining the purity, crystallinity, and luminescent properties of the phosphors. With the development of full-spectrum LEDs, a superior performance is a requisite for the phosphors, and the control of these parameters during the preparation becomes increasingly strict. The requirements of related equipment are also increasingly complex. The continuous preparation technology and equipment of high hydrogen/low hydrogen/air, which is widely used in the oxide phosphors (such as garnet aluminate yellow-green phosphor), is available now. The phosphors of nitride and oxynitride systems usually need to be prepared at high temperatures and pressures. In particular, β-SiALON: Eu2+ has high requirements for pressure, vacuum, and temperature for equipment. At present, the key preparation technology and market for β-SiALON: Eu2+ green phosphors are monopolized by a Japanese company. Furthermore, the equipment for preparing nitride and oxynitride phosphors generally faces several problems, such as high energy consumption, low work efficiency, and batch instability. Therefore, the development of a key preparation technology for phosphors for full-spectrum LEDs is imperative, especially in terms of high-quality, ultra-high-temperature, high-pressure, and continuous nitriding equipment.
《5 Future development trends and industrial development suggestions》
5 Future development trends and industrial development suggestions
At present, full-spectrum LEDs are used primarily in indoor lighting and plant lighting. Due to the high cost and low performance of full-spectrum LEDs, the market for full-spectrum LED lighting lamps is not well developed. With the continuous improvement of full-spectrum technology, the cost of full-spectrum LED will decrease and the performance will increase, thereby causing a gradual growth in its market share.
High-quality full-spectrum lighting presents a challenge and, hence, is an opportunity for the LED industry. Phosphors with high luminous efficiency, good thermal stability, and wide emission spectra are invaluable for the development of full-spectrum LEDs. The phosphors that are currently available for full-spectrum lighting contain many problems, such as blue phosphors with narrow emission spectra, green phosphors with low luminous efficiency and thermal stability, lack of far-red phosphors, and inadequacy of key equipment and technology. These unsolved issues limit the industrial development of full-spectrum LEDs. Therefore, the development of broad-spectrum blue, high-efficiency green, and far-red phosphors is critical, and an efficient matching technology between the above phosphors and violet or near ultraviolet chips should also be achieved to promote the development of high-quality full-spectrum LEDs. The key preparation technology and continuous equipment for oxynitride green phosphors and nitride red phosphors must be advanced to enhance the quality and batch stability and reduce the cost of the oxynitride and nitride phosphors. The application technology of these phosphors in full-spectrum LEDs should also be improved to stimulate the development of full-spectrum LEDs.














 京公网安备 11010502051620号
京公网安备 11010502051620号




