《1. Introduction》
1. Introduction
Soil contamination by petroleum and other heavy hydrocarbons is a major global environmental problem. For example, over 100 000 barrels of oil are spilled on average every year in the US, contaminating soils with a range of petroleum hydrocarbons, from crude oils and sludge to refined fuels such as gasoline [1,2]. While a cornucopia of remediation technologies exist, technologies that can quickly treat soils contaminated with a wide range of petroleum hydrocarbons are especially desirable. For example, in situ bioremediation of sites impacted by petroleum release can take years, particularly when recalcitrant species such as high molecular weight hydrocarbons are present [3–6]. In contrast, thermal technologies can remediate sites quickly and efficiently (hours to months), often removing over 99% of a wide range of hydrocarbon fractions [7–13]. The latter processes result in high removal efficiencies of both total hydrocarbon mass and total petroleum hydrocarbon (TPH) concentrations, which is a more common regulatory metric.
Quite frequently, the selection of remediation method is driven by considerations that require expeditious completion (e.g., compliance issues, impending property transactions, and impacts on third-party property). Thus, thermal technologies fill an important niche in petroleum hydrocarbon remediation.
Despite these advantages, the high treatment temperatures required by most thermal technologies can pose several downsides. First, heating contaminated soils to high temperatures is energy intensive and, thus, a relatively costly endeavor. Secondly, soil minerals and organic matter (OM) decompose and may be totally destroyed at high temperatures, potentially limiting the ability to restore soils and ecosystems to their original state [8,14,15]. While it is clear that thermal remediation technologies effectively remove contamination, the impacts of high temperatures on ecosystems (i.e., plant growth and soil organisms) and re-greening efforts are relatively unexplored. Furthermore, there is a growing need to establish a framework for optimizing thermal remediation with sustainable objectives such as energy and water conservation and ecosystem preservation. The development of a holistic approach toward thermal remediation is imperative in order to ensure that environmental cleanup efforts do not incur unnecessary environmental damage, but rather align with global efforts to enable a more sustainable future [12].
This paper reviews thermal treatment technologies for remediating soil contamination by petroleum hydrocarbon release, and summarizes both the current state of knowledge and potential unintended impacts on ecosystem health. We consider the relative importance of different removal mechanisms for different types of hydrocarbons and operating temperatures, as well as the associated energy and water requirements to discern opportunities to enhance process sustainability in tandem with hydrocarbon-removal efficiency.
《2. Thermal technologies》
2. Thermal technologies
《2.1. Thermal desorption》
2.1. Thermal desorption
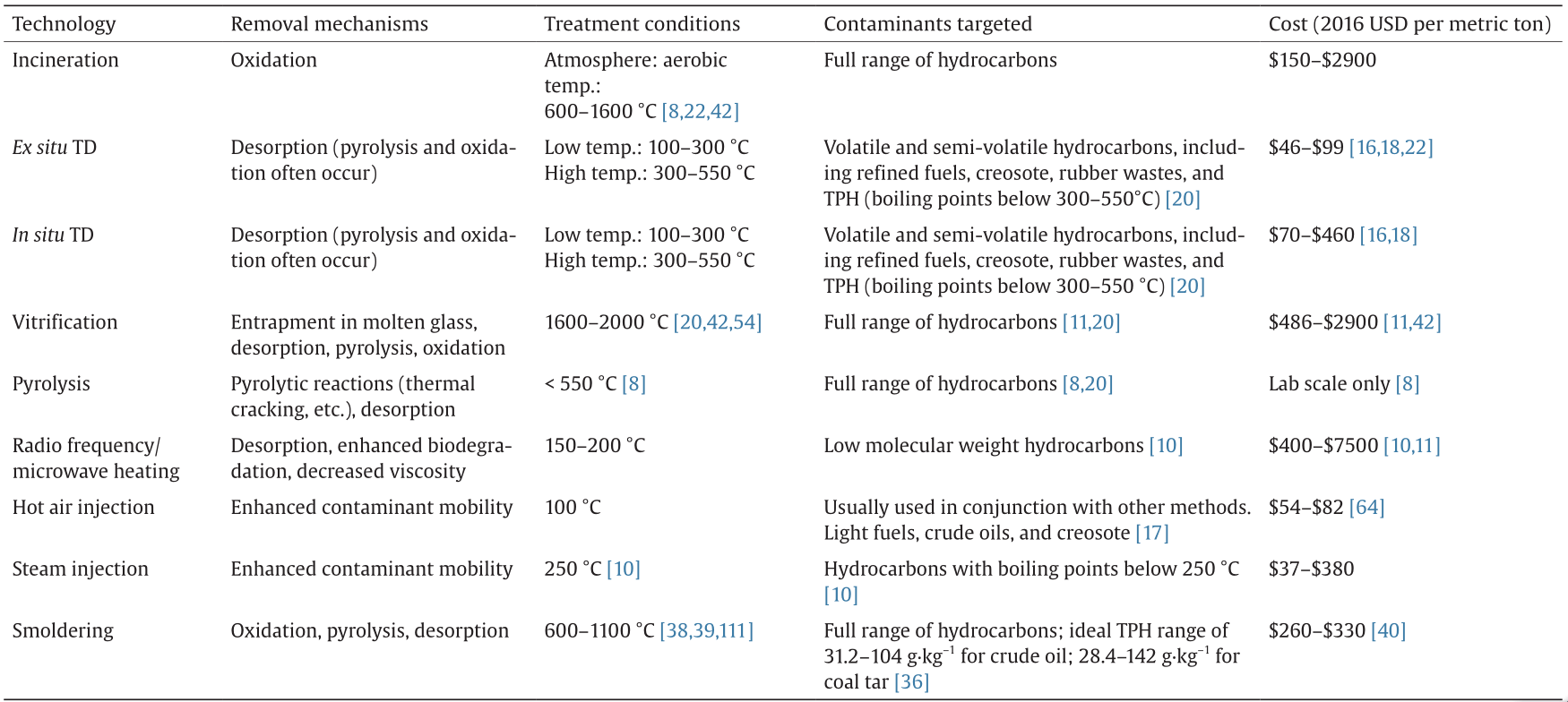
Thermal desorption (TD) involves the application of heat to contaminated soils with the intention of volatilizing/desorbing hydrocarbons, which are then carried away by a sweep gas or vacuum and eventually destroyed via incineration or carbon adsorption [16–18]. TD can be divided into low-temperature thermal desorption (LTTD, 100–300 °C) and high-temperature thermal desorption (HTTD, 300–550 °C).
In concept, TD consists of hydrocarbon desorption alone, but in reality, TD systems often achieve hydrocarbon removal through multiple mechanisms, including oxidation/incineration and pyrolytic reactions (thermal cracking, etc.) [16,18]. The dominance of these mechanisms depends on temperature and oxygen distribution [16]. Heavy hydrocarbons in areas containing low oxygen may be pyrolyzed (thermal cracking, etc.) at corresponding temperatures, whereas hydrocarbons in high-temperature, oxygen-containing regions may be incinerated.
2.1.1. Ex situ TD
During these processes, soil is excavated and heated in TD units such as thermal screws or rotary drums (Fig. 1). Desorbed hydrocarbons are carried away from the main reactor chamber by a sweep gas and incinerated or adsorbed onto activated carbon for final disposal and air pollution control. Fuel and heat recovery may be possible if soil moisture is low and hydrocarbon British thermal unit (BTU) content is high. Treated soils must then be re-moisturized to control dust.
《Fig. 1》

Fig.1 Ex situ TD includes the excavation of contaminated soils, which are heat-treated in a desorption unit (gas flow conditions may vary). Off-gases are collected for reuse or disposal.
In TD processes utilizing dryers (or kilns) with rotary drums and direct heating, contaminated soils are heated with an open-flame burner that typically requires excess oxygen [19]. In counter-current operation, the heater is located at the end where solids exit the TD unit and combustion gases flow against the direction of the solids. Solids entering the rotary drums first come into contact with gases that may have little or no oxygen. Desorption and/or pyrolysis of contaminant hydrocarbons may take place as the soils are heated in this anoxic or hypoxic zone. As the solids ap proach the exit, however, they enter an oxygen-rich zone where the remaining hydrocarbons and any char produced during pyrolysis are combusted and destroyed.
2.1.2. In situ TD
TD is achieved in situ through the application of dual heater/vacuum wells to desorb and remove contaminants via vapor extraction (Fig. 2). Thermal conduction heaters are effective for uniformly heating the entire contamination zone. Because thermal conductivity varies very little between soil types, heating is minimally affected by heterogeneity in soil structure or contaminant dispersal [18]. However, because soils have relatively low heat capacity, initial heating of the contaminated zone may require long periods of energy input before desorption will occur [7]. Moving radially from the heater/vacuum wells, radiative heat transfer dominates initially. However, thermal conduction (heat transfer via direct contact of soil particles) is the dominant form of heat transfer overall [7]. These wells may be either horizontal or vertical to suit the depth of contamination [18]. Shallow soil contamination (less than three feet deep) may be treated by thermal blankets or horizontal wells. Once extracted, hydrocarbon-laden air can be incinerated, reused, or adsorbed on activated carbon [20].
《Fig. 2》

Fig.2 In situ TD utilizes dual heater/vacuum wells to heat soils and remove contami-nants. Off-gases are collected for reuse or disposal.
In practice, heating and removal mechanisms for in situ methods vary spatially depending on proximity to heat/vacuum wells [7,18]. Although precautions are sometimes taken to maintain anoxic conditions and prevent combustion, practices in the field vary widely and the lack of standardization often fails to ensure consistent gas flow conditions [13,21]. Furthermore, to ensure sufficient temperatures throughout the contaminant zone, soils near the heaters reach higher temperatures (800–900 °C). Heavy hydrocarbons in this region will preferentially undergo thermal cracking reactions over desorption if oxygen levels are low, and be incinerated/combusted if oxygen levels are high. Thermal cracking will not occur where temperatures are below a critical threshold, set to 300 °C by some reports and 500 °C by others [7,8,18]. Furthermore, over time, oxygen levels change due to gas flow and smoldering. Thus, areas where thermal cracking/pyrolysis previously occurred may eventually be exposed to oxygen, leading to the burning of char formed during pyrolysis. This burning makes temperature regulation more difficult, but may also lead to energy savings if it can be controlled. For example, up to 25% of the energy used to desorb polycyclic aromatic hydrocarbons (PAHs) in one study were generated in situ through coke combustion [18].
2.1.3. Applications of TD
Whether due to desorption alone, or to a combination of removal mechanisms, both in situ and ex situ TD are highly effective. LTTD and HTTD can result in> 99% removal efficiency, although treatment time varies significantly by process configuration and contaminant [7]. The costs for TD (and all remediation technologies) vary considerably according to site-specific conditions. Reported costs for ex situ TD range from $46 to $99 per metric ton (adjusted for 2016 USD), while in situ TD costs between $70 and $460 per metric ton (adjusted for 2016 USD) [7,11,18,22–27]. Theoretically, desorption will occur for contaminants with a temperature below the chosen TD treatment temperature, and thus hydrocarbons with boiling temperatures below 300–350 °C can be desorbed with HTTD or LTTD (although if oxygen levels are high enough, thermal oxidation may remove even more hydrocarbons). Whereas boiling temperatures can be used to approximate desorption temperatures (Fig. 2), higher temperatures may be required. TD-treated soils become desiccated, allowing “even tight clays to become permeable enough for adequate vapor extraction [18].”
TD can be successfully applied to a wide range of volatile and semi-volatile hydrocarbons, including refined fuels, tars, creosote, rubber wastes, and TPH [20]. Due to lengthy heating times, in situ TD can take weeks or years, while ex situ TD has a contact time of several minutes to complete treatment [19]. For example, 45% of benzo(a)pyrene was removed in two years in situ, and it has been suggested that high molecular weight PAHs cannot significantly desorb in less than one year of in situ treatment [18,28]. However, low molecular weight hydrocarbons can be desorbed much faster [29]. In situ treatment was shown to remove> 99% of coal tar in several days [30]. Besides treatment temperature, soil moisture is a major variable affecting TD effectiveness and cost, because water has a high heat capacity, requiring a large energy input to raise soils past 100 °C [20,31,32]. Desorption kinetics (and treatment time) are also affected by soil properties such as compaction and density [33].
《2.2. Smoldering》
2.2. Smoldering
Natural smoldering in peat and coal deposits represents the largest and longest-lasting fires on Earth, highlighting the potential of smoldering in remediation [34]. Smoldering is a flameless combustion process that propagates a self-sustaining wave of exothermic combustion if fuel and oxygen needs are met (Fig. 3). Combustion converts the contaminants into heat, carbon dioxide, and water, thereby eliminating the need for additional fuel to complete remediation [5,34–38]. Although temperatures created by smoldering vary spatially and temporally, the average temperature is 600–1100 °C [37,39]. Smoldering is also used in enhanced oil-recovery methods, in which a smoldering front lowers the viscosity of the oil ahead of it and pushes it toward extraction wells [38].
《Fig. 3》

Fig.3 In situ smoldering utilizes a self-sustaining smoldering wave to destroy hydrocarbons without excavation. The smoldering reactions are started at a central ignition well. Off-gases are collected for reuse or disposal.
To initiate smoldering remediation, air injection and heating are required to begin combustion. Following ignition, heat injection can cease, while air injection continues for the duration of the remediation project. As long as oxygen and fuel levels are sufficient and heat loss is minimal, smoldering oxidation will be self-sustaining, with the smoldering heat wave moving in the direction of air flow [5]. Smoldering reactions are sustained by heat transfer through the soil/contaminant matrix. Contaminant removal occurs through several mechanisms. While the exothermic combustion reactions are the dominant removal mechanisms, desorption and pyrolysis (endothermic) also occur [5]. This heterogeneity of removal mechanisms has a spatial basis. Ahead of the smoldering wave, convection and conduction heat the soil, leading to desorption of hydrocarbons when the temperature exceeds their boiling point [5,34]. Organic matter (OM) is destroyed via combustion when the oxygen supply is sufficient [5]. Pyrolysis may occur in any anoxic or hypoxic region such as deep within dense non-aqueous phase liquids (DNAPLs). Conduction and convection cause heating to occur ahead of the smoldering wave; hydrocarbon destruction may occur in this region as well [5]. The speed of smoldering reactions—and overall remediation—varies by site conditions. The velocity of the smoldering front (and subsequent contaminant removal rate) is directly related to the air flow rate, while heat loss may also influence the propagation velocity [5,34].
The ignition of contaminants to initiate smoldering may take several hours [5]. Once started, treatment times for smoldering sites may be on the scale of hours to days, and can be controlled through air injection rates [5,37]. Ignition and hydrocarbon extraction can be achieved using electric heater/vacuum wells such as those found in in situ TD. As with other in situ thermal technologies, air pollution control is required to capture and destroy gases such as vaporized hydrocarbons and CO, which can be accomplished by using vapor extraction/vacuum systems followed by activated carbon adsorption or incineration [5].
Applications of smoldering
Most of the attention given to smoldering remediation has focused on the remediation of coal tar DNAPLs, with a limited number of field-scale pilot projects. However, due to the high temperatures that are achievable, it is likely that smoldering can be used for treating soil that has been impacted with heavier hydrocarbons. Studies have been successful in removing hydrocarbons to very high levels. For example, 98.5% average removal of coal tar was found using smoldering in shallow and deep sediments in a field-scale trial, and laboratory column measurements often find total hydrocarbon-removal efficiencies> 99% [5,36–38].
Although smoldering can successfully destroy hydrocarbons in lab-scale studies, comparatively large heat losses for these studies may not accurately simulate field conditions and results. Smoldering was effective in remediating heavy hydrocarbons from tar sands, suggesting that these hydrocarbons generate enough energy to overcome heat losses [38]. Due to the self-sustaining nature of the smoldering front, larger sites will become more energy efficient as the volume of treated soil increases [37]. The ideal TPH range in order to sustain smoldering reactions has been estimated at 31.2–104 g·kg–1 for crude oil, and 28.4–142 g·kg–1 for coal tar [36]. Costs were estimated at $260 to $330 per metric ton (adjusted for 2016 USD) [40].
The full range of concentrations within which the technology can be efficiently deployed is yet to be determined. Furthermore, smoldering effectiveness may vary in different soil environments. Although soil water does not prevent smoldering propagation, high water content has been shown to reduce temperature [5,37]. Ideal soils for smoldering possess sufficient pore space for oxygen transport, but do not shrink in the presence of heat, restricting heat transfer [37,38]. Fine-grained soil particles may limit oxygen levels to the smoldering front, thus leading to slower front propagation [38].
《2.3. Incineration》
2.3. Incineration
Incineration involves the total destruction of contaminants through high-temperature combustion of impacted soils. Incineration is an established technology, not just for hydrocarbon removal, but also for the treatment of many hazardous and municipal wastes.
Onsite incineration without excavation, known as on-land burning or open burning, can be difficult, costly, and unpredictable [41]. Therefore, incineration is typically applied as an ex situ technology that involves the excavation of contaminated soils and combustion in one of four major types of incineration units: ① rotary kilns, ② fluidized bed reactors, ③ liquid injection, or ④ infrared heaters [20] (Fig. 4). Many alternative heating technologies have been used such as infrared incineration using silicon carbide rods [11]. Following treatment, soils must be moisturized for dust control before reuse as backfill for construction projects or other non-agricultural applications. Although treatment conditions vary by target contaminant, incineration is typically conducted at 600–1600 °C [8,22,42]. Inflowing oxygen levels are maintained at approximately 10% for volatile organic compounds (VOCs) combustion. However, both oxygen levels and soil-loading rates must be considered along with the contaminant lower explosion limit (LEL) in order to ensure safe treatment [43].
《Fig. 4》
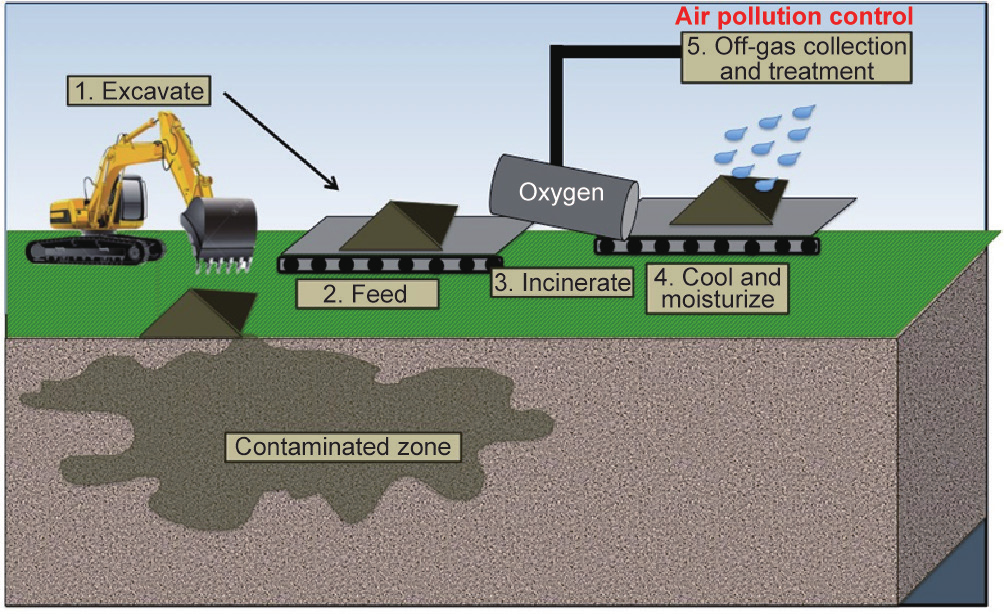
Fig.4 Ex situ incineration includes the excavation of contaminated soils, which are incinerated under oxygen-rich conditions. Off-gases are collected for reuse or disposal.
The exhaust gases are filtered in scrubbers, electrostatic precipitators, or baghouses, and subsequently incinerated to remove any gaseous products that cannot be vented due to air pollution and soil deposition concerns [42,44]. Depending on soil moisture and contaminant levels, these gases may be suitable for energy recovery as well. In addition to the soil and gaseous products, the incineration process produces ash that is typically disposed in landfills [11,13,21,42].
Applications of incineration
Due to its high temperatures, incineration is often one of the most expensive thermal technologies to operate. Still, it is a valuable technology because of its effectiveness and applicability to remove a wide range of target contaminants. Incineration can destroy nearly all hydrocarbons, due to high hydrocarbon flammability and high temperatures. Contaminant mass removal efficiencies greater than 99% are typical, with costs ranging between $150 and $2900 per metric ton (adjusted for 2016 USD) [11,21,41,42,45,46].
《2.4. Pyrolysis》
2.4. Pyrolysis
Pyrolysis involves the heating of impacted soils in anoxic atmospheres, typically to 400–1200 °C for a variety of hazardous wastes (< 550 °C for hydrocarbons) [8]. When applied to soils contaminated with petroleum crudes, pyrolysis removes hydrocarbons via two different mechanisms. As the soil temperature rises, low molecular weight hydrocarbons thermally desorb when heated to their boiling temperature. When the temperature rises above 250–300 °C, chemical bonds break and may form highly reactive free radicals. C-heteroatom (i.e., C–S) bonds break first, followed by C–H and C–C bonds. The free radicals rapidly react again to either continue their own cracking (beta-scission) or start a sequence of aromatic condensation reactions that leads to the formation of a carbonaceous material (char) with very low H/C ratio [47–49]. As discussed in Vidonish et al. [8], this complex process includes “① the cracking of alkyl chains from aromatic groups, ② the dehydrogenation of napthenes to form aromatics, ③ the condensation of aromatics to higher fused-ring aromatics, and ④ dimerization and oligomerization reactions [8,50–52].” The light hydrocarbons produced from both reaction pathways are swept away by the oxygen-free gas passing through the pyrolysis reactor. For most petroleum hydrocarbons, char formation is complete by the time the temperature reaches 450–500 °C [18,30,53]. Through char formation, therefore, pyrolysis is able to remove high molecular weight hydrocarbons without reaching their high boiling temperatures.
Pyrolysis is set up similarly to ex situ incineration or TD, with the notable exception of maintaining an anoxic atmosphere (Fig. 5). Oxygen exclusion can be achieved through indirect (electric) heating rather than direct heating via a gas fuel burner as with ex situ TD. The volatile products of desorption and pyrolysis are incinerated or reused as in TD [20], while the char produced remains on the treated soils. Char remaining in the soil may provide carbon content and can potentially facilitate re-greening efforts.
《Fig. 5》

Fig.5 Ex situ pyrolysis includes the excavation of contaminated soils, which are heat-treated under low-oxygen conditions. Off-gases are collected for reuse or disposal. Soils may be used for re-greening.
Applications of pyrolysis
Although pyrolysis is a relatively new application for hydrocarbon remediation, lab-scale experiments showed> 99% removal of solvent-extractable TPH from heavy crudes while preserving nutrients and soil properties that are lost when soils are incinerated [8]. Because heavy hydrocarbons react to make char at lower temperatures than their boiling points, pyrolysis can effectively treat high molecular weight hydrocarbons at lower tempera tures (< 500 °C), saving energy and possibly sequestering a small amount of carbon in the form of char. Thus, heavy crude oils, petroleum sludges, tars, PAHs, and refined fuels and fuel oils can be effectively remediated using pyrolytic treatment [8,20]. Because remediation of very high molecular weight hydrocarbons can be achieved in the temperature range of HTTD (< 500 °C), field-scale pyrolysis is expected to have a similar energy footprint and similar costs as HTTD, but with fewer detrimental effects on the treated soil [8].
《2.5. In situ vitrification》
2.5. In situ vitrification
Vitrification uses very high temperatures (1600–2000 °C) to melt and fuse contaminants and soil into a glass-like solid. It is especially common for the treatment of radioactive wastes in addition to petroleum [20,42,54]. The molten soil/contaminant solid possesses properties similar to obsidian and is over 10 times stronger than concrete [42]. By rapidly cooling molten soil and contaminants, crystallization is prevented and stable glass is formed from non-volatile materials. As with TD and pyrolysis, volatiles desorb and are treated as a waste gas stream. Much of the soil organic material is pyrolyzed in the low-oxygen molten center of the glass before migrating to the glass surface and undergoing oxidation [42].
Soil vitrification is usually done as an in situ process [20] (Fig. 6). In situ vitrification usually delivers heat to soil via molybdenum electrodes, often with additional graphitic or glass material mixed in to initiate the melting process [42,54,55]. After the soil minerals melt, the electrodes are turned off, allowing the soil (and trapped contaminants) to cool into a vitreous, glass mass. Depending on the size of the molten area, cooling can take up to one year to complete. The glass block is left in place and, because vitrification causes subsidence/shrinkage of soil volumes by 20%–40%, additional backfill soil must be added [42,54]. The vitrified solid can undergo repeated freeze/thaw cycles without the risk of releasing contaminants [42]. Air pollution control is similar to other in situ thermal technologies, including baghouses, electrostatic precipitators, and incineration employed for gaseous wastes [42].
《Fig. 6》

Fig.6 In situ vitrification involves the melting and fusing of contaminants and soil into a stable glass end-product. The glass can remain in soil, with clean soils used to fill in the subsidence area near the surface.
Applications of vitrification
While vitrification can be applied to soils with a variety of organic contaminants including petroleum, it is more commonly used in the treatment of hazardous inorganic wastes such as heavy metal contamination [11,20]. Costs for vitrification range from $486 to $2900 per metric ton (adjusted to 2016 USD) [11,42,45,56].
《2.6. Radio frequency heating/microwave heating》
2.6. Radio frequency heating/microwave heating
Radio frequency heating (RFH, i.e., microwave heating) was first developed to enhance oil recovery in shale and tar sands in the 1970s [57]. This technology can be used as a stand-alone remediation technique or to enhance other processes such as bioremediation, air sparging, and enhanced vapor recovery [58]. RFH volatilizes and desorbs low molecular weight hydrocarbons, decreases viscosity, improves bioavailability, and speeds microbial degradation rates [58].
RFH transfers heat on a molecular level, by imposing an electric field on electric dipoles (unbalanced charges) in soils, contaminants, and water. Because RFH relies on molecular dielectric interactions, this method of heating is reliable regardless of heterogeneous soil properties that may interfere with convective heating strategies [58]. However, deployment of RFH requires consideration of the different dielectric properties of soil types, hydrocarbon species, and soil water in heating calculations [58]. Water, in particular, is a major factor in microwave heating due to its dielectric properties, and thus high soil moisture is needed in RFH-enabled remediation [59]. Amendments such as carbon fiber or nanoparticles have been tested to improve or enhance the dielectric heating properties of soils [60].
RFH is most often applied in situ (Fig. 7). Heat is supplied by electrodes and antennae powered by a radio frequency (RF) generator [61]. RFH is often employed when temperatures higher than steam or hot air injection are desired, usually 150–200 °C [10,61]. Treatment times are usually on the order of days and vary according to the contaminant, temperature, and whether the RFH is used as a stand-alone or auxiliary technology [10].
《Fig. 7》

Fig.7 In situ RFH uses heating antennae to heat the subsurface. Off-gases are collected for reuse or disposal.
Applications of RFH
Stand-alone RFH is generally used to remediate low molecular weight hydrocarbons [10]. More than 99% removal of hydrocarbons has been achieved with RFH [9]. Microwave heating (4 kW) has also been employed as a pretreatment step to remove water from petroleum-contaminated soils before applying TD at 300 °C [9]. Studies have also tried to use RFH to pyrolyze soils, but encountered challenges due to insufficient soil moisture [59]. Cost estimates for RFH are widely variable, ranging from $400 to $7500 per metric ton (adjusted to 2016 USD) [10,62,63].
《2.7. Hot air injection》
2.7. Hot air injection
Hot air injection is used to increase contaminant mobility and extraction efficiency in soil vapor extraction remediation [20]. Due to the low heat capacity of air, large volumes of air at high temperatures (and thus high energy usage) are required to heat soils to the levels required for hydrocarbon desorption [10].
Injection is an in situ process that is accomplished through wells or auger injection pathways (Fig. 8) [10,61]. Solar panels, in conjunction with blowers and injection wells, can be used as a sustainable method of hot air production in sunny and warm areas [10]. Steam is often used in conjunction with hot air to more effectively carry desorbed organics into the vacuum well [10].
《Fig. 8》

Fig.8 In situ hot air injection uses injection/vacuum well systems to heat the subsurface. Off-gases are collected for reuse or disposal.
Applications of hot air injection
Hot air injection is typically used with bioremediation or other processes, and can be applied to any hydrocarbon contaminants, from light fuels to crude oils and creosotes [17]. Typical costs range from $54 to $82 per metric ton (adjusted to 2016 USD) [64]. Hot air injection has been found to increase petroleum removal efficiency by 9% over ambient-temperature air sparging [65].
《2.8. Steam injection》
2.8. Steam injection
Steam injection was first developed by the energy industry for enhanced oil recovery, and can be used alone or in tandem with other technologies to enhance the efficiency of other remediation approaches by lowering contaminant viscosity, increasing mobility, or warming cold soils to improve biodegradation rates. Steam has a higher heat capacity than hot air, providing more efficient means for heating soils, when steam is easily accessible [10,17,65]. Steam injection utilizes a pressure differential to encourage condensation of the steam and the subsequent desorption and evaporation of volatile hydrocarbons [10,61]. Steam injection creates three distinct treatment zones [66]. Zone One, the “steam zone,” is a near-isothermal zone near the injection site where removal is typified by steam distillation and stripping. Downstream of this region, a “contaminant bank” forms. Zone Two, the “variable temperature zone,” forms downstream where the temperature cools and steam/contaminants condense. Zone Three, the “ambient temperature zone,” includes the flow of water and mobile contaminants [66].
There are three primary steam-delivery methods applied in the field: ① steam/vacuum wells, ② steam injection through drill bits, and ③ steam injected beneath the contaminant zone, which condenses and flows upward as hot water (Fig. 9) [10]. Once extracted, vapors are treated with activated carbon adsorption, filtration, and so forth. Vapors are contained and removed in situ using a vacuum extraction system [10]. Effluent must also be monitored to ensure environmental safety, especially as condensation flow can risk secondary groundwater contamination [17,66].
《Fig. 9》
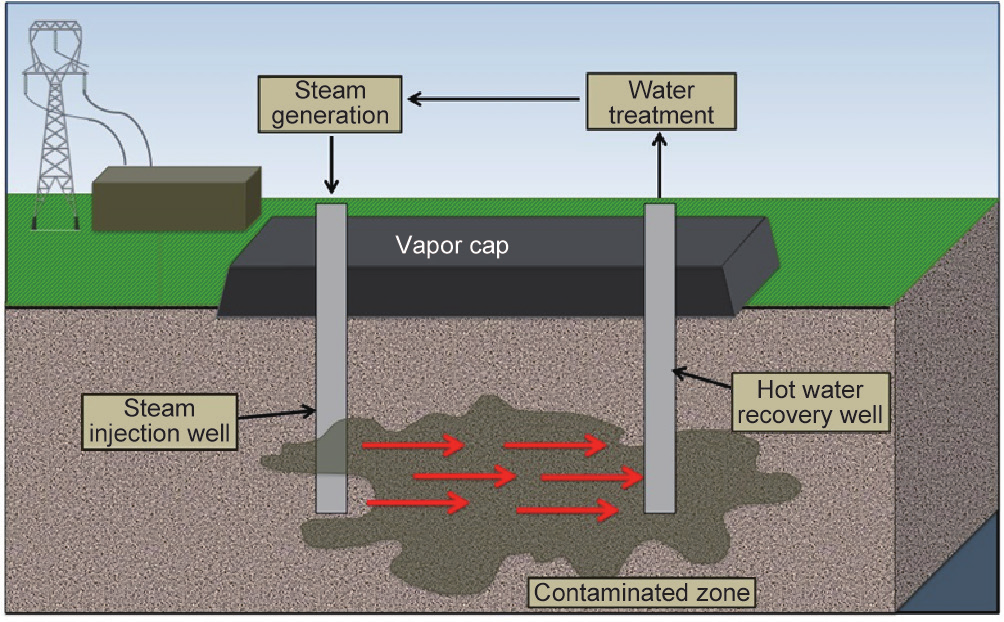
Fig.9 Steam injection uses injection/vacuum well systems to heat the subsurface. Off-gases are collected for reuse or disposal.
Applications of steam injection
Depending on its configuration, steam injection can be successfully applied to soil remediation for a wide range of organic contaminants such as heavy fuel oils [66]. Steam injection is most effective for the removal of organic contaminants with boiling points less than 250 °C, although extraction efficiency varies widely from 11% to> 99% depending on soil type (particularly clay mineralogy), contaminant polarity, and vapor pressure [10]. Hot water can also be utilized for a similar effect. Removal efficiencies vary in different soil environments. For example, studies have reported 20% removal of benzene, toluene, ethylbenzene, and xylene (BTEX) in clay versus 99.5% in sand, 60% removal of naph-thalene in clay versus 99.9% in sand, 35% removal of PAHs in marsh versus 97% in sand, 20%–80% removal of phenol in either clay or sand, and greater than 98% removal of acetone, xylenes, ethylbenzene, and 1,2-dichlorobenzene [10,67,68]. The mass transfer coefficient for trichloroethylene (TCE) removal has been shown to increase by a factor of two when hot water flushing was used to increase temperatures from 5 °C to 40 °C prior to air sparging [69]. However, when used in tandem with other remediation technologies such as bioremediation, steam can be a successful part of the remediation of nearly any hydrocarbon. Costs for steam injection vary from $37 to $380 per metric ton (adjusted to 2016 USD) [45,66].
《2.9. Modeling considerations》
2.9. Modeling considerations
Numerous modeling studies of both non-catalytic and catalytic thermal processing of hydrocarbons have been published [70–72]. However, the vast majority of these studies consider thermal cracking to produce feedstock for polymerization reactions, or lighter hydrocarbons in order to increase the yield of liquid fuels from petroleum crudes. Modeling the thermal treatment of contaminated soils has received much less attention in the literature. Several investigators have modeled transport processes occurring during the evolution of light hydrocarbons from beds of soil particles at temperatures lower than 300 °C, and validated these simulations through comparisons with data obtained with laboratory reactors [33,73,74]. While these efforts are applicable to the TD of light hydrocarbons, they only considered mass transport processes that constitute a subset of the chemical reactions and physical processes that occur during the thermal treatment of contaminated soils using the technologies considered in this review. Therefore, there is a need for developing computational models that also consider the pertinent chemical reactions to determine how key characteristics of the contaminated soil (e.g., type and amount of contaminants, soil composition, and moisture content) and of the processing conditions (e.g., reaction temperature and contact time) affect the effectiveness and efficiency of various thermal remediation technologies.
《3. Environmental compatibility and sustainability considerations》
3. Environmental compatibility and sustainability considerations
《3.1. Thermal treatment leads to decomposition of soil constituents》
3.1. Thermal treatment leads to decomposition of soil constituents
Remediation efforts tend to focus on the effect of thermal treatment on contaminants and the ability of the technology to meet treatment goals. The effects of thermal treatment on the soil are not typically considered. Only a few studies have considered the effect of high heat on soil minerals and OM [14,15,75–79].
OM undergoes structural changes when heated from 200 °C to 460 °C [79]. Fire has been shown to alter the structure of OM by removing oxygen groups (which decreases solubility), breaking molecular chains, increasing aromaticity, and producing char [79,80]. In a study of how soil decomposition affects remediation results, the pyrolysis of uncontaminated, low-organic content soils from 350 °C to 1050 °C resulted in weight loss from various soil fractions. Gas chromatography-mass spectrometry (GC-MS) analysis showed that CO, CO2, and tars were the major products, comprising over three quarters of total volatile losses that increased drastically over 500–600 °C [8,14]. Carbon, oxygen, and hydrogen content generally decreased as pyrolysis temperature increased [8,14]. In particular, carbon content dropped significantly, with 49% lost at 955 °C and 98% lost at 1033 °C [8]. CO2 evolution occurred in two distinct steps. Above 700 °C, CO2 is expected to have been derived from carbonate degradation. A smaller CO2 peak is observed from 350 °C to 550 °C, where several carbonates such as nahcolite and humic substances degrade [8,81]. Furthermore, OM decomposes into light hydrocarbons such as C2H2, C2H4, and CH4, especially between 300 °C and 600 °C [14,15,76,78,82]. The fulvic acid portion (polysaccharides, carbohydrates, and polyphenols) of OM decomposes more readily than humic acids (polysaccharides, lignins, peptides, and lipids), which display higher thermal stability [15,78]. In the presence of metals, humic acids form coke with a wide range of surface areas [75]. Due to the complex composition and varied origins of organic and inorganic soil matter, predicting decomposition can be difficult. Soil with high OM may undergo more drastic chemical, morphological, and structural changes than low-OM soils [76] and such changes are amplified with temperature. High temperatures have been associated with increases in particle surface area, porosity, and surface fractures, although these responses are highly variable [76].
Because OM plays an important role in agricultural soils, more researches are needed to understand the severity of high-OM induced soil decomposition on agricultural properties, as well as possible mitigation strategies to salvage valuable soil resources [82]. In addition, the complex composition of organic and inorganic soil matter makes predicting thermal decomposition difficult.
Knowledge of thermal decomposition of soil constituents can be used to determine the least damaging thermal treatment to reach desired cleanup levels. The clearest lesson that can be gleaned from this information is that the lowest effective treatment temperature should be used to minimize soil decomposition. The high temperatures utilized by incineration, vitrification, and smoldering, for example, allow for effective treatment of nearly all hydrocarbons, are also likely to cause extensive soil damage such as decomposition of clays, carbonates, and organic content. This decomposition will physically alter the soil and change its geochemical, biological, and fertility properties [39].
Heating rate, treatment time, and soil/contaminant type also affect reactions, and must be considered in addition to peak temperature [15,30,83]. Petroleum-contaminated soils are usually incinerated at temperatures higher than 700 °C, causing common carbonate minerals (calcite, dolomite, and siderite), carbonate salts, and metals to decompose [81]. Carbonates decompose upon heating, releasing CO2, and leaving metal oxides behind [84]. Since calcium (particularly in the form of carbonates) represents 75%–85% of exchangeable bases in soils, carbonate decomposition thus decreases soil acidity [39,85,86]. Soil pH may also increase at lower temperatures due to the denaturation of organic acids, but below 500 °C, the pH can usually be buffered adequately (by carbonates, etc.) to prevent extreme pH changes [79]. The alteration of soil pH affects many soil interactions, including plant tolerance, root and leaf growth, and cation-exchange capacity (CEC) [86]. Following incineration at 650 °C, two contaminated soils underwent pH increases, from 7.2 to 11.1 and from 7.7 to 11.9, respectively [8]. Increases in soil pH have also been observed after forest fires, and have been attributed to the presence of wood ash [8,87]. Although not a perfect analog for thermal remediation, forest fires induce similar mineral and OM changes in soils. Generally, water repellence, bulk density, pH, and inorganic nitrogen increase, while CEC, soil structure, and the quality of OM decrease. The extent of these effects, however, can vary according to site conditions and fire temperature [79].
High-temperature technologies such as incineration may be better suited for sandy soils, because quartz is more thermally stable than other soil minerals. Thus, sparsely vegetated sandy areas such as coastal beaches and deserts may be good candidates for high-temperature remediation. On the other hand, lower temperature methods such as HTTD and pyrolysis should be used for high molecular weight hydrocarbons in more sensitive ecosystems.
Soil type and particle size may also affect remediation efficacy. Steam injection has been shown to be far more effective on sandy soils than on clayey soils, and thus may be more efficient for spills in sandy areas [10].
Although treatment temperature affects the energy use of thermal remediation and the agronomic quality of the treated soil, site configuration is also influential. In situ techniques such as in situ TD, RFH, and hot air/steam injection have the potential benefit of minimizing soil disturbance and the disruption of soil aggregates, which are essential for soil/water dynamics, gas flow, and root penetration [41,61,88–92]. Hot air and steam, due to their lower temperatures, are thought to be minimally disruptive beyond some temporary sterilization of microbial communities. However, it is challenging for in situ methods to provide spatially even heating, which can also affect hydrocarbon removal and energy use. For example, the lack of control of oxygen levels in most TD processes means that accurate temperature regulation is difficult, possibly resulting in uneven heating profiles. The under-heating or overheating of soils may increase soil damage or energy usage, or may even jeopardize remediation results.
Consideration of the final (treated) soil that is delivered to the environment is important in order to enhance ecosystem restoration and public acceptance. Successful remediation projects should consider the possibility that simply meeting legal remediation goals will be insufficient to produce soil that meets local community expectations for fertility. While it may be possible to narrowly meet legal chemical thresholds for remediation, remediation teams should be aware that long-term success is most likely when local community expectations are taken into account.
The choice of off-gas collection and treatment technologies is critical for energy efficiency and water conservation. To our knowledge, however, there are no published studies that rigorously address these important factors, underscoring the need for developing process simulators that estimate the effect of various process options on the energy and water requirements of different thermal treatment technologies.
《3.2. Challenges and opportunities for ecosystem restoration》
3.2. Challenges and opportunities for ecosystem restoration
Alteration of soil properties through chemical decomposition or mechanical disturbance as part of thermal remediation leads to changes in soil fertility. Fertility alterations in turn can affect the ability to re-vegetate the treated area, or can change site ecosystem dynamics. Although conclusive and quantitative research on the role of soil decomposition in plant growth is sparse, changes such as pH increase with carbonate decomposition suggest that the lowest effective treatment temperature should be employed. Beyond this, the data on temperature impacts on soil fertility are varied and inconclusive. For example, while some studies suggested that TD-treated soils may sustain plant growth, these soils are still typically recommended only for backfilling and construction applications [88]. Few quantitative studies on the impacts of TD on soil properties or plant growth exist. Some studies made conjectures about soil quality based on visual inspection, or suggested, as in Ref. [16], that TD is “fairly environment friendly” without studying or citing this information directly [16,93]. A few studies on PAH-impacted soils treated with TD exist and find mixed results. One such study reported increased genotoxicity to earthworms in treated soil that was contaminated with PAHs from a coking plant, possibly due to the increased bioavailability of residual contaminants [94]. Another study, however, found that soil contaminated with PAHs and metals could be used for re-greening efforts following TD [95]. Clearly, systematic studies with well-designed controls are needed before the true impact of TD on soil and plant health can be ascertained. Because TD in practice often includes not only desorption, but also ancillary oxidation and/or pyrolysis, it is likely that impacts on soil properties vary spatially and from project to project, particularly for in situ applications. On the other hand, in situ TD may be more eco-friendly in sensitive ecosystems due to the lack of soil disturbance.
Similarly, incineration has the reputation of limiting soil reuse possibilities, as incinerated soils are typically recommended for construction/backfill applications rather than agricultural use or re-greening [21]. The existing literature on this topic is sparse and inconclusive. In one case, no significant difference for re-greening was found for soils treated by surface land burning, phytoremedia-tion, or lime addition [96]. In contrast, severely stunted growth of plants in incinerated soils has also been demonstrated. In fact, germination, growth, and mortality rates were worse in incinerated than in contaminated soils [8,43]. Other studies found short-term (5–6 weeks) negative effects on a local marsh plant, Sagittaria lancifolia, before the vegetation was able to recover to pre-burn levels [43].
Soil aggregation is crucial to fertility, and aggregate stability is highly dependent on moisture content, microbial communities, and OM, which are destroyed by incineration [92,97–100]. However, crude cleanup via on-land burning, which does not disturb soil as much as ex situ incineration, has also been demonstrated to have no significant impacts on the physical properties of soils [101].
Lab-scale pyrolysis studies suggest that preventing oxidation tempers the loss of fertility and improves plant performance and soil properties, though this has not been demonstrated at the field scale [8]. Very low temperature technologies such as steam/air injection and LTTD are more likely to be performed below the decomposition temperature of many soil constituents, emphasizing the importance of using the lowest required treatment temperature. These studies suggest that performing thermal remediation at the lowest possible temperatures is least destructive to soils and re-greening efforts [14,76]. Overall, more studies that consider the environmental impacts of incineration from a soil science and plant biology perspective are needed.
《3.3. Challenges and opportunities for energy efficiency》
3.3. Challenges and opportunities for energy efficiency
Minimizing treatment temperature is not only beneficial for protecting soil properties and fertility, but also important to keep energy usage and associated costs low (Table 1, Fig. 10). Thus, we recommend considering lower temperature alternatives to high-temperature processes such as incineration and vitrification. Refined fuels, most crude oils, and PAHs can be remediated using technologies that do not incur the high energy costs and soil quality impacts of incineration (Fig. 11) [8]. High-temperature technologies should be a last resort for complicated and hazardous waste mixtures such as radioactive contaminants or chlorinated solvents [42]. Note that, while thermal technologies can safely and effectively treat mixed wastes, care must be taken to avoid toxic byproducts such as dioxins from chlorinated solvents. Two mechanisms have been proposed for dioxin formation [102–105]. The first is a de novo mechanism in which carbon, oxygen, hydrogen, and chlorine combine and react to (eventually) form dioxins and dibenzofurans (PCDD/Fs). De novo formation of dioxins is the dominant route in combustion systems [102]. The other route is the precursor mechanism, which involves the surface-catalyzed reactions that convert precursor compounds such as chlorobenzenes and chlorophenols to PCDD/Fs. Under certain conditions, gas-phase formation of dioxins via the precursor mechanisms can also be observed [106]. Thus, we do not expect PCDD/Fs to form when contaminated soils that do not contain the precursors are thermally treated in anoxic atmospheres (pyrolysis). Even when these conditions are not met, however, thermal technologies can still be successfully applied if accompanied by end-of-pipe pollution control systems that remove dioxins from the exhaust gases [102,107–110].
《Table 1》
Table 1
Overview of thermal treatment technologies.
《Fig. 10》

Fig.10 Cost range of various thermal remediation technologies. Due to factors such as contaminant type, moisture content, and operating differences, reported costs can vary widely.
《Fig. 11》

Fig.11 (a) Boiling temperatures of various petroleum hydrocarbons; (b) boiling temperatures of various hydrocarbon classes. Boiling temperature can be used to approximate effectiveness of different treatment technologies for various hydrocarbons. Incineration, pyrolysis, and vitrification also rely on mechanisms beyond desorption/boiling, but their high temperatures make them reliable for nearly all hydrocarbons.
For all technologies studied, hydrocarbons are destroyed either in reactors (incineration) or as off-gas as part of air pollution control. If possible (i.e., given low moisture and high BTU content), energy recovery should be considered in order to reduce the carbon footprint of these technologies.
Because TD requires lower temperatures than incineration, operation costs for this process tend to be lower. In one case of polychlorinated biphenyl (PCB) remediation, LTTD was to be 75% less expensive than incineration [93]. Although costs depend on contaminants and other variables, in situ TD is generally more expensive due to subsurface heating challenges, with an average cost of $70 to $460 per metric ton (adjusted to 2016 USD) [16,18].
TD has several disadvantages. The lack of control of oxygen levels in most TD processes means that accurate temperature regulation is difficult, possibly resulting in uneven heating profiles. Under-heating or overheating of soils may increase soil damage or energy usage, or may even jeopardize remediation results. Again, it is important to keep the treatment temperature at the lowest possible level for effective contaminant removal in order to minimize energy usage and maximize re-greening potential by limiting the decomposition of soil minerals, OM, and nutrients.
Smoldering, on the other hand, possesses the benefit of being self-sustaining, although its heat may be more difficult to control (as are the subsequent remediation mechanisms). Due to the self-sustaining nature of smoldering remediation, energy costs for this technology can be much lower than those of other thermal treatments. For example, 1.1 kJ·kg–1 of remediated soil was required to ignite smoldering soils, compared with 300–700 kJ·kg–1 for in situ TD [5]. Furthermore, because ignition is only required at the beginning of the project, larger sites become more and more efficient, both in energy ignition requirements per kilogram of soil and through reduced heat losses (i.e., faster front movement and treatment times) [5]. Thus, smoldering is a highly sustainable choice for large sites, particularly in remote areas without access to the energy grid.
When location permits, alternative energy sources may lower costs and the carbon footprint of thermal treatment. For example, solar power can be used to more sustainably heat the air [10]. However, because air is not a good heat conductor, it may be less sustainable in areas without abundant cheap energy such as onsite solar energy. The ability to use alternative energy sources could make a technology such as hot air injection more sustainable, despite the relatively poor heat conduction of the air. Warm areas with heavy rainfall such as tropics may also be able to take advantage of dual sun/water resources to efficiently apply solar steam generation.
《3.4. Challenges and opportunities for water conservation and reuse》
3.4. Challenges and opportunities for water conservation and reuse
Virtually all the thermal treatment technologies reviewed in this paper will desiccate soils, with the exception of steam injection. Thus, sites where re-greening is desired will require post-treatment with water. In the case of steam, care must be taken to monitor groundwater, so that contaminants do not percolate into aquifers. Thus, steam may be best applied in areas with deep aquifers.
RFH relies heavily on water content to heat, and thus is more sustainable in wet areas than in dry climates. Its use in dry soils may require additional water, which may be environmentally and economically prohibitive in some areas [9,112]. It is a viable alternative to LTTD for low molecular weight hydrocarbons, and is a more appropriate choice than incineration for low molecular weight contaminants.
The water demands of a remediation project should be considered when determining remediation strategy for a site. For example, hot air injection may be preferable to steam in arid areas. When possible, water recovery from off-gases should be considered to lower the water footprint. Finally, disrupting soil aggregates may alter soil water dynamics. In situ technologies may help preserve some soil structure and lessen these effects. However, further studies on the aggregation dynamics of thermal treatment are needed.
《4. Conclusions》
4. Conclusions
Thermal remediation technologies are unparalleled in accomplishing fast and effective remediation, and will continue to occupy an important niche for the remediation of soils impacted by petroleum hydrocarbons over the long term. However, thermal technologies will not reach the goal of ecosystem restoration without a holistic consideration of soil, plant, and ecosystem impacts. Reaching this goal will require further research beyond simply determining whether hydrocarbon removal has been achieved.
Primarily, treatment temperatures should be minimized to save energy and mitigate soil damage. Despite their effectiveness, high-temperature technologies such as incineration and vitrification should be reserved for highly recalcitrant and hazardous contaminants, including mixed wastes. Water requirements should be considered in tandem with site parameters; steam injection and microwave heating are ideal when water is abundant, while hot air injection, TD, smoldering, and pyrolysis require less water. The thermal properties of the contaminated soils must be considered along with treatment temperatures in order to understand the effect of treatment on subsequent re-greening or ecosystem restoration efforts.
Overall, the convergence of treatment process engineering with soil science, ecosystem ecology, and plant biology research is essential in order to select and design appropriate thermal treatment technologies that effectively remove contaminants while minimizing unintended environmental impacts.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Julia E. Vidonish, Kyriacos Zygourakis, Caroline A. Masiello, Gabriel Sabadell, and Pedro J. J. Alvarez declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




