《1. Introduction》
1. Introduction
The use of fluorinated graphite (CF x) materials as the active cathode material in primary lithium batteries with the theoretical energy density reach to 2189 W.h.kg−1 was first demonstrated by Watanabe et al. [1,2]. A few years later, the Li/CFx batteries with energy density up to 560 W.h.kg−1 were first commercialized by Matsushita Electric Industrial Co., Ltd. in Japan [3]. The high energy density is attributed to the large Gibbs free energy change of the Li intercalation reaction, which can be written as (CF)n + nLi → (C)n + nLiF [4]. In addition, the CF x has other advantages as electrode material such as high average operating voltage, long lifespan, stable operation, and high efficiency in primary lithium batteries [1,2]. These advantages make it the superior cathode material in primary lithium batteries.
Recently, there are many attempts to extend the application of CFx in lithium ion batteries (LIBs). However, Li is easy to react with the fluorides and forms thermodynamically very stable LiF compound, as it is shown in the primary Li/CFx batteries, and therefore the electrochemical reaction is irreversible [5]. This makes it very difficult for the application of CFx as electrode material in LIBs, in which Li ions should be able to reversibly intercalate and extract [6]. Recently, Liu et al. reported that Na can be reversibly intercalated into and extracted from the CFx compound and the cycling of the Na-CFx batteries is good under room temperature [7]. The authors showed that the formation and decomposition of NaF are reversible in the electrochemical reaction, indicating that the structure of the CFx is not destroyed or the structural change is reversible upon Na intercalation.
As Na and Li are belong to the same main group on the periodic table, it might be very interesting to know why Na can be reversibly intercalated/extracted into/from the CFx . What are the different characteristics between Li and Na that make the different performances upon Li and Na intercalation into the CFx compound? In the present work, we analyzed the structural evolution behavior of the CFx upon Na/Li intercalation by first-principles calculations.
《2. Method and computational details》
2. Method and computational details
All calculations are performed using the Vienna Ab-initio Simulation Package (VASP) [8] within the projector augmented-wave (PAW) approach [9], with which the ion-electron interactions are described. The ground state of the electronic structure is described within the density functional theory (DFT) using the generalized gradient approximation (GGA) with PW91 exchange correlation functional [10]. We mention here that other functionals are also tested and no obvious difference is found in our calculations. The cutoff energy for expansion of the wave functions is 550 eV. The Monkhorst-Pack [11] scheme k-points sampling method is used for the integration in the first Brillouin zone and a 6 × 6 × 1 k-mesh is used in this study. The atomic positions are fully relaxed until the force on each atom is less than 0.02 eV.Å−1. We used a 3 × 3 × 1 CF supercell (including 36 C and 36 F atoms) to simulate the bulk phase of the chair configuration (the lowest energy configuration) with AB stacking. The bulk phase of the CF with AA stacking was also tested, and the results showed that the total energy of the AA stacking is higher than that of the AB stacking, and therefore, all results presented in this work is based on the AB stacking model. The single CF layer model is obtained from the bulk model and the CF layers are separated by a 15 Å thick vacuum layer, in order to ensure that the interactions between layer and layer are negligible.
《3. Results and discussion》
3. Results and discussion
《3.1. Adsorption of Li/Na on single layer CF》
3.1. Adsorption of Li/Na on single layer CF
In order to find out the interaction between the Li/Na and the CF layers, the single layer of CF with one Li/Na atom adsorption on it has been calculated. Different adsorption sites are tested and the stable adsorption site for single Li/Na atom is the hollow site. The optimized structures are shown in Figure 1. The distances from Li atom and Na atom to the nearest F atom (No. “1” in Figure 1, denoted as F“1”) are 1.859 Å and 2.207 Å, respectively. As both the Li and Na ions are univalent cations, the Coulomb interactions between the Li/Na and F are mainly determined by the distance, and thus the Li-F interaction is stronger than the Na-F interaction in the CF layer.
《Fig. 1》
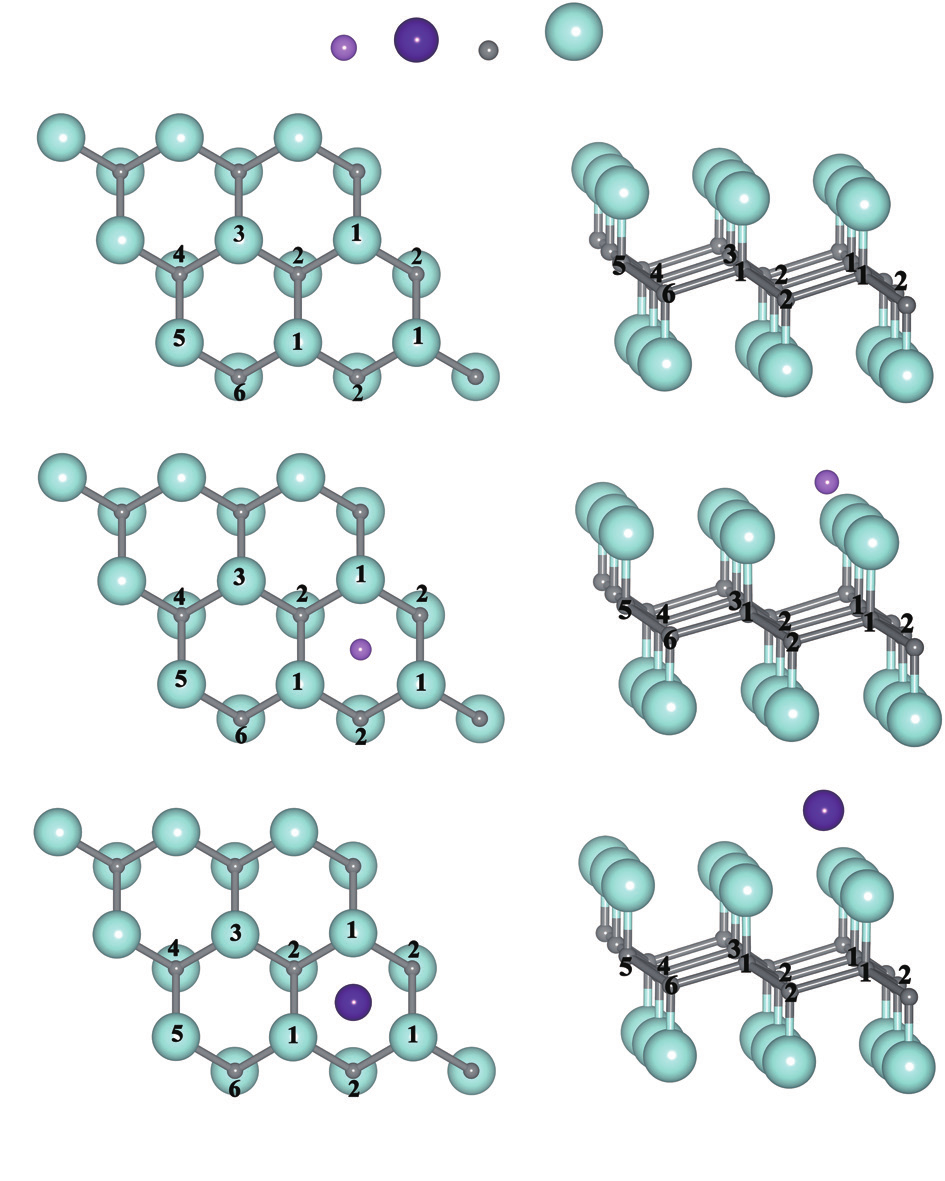
Fig.1 The optimized structures with one Li/Na atom adsorption on single CF layer. The numbers from 1 to 6 denote different F atoms close to the adsorbate. (a) C18F18; (b) LiC18F18; (c) NaC18F18.
Then, we further analyzed the change of C—F bond lengths upon the adsorption of the Li/Na atom. The data are summarized in Table 1, from which we can see that the C—F bonds are elongated substantially upon Li/Na adsorption. As the F“1” atom, as shown in Figure 1, is closest to the Li/Na atom, the corresponding C—F bond length becomes the longest due to the strongest Coulomb attraction. On the other hand, the C—F bond length in the case of Li adsorption (1.493 Å) is obviously longer than that in the case of Na adsorption (1.458 Å), indicating that the interaction between the Li and F“1”atoms is much stronger than that between the Na and F“1” atoms. The distance of Li-F and Na-F is one important reason for this, as discussed above, but it is not the only one. Our Bader charge [12] analysis also demonstrated that the Li is more ionized than Na upon adsorption on the CF layers. The Bader charges of Li and Na atoms adsorbed on the CF layer are 0.103 e and 0.152 e, respectively. That is to say, the charge transfer from Li to F is more than that from Na to F. Therefore, the stronger Li-F attraction is also contributed by the more charges they possess (Li is positively charged while F is negatively charged), comparing to that of the Na-F case.
《Tab.1》
Tab.1 C−F bond lengths (unit: Å).
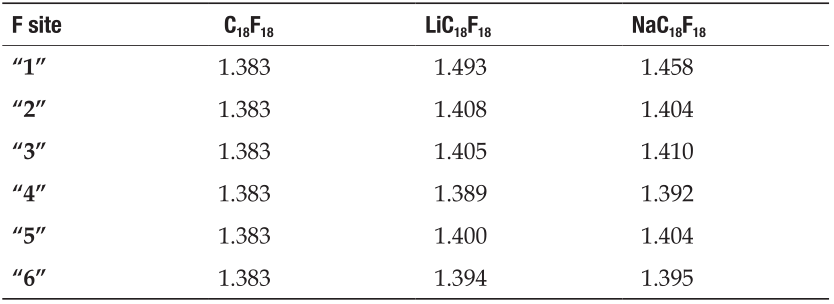
Very interestingly, the C—F“2” bond length is also slightly elongated upon the adsorption of Li and Na atoms, although the F“2” is located at the opposite side of the adsorbate. The reason is also related with the charge transfer from the Li/Na atoms to the F atoms. In the CF layer, the electronic configuration of the C atom is in sp2 hybridization, and the pz electron is transferred to the F atom. Therefore, the planner hexatomic carbon ring is distorted and the buckling of the structure occurs. Upon Li/Na adsorption, charges transferred from Li/Na to F atom, and in turn the amount of the charges transferred from C to F becomes small, which also leads to a weakened C—F bonding. As a result, all of the C—F bond lengths become longer, as shown in Table 1. As it can be seen, the C—F bond lengths are sensitive to the distance between the adsorbate and the F atom. Furthermore, we can also see that this effect is more clearly around the Li atom, indicating that the charge distribution (F-Li interaction) is more localized for Li adsorption than that of Na adsorption.
In order to quantitatively describe the interactions between the adsorbate and F atoms, we calculated the binding energies defined as
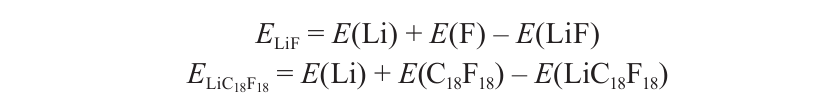
where the ELiF and ELiC18F18 are the binding energies of LiF molecule and Li on CF layers, respectively; the E(Li) is the total energy of LiF molecule in vacuum; E(LiC18F18) and E(C18F18) are the total energies of the single layer of CF with and without Li atom adsorption, respectively; E(Li) and E(F) are the total energies of the corresponding atom in vacuum. The definition for Na adsorption is the same as that of Li. The results are given in Table 2. As it can be seen, the binding energy of LiF crystal is larger than that of NaF crystal, indicating that the Li-F interaction is stronger than the Na-F interaction in the ionic crystal. Similarly, the binding energy for Li adsorption on C18F18 is also larger than that of Na adsorption. The binding energy difference between LiC18F18 and NaC18F18 is 0.72 eV. These data demonstrate that the interactions between Li and F are stronger than that between Na and F.
《Tab.2》
Tab.2 Binding energies (unit: eV).

Then, different concentrations of Li/Na adsorption on single CF layer have been considered. Several optimized structures are shown in Figure 2, which includes the one-side adsorption with three Li/Na atoms and nine Li/Na atoms, the two-side adsorption with two Li/Na atoms and six Li/Na atoms. As it is shown, both Li atom and Na atom have strong interactions with F atom, the C—F bonds are broken and F atoms are pulled out by Li and Na. The more Li/Na atoms are adsorbed on the CF layer, the more C—F bonds are broken. This is reasonable since more Li atoms contribute more charges to F atoms and thus fewer charges possessed by F atoms are obtained from C atoms, which in turn weakens the C—F bonding strength. On the other hand, this effect is more obvious in the case of Li adsorption, comparing to that of Na adsorption.
《Fig. 2》
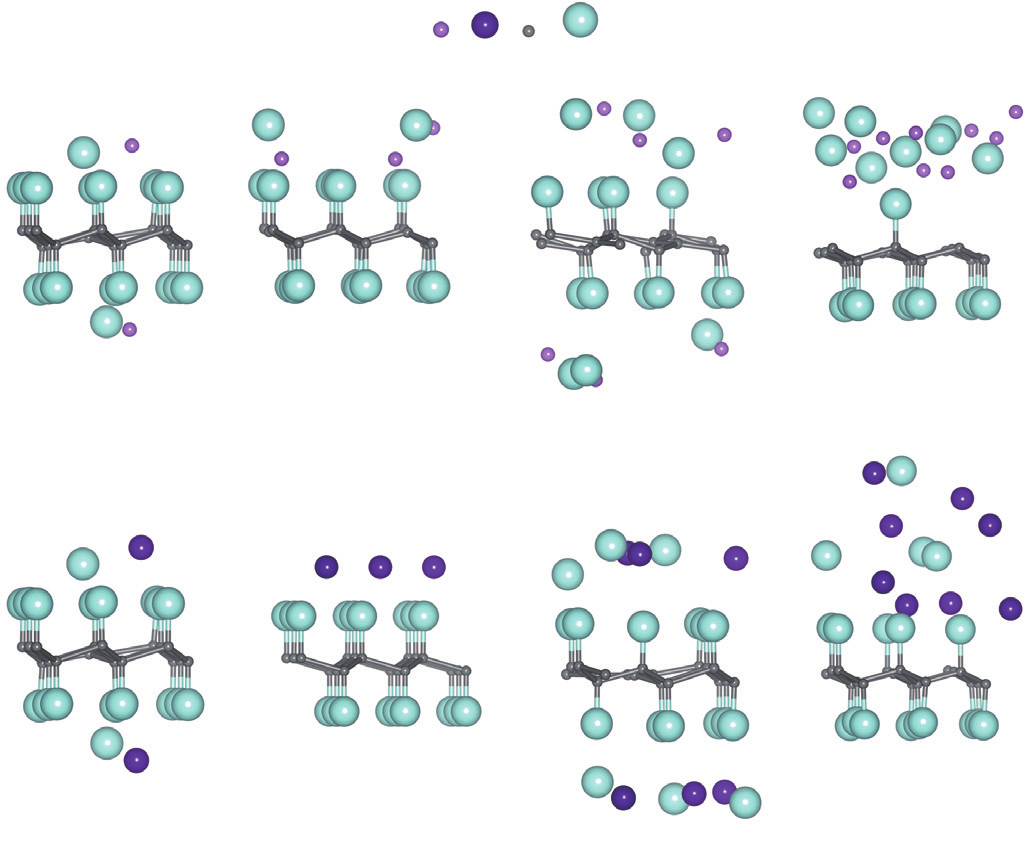
Fig.2 The optimized structure with different concentrations of Li/Na adsorption on single CF layer.
《3.2. Intercalation of Li/Na in bulk phase CF》
3.2. Intercalation of Li/Na in bulk phase CF
Finally, in order to simulate the CF x as the cathode material for lithium batteries and sodium batteries, we analyzed the situation of the Li/Na intercalation into bulk phase CF. Eighteen Li/Na atoms have been intercalated among the two layers of CF and the lattice constant c (perpendicular to the layer plane) was initially fixed as 12 Å, which is the lattice constant of the CF. Figure 3 gives the optimized structures. For the Li intercalation, as shown in Figure 3(a), it is very interesting that all F atoms of one CF layer have been extracted to form one graphene layer. Meanwhile there is no valence electron of one layer carbon contributing to F atoms, the hybridization of that carbon layer from the original sp3 to sp2, and the structure of CF became the flatting graphene. On the other hand, the remained CF layer keeps its original structure well. However, the Na intercalation gives the different appearance, as shown in Figure 3(b), the number of broken C—F bonds is less than that of upon Li intercalation.
《Fig. 3》
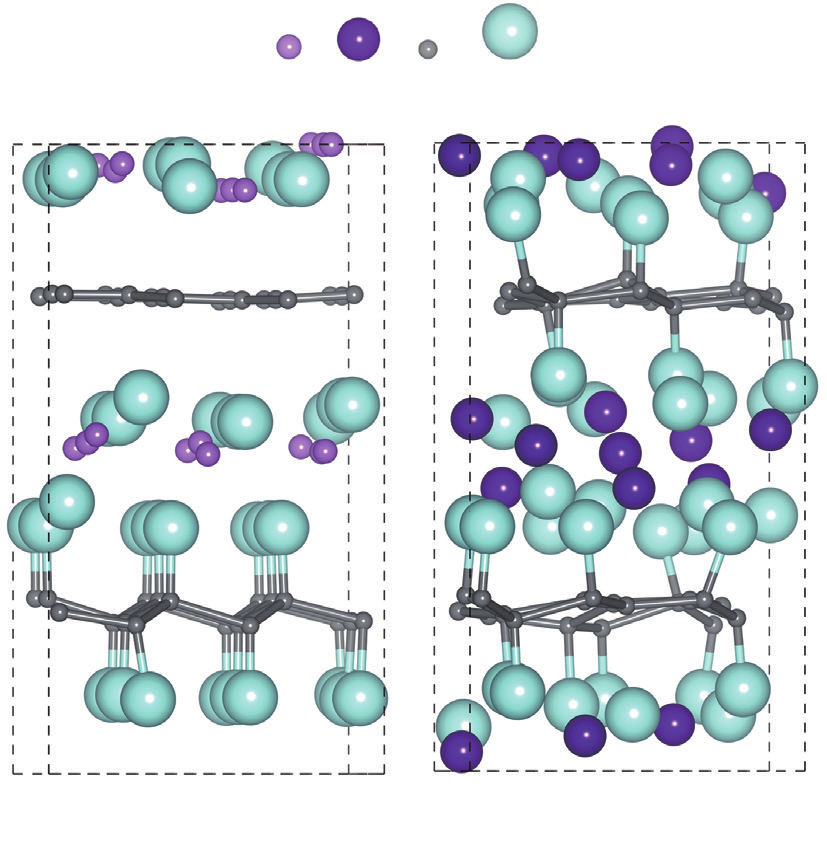
Fig.3 The optimized structures of (a) Li18C36F36 and (b) Na18C36F36 in the chair configuration with AB stacking.
In order to make it clear that whether the initial distance between Li atom and F atom is important to the structure evolution, we enlarged the initial lattice constant c to be 13 Å, 14 Å and 15 Å. Our results show that the same thing happens: One graphene layer is formed upon Li intercalation; on the other hand, graphene sheet was not formed upon Na intercalation. This result confirms that the interactions between Li and F are more localized comparing to that between Na and F. As the interaction between Li atom and F atom in the CF is stronger and more localized, C—F bonds can be more easily broken by Li in local places, and F vacancy sites are formed on the CF. This will accelerate the damage to the layered structure. When F vacancies are formed, fewer charges are transferred from the C layer to the F layer, and therefore, the Coulomb attraction between the C layer and the F layer becomes weakened, which further accelerates the C—F bond breaking. The more available the F vacancies are, the weaker the C—F bonding is. As a result, when one F atom is escaped from one CF layer by the strong and localized attraction of Li, breaking of other C—F bonds of that CF layer becomes easier and finally all F atoms can be escaped from the CF layer. This is why we observed the graphene sheet in the case of Li intercalation.
In order to enhance the cycling performance of the CF x as cathode materials, one strategy is to enhance the binding interaction between C atom and F atom. Doping and replacing C atoms with elements that are less electronegative is a good choice. For example, when the dopant is positively charged and has stronger Coulomb attraction to F atoms than that C atoms do, the CF layer will become more stable upon Li/Na intercalation.
《4. Conclusions》
4. Conclusions
In summary, we have studied the Li and Na intercalation from first-principles calculations. Results from Li and Na atoms adsorption on single CF layer reveal facts: ① Li atom transfers more charges to the CF layer than Na does; ② the distance between Li and F is smaller than that between Na and F in the equilibrium states. These results demonstrate that the interaction between Li and F is much stronger than that between Na and F. On the other hand, the interaction between Li and F is more localized comparing to that between Na and F. As a result, C—F bonds can be broken by Li adsorption and intercalation more easily comparing to that of Na does. Upon Li intercalation, one C—F bond is broken randomly and one F vacancy is formed in one CF layer. However, F vacancy will accelerate the C—F bonds breaking process in the same layer and more F vacancies can be formed, which in turn weakens the C—F bonding. As a result, we observed the formation of graphene sheet when 18 Li atoms are intercalated into the C36F36 bulk structure.
《Acknowledgements》
Acknowledgements
Thanks to the support of National High Technology Research and Development Program of China (“863” Program) (2015AA034201), the National Natural Science Foundation of China (11234013 and 11264014), Natural Science Foundation of Jiangxi Province (20133ACB21010, 20142BAB212002, and 20132BAB212005), and Foundation of Jiangxi Education Committee (GJJ14254 and KJLD14024).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Fengya Rao, Zhiqiang Wang, Bo Xu, Liquan Chen, and Chuying Ouyang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




