《1 The rise of optical therapy》
1 The rise of optical therapy
Neurodegenerative diseases are a group of illnesses that are caused by the progressive and irreversible loss of neurons in specific brain regions, leading to cognitive and movement impairments. Neurodegenerative diseases include amyotrophic lateral sclerosis, Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease, and spinal muscular atrophy. The number of patients diagnosed with age-related neurodegenerative diseases worldwide is rapidly increasing every year, and these diseases are gradually becoming a more common cause of death in the aging population. AD and PD are the two most common neurodegenerative diseases. In China, the prevalence of PD in people over 65 years is 1.7%, with the number of people with PD now over 2.5 million. Meanwhile, the prevalence of AD is 3.21%, with the patient population exceeding 8 million. With the rapid growth of the aging population in China, the incidence rate is increasing each year. Moreover, AD and PD cause both physical and emotional distress to patients and caregivers and are an economic burden on society. However, currently there is no effective treatment to prevent or delay the pathological progression. Due to the rapid increase in the prevalence of AD and PD, China is facing significant societal and economic challenges. Therefore, there is an urgent need to develop non-invasive techniques for early diagnosis and treatment of such diseases. However, non-invasive diagnosis and treatment of neurodegenerative diseases have proven to be a significant challenge for medical researchers globally.
Light therapy is a non-invasive or minimally invasive physical therapy approach that utilizes red and near-infrared light to irradiate damaged tissue or cells and ameliorate disease. In 1903, Niels Finsen from Denmark won the third Nobel Prize in Physiology and Medicine for the treatment of lupus erythematosus with red light, paving the way for the application of light therapy. The biological effects of low-level lasers on tissue or the body were first illustrated by Endre Mester, a Hungarian physician. In 1967, Mester et al. discovered that a ruby laser (694 nm) could promote hair regeneration and wound healing in mice. This discovery led to the development of “low-dose laser therapy,” also known as low-level laser treatment. Low-dose laser therapy irradiation can result in a series of physiological and biochemical responses to regulate tissue or body functions, ultimately ameliorating or curing the target disease. Light therapy, which consists of three major light treatment technologies, i.e., high-power laser therapy, photodynamic therapy, and low-level laser therapy, has been widely used in the clinical treatment of various diseases. It is considered to be a minimally invasive/non-invasive procedure that requires precision and is helping to transform a number of clinical treatment strategies.
In recent years, many studies have reported that low-level light therapy can regulate the activity of neurons and microglia in animal models of neurodegenerative diseases, effectively reduce neuronal death, and delay disease progression [1,2]. Clinical trials using light therapy have also demonstrated that low-level light can improve the pathological symptoms of neurodegenerative diseases. Due to developments in optoelectronic technology, low-level light equipment is gradually becoming smaller, more intelligent, and more precise. As a non-invasive physical therapy with few side effects, low-level light therapy is promising in the treatment of neurodegenerative diseases. The exploration of its potential application and development of low-level light therapies for neurodegenerative diseases will help to promote its clinical application, standardize its market transformation, provide effective therapies and strategies for patients with neurodegenerative diseases as well as ease the nursing and medical burdens due to the growing aging population.
《2 Development of low-level light therapy》
2 Development of low-level light therapy
Low-level light therapy, also known as photobiomodulation (PBM), refers to red or near-infrared light that is used to treat a disease or injury by regulating damaged tissue. Many studies have reported that PBM is capable of promoting wound healing, relieving pain, and reducing inflammation. Moreover, PBM can play an important role in neuroprotection. However, its photobiological mechanism is not yet fully understood. Studies by Professor Hamblin’s team have shown that an 810 nm low-level laser can enhance mitochondrial function and increase adenosine triphosphate (ATP) production via the upregulation of neuronal cytochrome c oxidase (CCO) activity, resulting in alleviation of neuronal death [3,4]. Research conducted by Da Xing’s team found that a 633 nm laser activates non-receptor tyrosine kinases on the surface of neuronal membranes, thereby activating a series of downstream signaling pathways, enhancing cell proliferation signals, suppressing cell apoptosis signals, and reducing neuronal death [5–7]. Besides, his team demonstrated that low-level laser therapy can reduce the activation of microglia by activating non-receptor tyrosine kinases, thereby reducing their neurotoxicity and enhancing their phagocytic abilities for clearing β-amyloid (Aβ) deposition [8]. The two mechanisms of action are shown in Fig. 1. An increasing number of research groups have been trying to prevent or slow the pathological progression using different wavelengths and patterns of light for regulating the activity of neurons.
《2.1 Low-level light therapy and AD》
2.1 Low-level light therapy and AD
AD is a chronic neurodegenerative disease characterized by progressive cognitive dysfunction. Symptoms include memory and cognitive impairment, emotional instability, and loss of function. The pathological hallmarks of AD are predominantly the abnormal accumulation of Aβ plaques and neurofibrillary tangles (NFTs). The cytotoxicity of Aβ and NFTs can cause neuronal death and further lead to cognitive impairment. At present, patients with AD are most commonly treated with pharmacological therapy. There are five kinds of drugs for AD treatment that have been approved by the United States Food and Drug Administration (US FDA). Among them, tacrine has been withdrawn from the market because of patients experiencing too many side effects. The remaining four are galantamine, rivastigmine, donepezil, and memantine, which can improve AD-induced impairments of cognition and memory to a certain extent but not cure AD. However, some patients have reported side effects, such as vomiting, headaches, and hallucinations [9,10]. Therefore, discovering treatments that have no adverse effects and significant curative effects are the focus of most current research. In recent years, worldwide preclinical and clinical studies have confirmed that low-level laser therapy can improve AD symptoms and thus, may be a promising and novel physical therapy for AD.
《Fig. 1》

Fig. 1. Potential cellular mechanisms of PBM.
In AD animal models, it was found that low-level lasers can significantly reduce the area and number of Aβ plaques in the cerebral cortex and hippocampus, effectively alleviating the symptoms of AD. Taboada et al. used an 808 nm (continuous wave laser) transcranial PBM (tPBM) approach to irradiate AβPP model mice for six months. They found that tPBM improved the performance of mice in the morris water maze; reduced the Aβ burden in the brain, cerebrospinal fluid, and plasma; decreased the levels of the proinflammatory cytokines interleukin 1β and tumor necrosis factor α; and improved mitochondrial and neuronal functions. In addition, their results revealed that the tPBM effect was related to the power density since the effects of a laser with suboptimal power density were not statistically significant. They found that a laser with a power density of 50 mW/cm2 had the optimal effect in AβPP mice [11]. Tao et al. found that a 1070 nm LED could significantly improve the morris water maze performance of APP/PS1 mice after 40-day irradiation and significantly reduce the area of Aβ plaque in the hippocampus [12]. Purushothuman et al. found that PBM (670 nm, LED) could reduce the level of Aβ plaque in the hippocampus, cortex, and brainstem of APP/PS1 mice. Moreover, it could reduce the NFTs in the hippocampus, cortex, and brainstem of K3 model mice and improve the damage caused by oxidative stress [13]. Farfara et al. found that PBM (670nm or 904nm) was capable of improving the differentiation ability of bone marrow mesenchymal cells to monocytes and enhancing the phagocytosis of soluble Aβ, thereby reducing Aβ load and improving cognitive function and spatial learning ability in four-month-old 5XFAD mice [14].
In addition to decreasing Aβ levels in the brains of AD model mice, PBM can effectively reduce a series of Aβ-mediated neuronal damage. Synaptic dysfunction caused by the binding of Aβ and tau oligomers to synapses occurs early in the progression of AD. PBM (670 nm) could improve synaptic dysfunction and reduce the suppression of long-term potentiation by decreasing the synaptic susceptibility to Aβ oligomers binding and increasing the synaptic mitochondrial membrane potential in the hippocampus and cortex of Tg2576 model mice [15]. In addition, PBM (808 nm) could also reduce the Aβ-induced neurotoxicity in rats injected with Aβ1-42. It was found that PBM could restore mitochondrial function by changing the levels of division and fusion proteins. This resulted in the suppression of the Aβ-mediated excessive fragmentation of the mitochondria, regulation of the Bax/Bcl-2 ratio, mitochondrial antioxidant expression to restore mitochondrial homeostasis, suppression of the Aβ-induced collapse of the mitochondrial membrane potential, and promotion of CCO activity and ATP production. PBM was found to be capable of suppressing Aβ-induced glucose-6-phosphate dehydrogenase and nicotinamide adenine dinucleotide phosphate oxidase activities, reducing oxidized mitochondrial DNA and excessive mitophagy, enhancing the antioxidant capacity of hippocampal CA1 neurons, and reducing oxidative damage. Furthermore, PBM was also found to reduce Aβ-induced reactive gliosis, inflammation, and hyperphosphorylation [16].
In addition to the commonly used red and near-infrared continuous light treatment modes, other wavebands and irradiation modes can also achieve the biological effects induced by traditional low-level lasers. However, the photoreceptors and corresponding mechanisms are still unclear. In 2013, Grillo et al. demonstrated that 1072 nm LED light could reduce the level of Aβ plaque in the dentate gyrus of TASTPM transgenic model mice. In addition, PBM significantly upregulated heat-shock proteins (HSPs), HSP60, HSP70, HSP105, and HSP27 and downregulated HSPαB-crystallin levels to reduce Aβ-induced toxicity. The regulation of HSPs in mice following PBM may be due to the process of thermal relaxation. The absorption of near-infrared light by photoreceptors causes the emission of a small amount of local thermal energy, thereby triggering intracellular signaling pathway [17]. Likewise, Zinchenko et al. found that tPBM (1267 nm, 32 J/cm2 ) can activate the lymphatic circulatory system of the brain and neck to promote the clearance of Aβ from the lymphatic circulation [18]. Intracellular Aβ can inhibit the large-conductance calcium-activated potassium (BK) channel of cortical pyramidal cells, thereby broadening the action potential peak width. Wider action potentials trigger an excessive per-spike calcium influx, which leads to endoplasmic reticulum stress. Zhang et al. reported that visible light (2 Hz) could improve the fear and spatial learning in mice by increasing the level of homologous protein 1a, thereby promoting the photic stimulation-induced BK channel facilitation in the cingulate gyrus and lateral amygdala. At the same time, no change was found in the Aβ42 burden of the cingulate gyrus cortex [19]. In 2016, Professor Li-Huei Tsai's team found that 40-Hz white LED light could non-invasively trigger gamma oscillations in the visual cortex of AD model mice and alter the morphology of microglia in the visual cortex to increase its phagocytosis of Aβ depositions [20]. In 2019, his team explored the mechanism of 40-Hz light therapy on AD mice and found that it could reduce neuronal and synaptic impairments in the brain by regulating the expression of genes related to neuronal and synaptic damage as well as microglia morphology to reduce microglia activation and neuroinflammation [21]. In the same year, the team explored the effects of the combined treatment of 40-Hz visual stimulation and 40-Hz auditory stimulation. They found that this combined stimulation could also reduce the Aβ levels in the mouse brain, decrease microglia and astrocyte activation, and improvecognition and memory [22]. PBM seems to improve AD symptoms through several mechanisms, making it a potentially useful therapeutic approach for AD.
Clinical trials have confirmed that PBM can effectively alleviate the pathological symptoms of AD. Chang Zhou’s group found that irradiating 670 nm light in the nasal cavity of patients with mild cognitive impairment could reduce the serum Aβ levels, affect the Aβ metabolism process, and delay the pathological progression [23]. Arakelyan et al. investigated the efficacy of five therapies, including PBM, magnetic field therapy (MFT), light chromotherapy (LCT), AD drug therapy, and combined PBM+MFT+LCT therapy in 154 patients with AD. They found that the Alzheimer’s Disease Assessment Scale (ADAS-cog) scores of patients on combination therapy, PBM, MFT, or drug therapy were significantly lower than those of the placebo group. The therapy effects weremost significant in patients on combination therapy, followed by those on PBM therapy and drug therapy, and finally those on MFT [24]. Maksimovich et al. also reported a difference in efficacy between patients treated with intravascular PBM (visible light, 20 mW, continuous/pulse, 46 cases) and patients treated with memantine and rivastigmine (43 cases). They demonstrated that PBM could significantly ameliorate cognitive and memory impairment in patients with AD by improving cerebral microcirculation and metabolism [25]. Lim et al. treated four patients with AD using 810 nm pulsed LED and 633 nm continuous LED light. Over one year of observation, the cognitive abilities of the four patients significantly improved, and one of them even improved from a baseline of “significant cognitive impairment” to “no cognitive impairment” [26]. Berman et al. conducted a small double-blind trial in 11 patients with mild cognitive impairment, of whom eight received 1070 nm light therapy and three a placebo. After 28 consecutive days of treatment, the execution ability (clock drawing, instant recall, exercise memory, visual attention, and task switching) of patients in the treatment group had significantly improved. Electroencephalogram recordings demonstrated that PBM also ameliorated the alertness, attention, and anxiety of patients in the treatment group [27]. Saltmarche et al. combined transcranial irradiation (810 nm, 10 Hz) and intranasal irradiation to treat five patients with dementia. After 12 weeks, the participants’ ADAS-cog scores had significantly improved and the mental state test score had increased by an average of five points. Moreover, participants with PBM displayed decreased anxiety and anger, improved sleep quality, and no adverse reactions [28].
《2.2 PBM and PD》
2.2 PBM and PD
PD is a neurodegenerative movement disorder, with an average age of onset of about 60 years. The dopaminergic neurons in the substantia nigra pars compacta (SNpc) and striatum gradually degenerate over time, leading to a static tremor and limb stiffness. There are more treatment options for patients with PD, including medication and surgery, than for those with AD. Most patients with PD choose dopamine replacement therapy at an early stage to reduce motor symptoms by increasing the reduced dopamine levels in the system. However, long-term medication can lead to drug resistance and side effects, such as movement disorders. Deep brain stimulation, also known as brain stimulation pacemaker implantation, a commonly used surgical treatment for advanced PD, can correct basal ganglia circuit function caused by the loss of dopamine and help to control the motor symptoms of PD. Although deep brain stimulation is a minimally invasive procedure, it is expensive, has a risk of infection, and requires life-long maintenance. Therefore, it is necessary to develop non-invasive methods that can be widely applied to cope with the rapidly growing number of people with PD because of the aging population.
Mitrofanis’ group from Australia has researched the effects of PBM on PD with different models. They found that PBM relieved the symptoms of PD and even prevented the occurrence and progression of PD in neurons through remote PBM on peripheral tissues. Toxicity induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in dopaminergic cells of the SNpc in the mouse brain was alleviated by PBM using invasive irradiation with 670 nm LED light. Moreover, increased PBM doses prevented toxicity caused by increased MPTP doses [29– 31]. PBM treatment (670 nm LED) effectively promoted the survival of neurons and improved the movement behavior of rats treated with 6-hydroxydopamine [32]. In K369I transgenic mice, PBM treatment (670 nm LED) was found to restore hyperphosphorylated tau protein, NFTs, and oxidative stress markers (4-hydroxynonenal and 8-hydroxy-2-deoxyguanosine) in the cortex and hippocampus to wild-type levels and recover the expression of the mitochondrial marker Cox in surviving neurons [33]. In addition, 810 nm PBM treatment improved the autonomous activities of MPTP-induced PD mice [34]. The delivery of light from a 670 nm LED or 670 nm laser by a surgically implanted intracranial optical fiber to the lateral ventricle of MPTP-treated mice was found to effectively preserve SNpc cells [35]. Mitrofanis’ group further studied the effects of PBM on monkeys with PD induced by MPTP. PBM treatment (670 nm, 25 J and 35 J) through an intracranial fiber optic reduced (by about 75%) the proliferation of SNpc and striatal astrocytes, increased the number of tyrosine hydroxylase-positive cells in the striatum and the expression level of glial-derived neurotrophic factor, and improved the PD clinical symptoms (posture, overall activity, exercise sluggishness, and face). However, high-dose PBM treatment (670 nm, 125 J) did not cause a significant improvement in the PD-associated clinical symptoms of the monkeys [36,37].
In addition, Mitrofanis’ group found that irradiation of peripheral tissue, which is termed as remote PBM, triggers a neuroprotective action. In BLAB/c mice with PD induced by MPTP, PBM (670 nm LED) irradiating the back of mice while their heads were covered with aluminum foil reduced the loss of dopaminergic cells in the SNpc [38]. These effects have also been confirmed in C57BL/6 mice; PBM (670 nm LED) irradiation of the back of the mice helped to protect from MPTP-induced loss of dopaminergic cells [39]. However, because PBM treatment and the MPTP injection were performed simultaneously, it remains unclear as to whether PBM acts by affecting the pharmacokinetics of MPTP, which in turn, delays the occurrence of PD. A recent study showed that pretreatment with PBM (670 nm LED) on the back and hind limbs of mice 10 days before MPTP injection significantly reduced the loss of tyrosine hydroxylase-positive dopaminergic cells in the midbrain and mitigated the increase of FOS-positive neurons in the caudate-putamen complex [40]. These results confirmed that PBM targeting peripheral tissues can prevent MPTP-induced PD damage, thereby clarifying the neuroprotective function of distal PBM. Hence, remote PBM pretreatment is expected to be a feasible neuroprotective intervention, but its mechanism of regulation of brain nerve function is still unclear.
Clinical trials of PBM in PD are underway. Santos et al. [41] reported a significant improvement in the gait of PD patients who received PBM treatment (670 nm LED, transcranial irradiation) (17 cases) compared to that of those who did not (17 cases). Researchers have studied the effect of white light irradiation on patients with PD using bright pulse light at an intensity of 1 000 to 10 000 lux and a duration of 30 min to 1.5 h twice a day (from 1 week to several months). Paus et al. in Germany and Willis et al. in Australia reported that white light therapy gradually improved the related motor and mental parameters of patients with PD [42,43]. Videnovic et al. in the United States and Rutten et al. in the Netherlands have reported that white light therapy improved the sleep quality of patients with PD and effectively relieved sleep disorders [44,45].
《2.3 PBM and normal brain tissue function》
2.3 PBM and normal brain tissue function
While numerous studies have confirmed the positive effects of PBM on cognition and memory retention in neurological diseases, few studies have explored the function of PBM on healthy subjects. To this end, Gonzalez-Lima and Barrett in the United States [46,47] determined the effects of tPBM on memory and annotation in 40 undergraduates. The subjects received non-contact 1064 nm laser irradiation (true or false PBM) on the right frontal lobe of the cerebral cortex. Two weeks later, the true PBM-treated subjects displayed faster reaction times and better memory according to their delayed match-to-sample task scores compared to the control subjects. The Positive and Negative Affective Program (PANAS-X) tracking self-reports showed that although the participants in both groups usually reported more positive than negative emotional states, the overall effect was significant in PBM-treated subjects because of more sustained positive emotional states. These data indicated that tPBM could be employed as a non-invasive and effective method to enhance brain function, particularly those related to cognitive and emotional dimensions. Professor Salimi’s group from Iran studied the effects of tPBM on the electrophysiological characteristics and attention function of healthy young people [48]. The subjects received true or false PBM irradiation through an 850 nm LED on the right prefrontal cortex. Quantitative electroencephalogram recordings showed that the cortical brain electrical activity changed after tPBM, which had a significant impact on attention function. The Chinese group of Song et al. found that tPBM with a transcranial 820 nm laser irradiating the left primary motor cortex and the right first dorsal interosseous muscle influenced the excitability of the human motor cortex [49]. In their study, the motor-evoked potential amplitude significantly increased according to the transcranial magnetic stimulation measurement, indicating that PBM temporarily increases the excitability of the motor cortex.
At present, a large number of studies have shown that PBM effectively improves the cognition, memory, and mobility of mice and patients with a neurodegenerative disease, with no apparent side effects. Most studies on the use of PBM to treat neurodegenerative diseases were conducted at an early disease stage. Similar to other treatments, PBM is not capable of rescuing degenerated neurons from apoptosis and facilitating complete recovery. Therefore, it is essential to apply PBM to patients at an early stage to limit disease progression. On the other hand, many of the effects of PBM, including the reduction of oxidative stress and the increase in blood flow, can be applied to a wider range of people. In general, it is believed that PBM has a beneficial effect on healthy people. As a non-invasively physiotherapeutic method, PBM can help to improve cognitive memory and decrease the incidence of brain diseases in healthy people. Collectively, PBM could be a promising therapy for the treatment and prevention of brain diseases. As PBM is a low-cost and easy procedure, it can be broadly promoted and applied.
《3 Problems in optical therapy to be resolved》
3 Problems in optical therapy to be resolved
After decades of development, phototherapy has been shown to be beneficial in the treatment of a number of diseases. However, the field of PBM is still in its infancy. There are still many questions that remain, including the unclear mechanism, different treatment models, inconsistent dose-effects, and insufficient clinical evidence, all of which could limit its development and application. Therefore, research on the use of phototherapy for neurodegenerative diseases is urgently required, along with development of new technical methods suitable for clinical use.
《3.1 Clarification of the mechanism and dose-effects of PBM》
3.1 Clarification of the mechanism and dose-effects of PBM
Regarding the mechanism of PBM neuroprotection, the teams of professors Wong-Riley and Hamblin have mainly focused on how 808 nm PBM increases ATP via regulation of CCO activity. Professor Da Xing’s group from China has studied the regulation of neuronal signal transduction pathways induced by the effect of 633 nm PBM on non-receptor tyrosine kinases expressed on the cell surface for many years. However, the above studies were all carried out at the cellular level, thereby ignoring the systemic mechanism of PBM in vivo. The alleviation of neurodegenerative disease symptoms has been achieved in a diverse number of PBM models, including intracranial, vascular, and ocular irradiation. However, the mechanism of action is still unclear. In particular, for remote PBM, it is unclear how irradiation on the peripheral tissue induces neuroprotection in the brain and whether there are optimal target tissues/organs and optimal modes of action. There are still many questions regarding this novel therapy. Therefore, a thorough exploration of the initial interactions between the laser and tissue is required, along with a clarification of the mechanism of action under different wavelengths and modes, to further guide the development and clinical application of phototherapy technology.
One of the best features of PBM is that it is non-invasive. Its power density usually ranges from 1 to 100 mW/cm2 , with the total energy ranging from 1 to 10 J. The local temperature rise by PBM does not exceed 0.10– 0.75°C. Many studies have shown that the biological effects of PBM vary with different parameters, including the light dose, wavelength, and action mode (continuous or pulsed). Furthermore, the penetration depth of PBM is an important consideration in clinical practice. Measurements using human autopsy samples have determined that the penetration range of red light to near-infrared light through the human scalp and skull was 1% to 3%, and less than 0.03% of the emitted light can penetrate 12 mm of the brain tissue [50]. Therefore, to provide better guidance on its clinical application, it is essential to optimize the parameters, including the wavelength, mode, dosage, and treatment model (extracranial, transcranial, nasal, or ocular irradiation) for application to a specific area.
《3.2 Development of novel treatment technologies》
3.2 Development of novel treatment technologies
The phototherapy equipment used for neurodegenerative diseases mainly utilizes red light and near-infrared light. The main models are portable nasal cavity irradiation and helmet phototherapy devices, such as the 670 nm semiconductor continuous laser intranasal therapy device and the 810 nm (10 Hz) pulsed LED intranasal/intracranial therapy device. With the transformation of medical treatment models and the expansion of application from common diseases to major chronic age-related diseases, PBM is increasingly being used for the prevention and treatment of diseases. In addition, PBM is increasingly being used in community settings, as well as in medical institutions. Therefore, smaller, more portable, and wearable PBM equipment is increasingly being sought. French scientists have developed a laser-knitted fabric to treat dermatitis through light irradiation, which is expected to result in a wearable and painless treatment option. By coupling blue light to a polymer fiber with a diameter of 160 μm wrapped in a fabric, this wearable light source can be used to treat neonatal jaundice. Furthermore, the blue light wearable device has been approved by the US FDA to treat mild psoriasis. With the rapid development of optoelectronic technology, the development of portable, smaller, and wearable devices for the treatment of neurodegenerative diseases is of great significance and commercial value. However, real-time monitoring technology and big data platforms are required to support such technology.
《3.3 Improvement of the clinical evaluation systems》
3.3 Improvement of the clinical evaluation systems
Many clinical studies in China and abroad have shown that PBM is efficacious in treating neurodegenerative diseases and may potentially be a novel, non-invasive treatment method. However, owing to differences in the light parameters and treatment modes used in each study, it is impossible to verify their treatment efficacies and thus, generate a standardized and generalized clinical treatment plan. The establishment of a clinical verification system along with evaluation indicators for phototherapy is required to effectively verify the therapeutic effects of different phototherapy parameters and their feasibility. This will help to clarify the treatment mode most applicable to neurodegenerative diseases, quickly evaluate the effectiveness of the phototherapy device, ensure wide application and development of the treatment technology, and facilitate the rapid transformation and promotion of treatment devices. Moreover, the development of big data cloud platforms to collect patient information in a timely manner could facilitate remote treatment. This would not only help to realize a personalized treatment model but also promote the development of wearable medicine.
Fig. 2 outlines the potential research directions of PBM and the relevance of each direction. First, it is necessary to clarify the reason for phototherapy having such a general neuroprotective effect and the basis of the light-body interaction. The understanding of the mechanism could fundamentally promote the application of phototherapy. Second, the types of light sources, parameters of phototherapy, and modes of action vary across studies. Clarifying the dose-effect relationship is pivotal to the clinical application of phototherapy. More extensive clinical trials are required to identify the most effective treatment models, develop portable treatment devices specific to different diseases, and establish a clinical evaluation system. Furthermore, a big data cloud monitoring platform that can enable monitoring and remote guidance of treatment should be developed to ensure the safety and effectiveness of portable and wearable devices.
《Fig. 2》
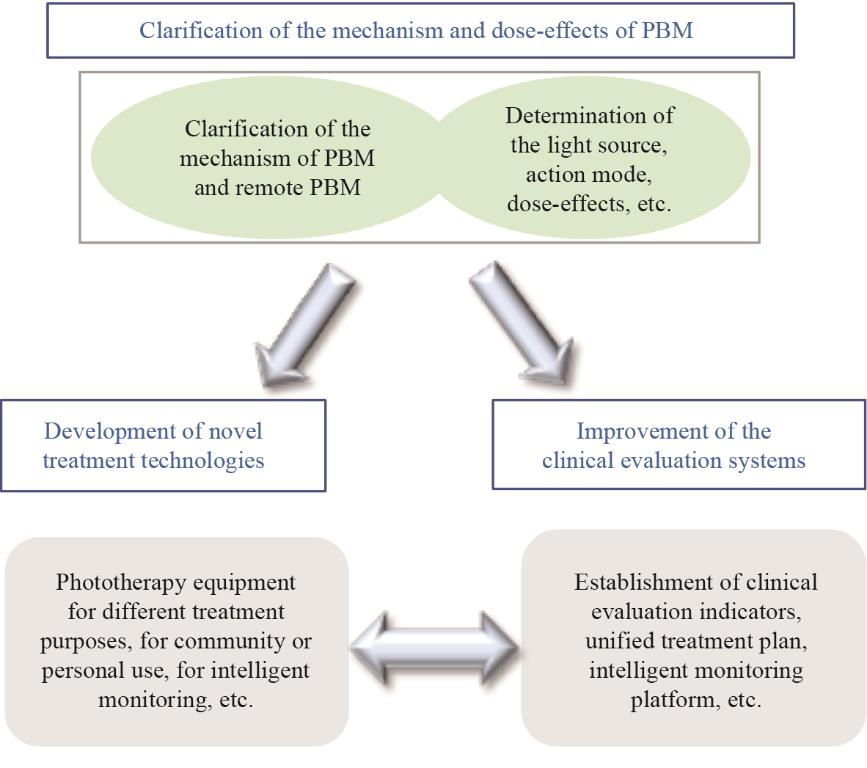
Fig. 2. Potential research direction and correlation of PBM.
《4 Conclusion》
4 Conclusion
The efficacy of PBM for patients with AD and PD and in different animal models has been shown in a number of studies, indicating that PBM is potentially an effective therapeutic strategy for age-related neurodegenerative diseases. Moreover, it could be used as an adjuvant therapy to the current treatments and be developed as a preventive physiotherapy method because of the few side effects associated with PBM. In general, phototherapy appears to have great promise in the treatment and prevention of brain diseases. However, to become a widely available treatment method, more research is required. Finally, to promote the clinical application and promotion of phototherapy and benefit patients and wider society, the mechanism of PBM and remote PBM, the development of novel treatment technologies, and the improvement of the clinical evaluation system should be cooperatively researched.













 京公网安备 11010502051620号
京公网安备 11010502051620号




