《1.Introduction》
1.Introduction
Zein is one of the major storage proteins of corn, and is the main byproduct in the corn-processing industry [1]. Its unique hydrophobicity causes researchers to study it for possible applications beyond the food industry [2]. Our group is the first to study zein as a biomaterial in tissue engineering, and we have proved its good biocompatibility and mechanical properties. The first product from a porous scaffold of zein may be used as a bone substitute [3–6]. As a Class III medical device, quality monitoring for raw materials, a suitable sterilization method, and biocompatibility are required.
Firstly, the quality of the raw materials must be stable and controllable. We found that zein purchased from different sources had a different appearance, affecting its scaffold shaping and mechanical properties; this prompted us to investigate the differences in zein from different sources.
Sterilization process control is important for the quality management system of sterile medical device manufacturers. The control level of the sterilization directly affects the quality and safety of the sterile medical devices. The most traditional method of sterilization for manufacturers is ethylene oxide sterilization. However, this method may cause the problem of ethylene oxide residue in the porous scaffolds [7,8]. Therefore, we investigated other traditional methods such as moist-heat sterilization, dry-heat sterilization, and gamma-ray (γ-ray) sterilization. However, these methods may also be problematic for the structural stability of zein because heat and pressure may influence the structure of zein [9]. For example, the use of gamma radiation involves a great deal of energy, which may cause molecule ionization [10].
A traditional way to assess the proliferation of cells is a 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [11,12], a method that we have applied in our previous work [3–6]. Recently, we found that the background optical density (OD) of zein scaffolds was significantly high in MTT assays, which may affect the evaluation of data [13,14]. Therefore, we tried another method, a cell-counting kit-8 (CCK-8) assay [15], and compared these two methods in the evaluation of cell proliferation on zein film. We also considered possible factors causing a high background OD of zein in the MTT assay.
《2.Materials and methods》
2.Materials and methods
《2.1. Amino acid analysis, sodium dodecyl sulfate polyacrylamide gel electrophoresis, and microscopy characterization》
2.1. Amino acid analysis, sodium dodecyl sulfate polyacrylamide gel electrophoresis, and microscopy characterization
Zein 1 was purchased from Kobayashi Perfumery Co., Ltd. (Japan), zein 2 (260-01283) and zein 3 (261-00015) were both purchased from Wako Pure Chemical Industries, Ltd. (Japan), and zein 4 (in sheet form) was purchased from Wujiang Bache Pharmaceutic Adjuvant Co. (China). We obtained the zein 4 powders by grinding sheets of zein 4 using a grinder (Q-100A2, Shanghai Bingdu Electric Co., Ltd., China) and then sifting. All were dispersed in 6 mol·L–1 HCl with a ratio of 1:6 (g·mL–1). The mixture was kept in an oil bath at 110 °C for 22 h. After acidolysis, the mixture was first rotary evaporated to concentrate it, and then lyophilized (FreeZone 4.5, Labconco, USA) to obtain the powder. The powder was dissolved in citric acid-sodium citrate buffer (pH = 2.2) and analyzed using a High-Speed Amino Acid Analyzer (L-8900, Hitachi, Japan) [16].
Samples of the four sources of zein were dissolved in 75 vt% ethanol solution at a concentration of 1 mg·mL–1. Next, 20 μL of the sample solution was mixed with 20 μL of the loading buffer, and the mixture was heated in a water bath at 90 °C for 15 min. The loading buffer was made of 2 mL glycerol, 2 mL 10 wt% sodium dodecyl sulfate (SDS), 1 mL 2-mercaptoethanol, 0.5 mL 0.1% bromophenol blue, and 0.625 mL stacking gel buffer. Denaturing gel electrophoresis was carried out with a vertical slab gel apparatus (Bio-Rad, USA). The gel was made of a stacking gel of 5% and a resolving gel of 12% acrylamide concentration. It was run at room temperature at 110 V for 30 min and then at 150 V for about 1 h, using a running buffer made of 3.05 g of Tris base, 14.4 g of glycine, and 1 g of SDS dissolved in 1 L of Milli-Q water. The bands were visualized by Coomassie blue R250 staining [17].
The micro-morphology of the zein powders was investigated using scanning electron microscopy (SEM) (S-3400N, Hitachi, Japan) and fluorescence microscopy (IX71, Olympus, Japan) with an optical filter (U-MSWB2, Olympus, Japan) to produce the excitation light.
《2.2. Porous zein scaffolds: Fabrication and sterilization》
2.2. Porous zein scaffolds: Fabrication and sterilization
Zein 1 was mixed with sodium chloride (particle sizes from 0.3 mm to 0.425 mm) at a mass ratio of 1: 1.5, and the mixture was molded into 3D scaffolds using a stainless steel mold (ϕ = 1.75 mm) at 0.1 MPa for 2 min on both sides. Next, the scaffolds were leached in a water bath at 55 °C for 30 min, cut to a cylindrical rod with a diameter of 2 mm and a height of 4 mm, and lyophilized for 6 h [4].
γ-ray sterilization was performed by keeping porous zein scaffolds under 20 kGy, 25 kGy, and 30 kGy [18]. The zein powder and the porous zein scaffolds were monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) before and after sterilization.
Dry-heat sterilization was performed by keeping the zein powder in an electro-thermostatic blast oven (DHG-9053A, Shanghai Jing Hong Laboratory Instrument Co., Ltd., China) at 160 °C for 3 h. Moist-heat sterilization was performed by keeping the zein powder in an autoclave sterilizer (HVE-50, Hirayama, Japan) at 121 °C for 20 min. The zein powders were monitored by SDS-PAGE before and after sterilization.
《2.3. Cell culture and proliferation》
2.3. Cell culture and proliferation
L929 cells (Type Culture Collection of the Chinese Academy of Sciences, China) were used in cell studies and cultured in GIBCO® Dulbecco’s Modified Eagle Medium (DMEM) (Life Technologies Co., USA) supplemented with 10% newborn calf serum (Shanghai Yuanmu Biological Technology Co., Ltd., China) and antibiotics (100 U·mL–1 penicillin and 100 μg·mL–1 streptomycin) (Sinopharm Chemical Reagent Co., Ltd., China) in a 37 °C 5% CO2 incubator (NU-4750E, NuAire, Ltd., USA).
Zein film slides were made by coating 10 μL of zein 1 glacial acetic acid solution at a concentration of 0.1 g·mL–1 on a glass slide of 8 mm in diameter. Before cell seeding, all slides were sterilized at 160 °C for 3 h, and then immersed in culture medium for 2 h in 48-well plates. L929 cells were seeded at a density of 2 × 104 mL–1 on both glass slides and zein film slides, and 500 μL of the cell suspension was added to each test well, while 500 μL of the culture medium was added to each background well. The plates were incubated for 6 d.
《2.4.MTT and CCK-8 assays》
2.4.MTT and CCK-8 assays
The proliferation of L929 cells on glass slides and zein film slides was assessed using an MTT assay and a CCK-8 assay. Before assessment, the solution in each test or background well was replaced with 500 μL of culture medium. Then 50 μL of MTT (AMRESCO, USA) at a concentration of 5 mg·mL–1, or a CCK-8 kit (YEASEN, China), was added to each well, and the plates were incubated for another 4 h. After incubation, 100 μL of the solution per well was transferred to a 96-well plate for the CCK-8 assay, while for the MTT assay, the solution was replaced with 300 μL of dimethyl sulfoxide (DMSO) and transferred to a 96-well plate at 150 μL per well, after shaking for 10 min. The OD of the plate was measured at 450 nm (n = 6) using a microplate photometer (Multiskan FC, Thermo Scientific, USA) for the CCK-8 assay, and at 490 nm (n = 6) for the MTT assay. After measurement, the wells for the CCK-8 assay were washed carefully with phosphatebuffered saline (PBS) solution; 500 μL of culture medium was then added per well to continue incubating. The assays were assessed at the same time every day for 6 d.
To investigate the background in the MTT assay, zein film slides and glass slides were immersed in culture medium or PBS for 12 h. After being incubated with or without MTT for another 4 h, the solution was replaced with 300 μL of DMSO and transferred to a 96-well plate at 150 μL per well, after shaking for 10 min. Next, the OD of the plate was measured at 490 nm (n = 6).
《2.5. Statistical analysis》
2.5. Statistical analysis
Where applicable, all data were expressed as means ± standard deviation (n = 6). The significance of the differences between data was assessed by one-way analysis of variance (ANOVA). Statistical significance was set at P < 0.05.
《3.Results and discussion》
3.Results and discussion
《3.1. Characterization of four sources of zein》
3.1. Characterization of four sources of zein
In appearance, both zein 1 and zein 2 are white, while zein 3 and zein 4 are yellow. The morphologies of the zein powders from the four sources could easily be observed due to the autofluorescence of zein protein, as shown in Fig. 1(a–d). As this figure shows, the powders of both zein 1 and zein 2 are pebble-like, while the powders of zein 3 and zein 4 are clastic. Regarding the size of the powders, the subsize powder of zein 3 appears to be much more prevalent than in zein 1 or zein 2, and much smaller in size. The SEM images shown in Fig. 1(e–h) show that both zein 1 and zein 2 have smooth surfaces, while both zein 3 and zein 4 are porous.
《Fig. 1》

Fig. 1. Images of zein powders. Fluorescence microscope images of (a) zein 1, zein 2, (c) zein 3, and (d) zein 4; SEM images of (e) zein 1, (f) zein 2, (g) zein 3, and (h) zein 4. The scale bar on each image represents 200 μm.
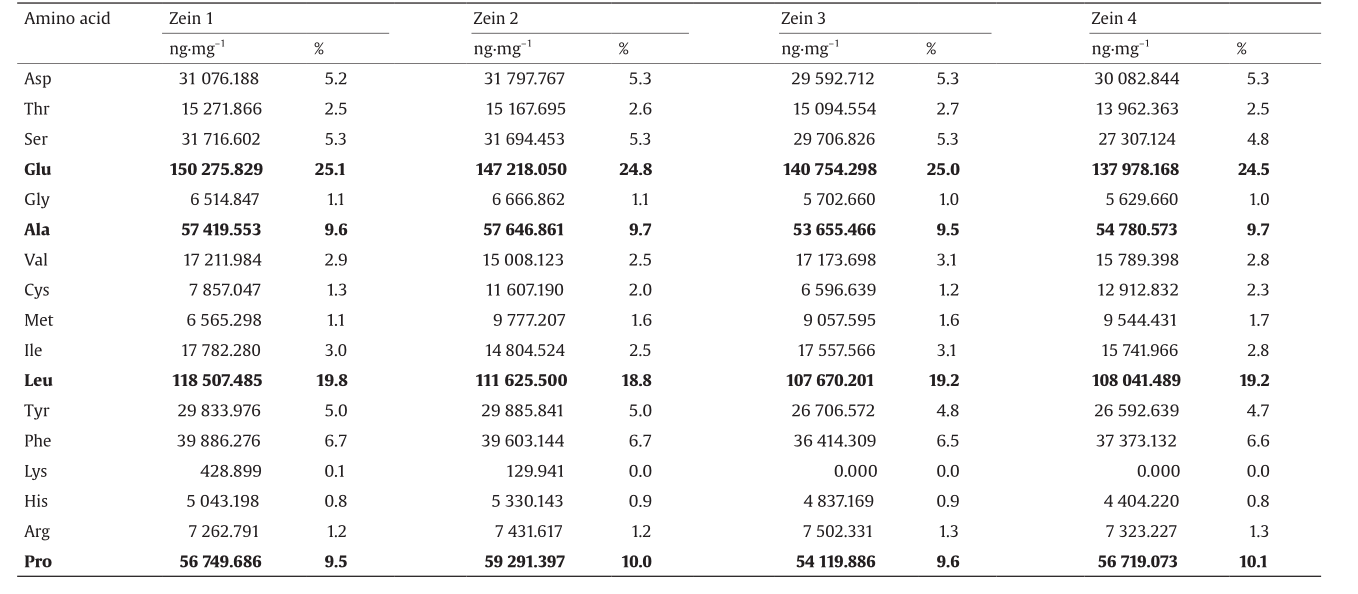
We then investigated the amino acid composition of the four sources of zein, as shown in Table 1. Fig. 2 shows the liquid chromatogram of zein 1 as a typical chromatogram of zein. Table 1 shows that even though the zein from the four sources differs somewhat in morphology, there is no significant difference in amino acid composition. Since the major amino acid composition of zein is glutamic acid (24.8% ± 0.3%), leucine (19.2% ± 0.4%), proline (9.8% ± 0.3%), and alanine (9.6% ± 0.1%), which are all hydrophobic amino acids, it is reasonable for zein to be a hydrophobic protein.
《Table 1》
Table 1 Amino acid composition of four zein sources.
《Fig. 2》

Fig. 2. Chromatogram of zein 1.
Fig. 3 shows the electrophoretograms of the four sources of zein. These show two major bands at 25 kDa and 22 kDa in all four sources of zein, with no significant differences.
《Fig. 3》

Fig. 3. SDS-PAGE images of zein from different suppliers. (a) The marker; (b) zein 1; zein 2; (d) zein 3; (e) zein 4.
《3.2. SDS-PAGE before and after sterilization》
3.2. SDS-PAGE before and after sterilization
Fig. 4 shows the electrophoretograms of the zein before and after thermal sterilization, and Table 2 provides the intensity analysis for Fig. 4. No new bands appeared after sterilization, which indicates that polymerization or breakage did not occur on peptide chains of the zein when suffering dry-heat sterilization and moist-heat sterilization. The results suggest that both sterilization methods are acceptable. Table 2 shows that the proportion of band 1, the larger molecular weight (25 kDa), is lower than the proportion of band 2, the smaller molecular weight (22 kDa).
Fig. 5 shows the electrophoretograms of the zein before and after γ-ray sterilization, and Table 3 provides the intensity analysis for Fig. 5. No new bands were produced, although the relative percentage of the two bands was slightly different in the 30 kGy dosage. Thus, gamma radiation of 25 kGy, which is the recommended dosage, is suitable for the sterilization of zein scaffolds [18].
《Table 2》
Table 2 Intensity analysis for Fig. 4.

《Fig. 4》

Fig. 4. SDS-PAGE images using different sterilization methods. (a) The marker; (b) zein 2 before sterilization; (c) zein 2 treated by dry-heat sterilization; (d) zein 2 treated by moist-heat sterilization.
《Fig. 5》

Fig. 5. SDS-PAGE images before and after γ-ray sterilization. (a) The marker; (b) zein 2; (c) zein 3; (d) zein 4; (e) the porous zein (zein 2) scaffolds sterilized with 20 kGy; (f) the porous zein (zein 2) scaffolds sterilized with 25 kGy; (g) the porous zein (zein 2) scaffolds sterilized with 30 kGy.
《Table 3》
Table 3 Intensity analysis for Fig. 5.

《3.3. Cell proliferation》
3.3. Cell proliferation
Fig. 6 shows the L929 cell proliferation on glass and zein film. We found that the curves obtained from the MTT assay and CCK-8 assay were slightly different. The MTT assay showed that L929 cells proliferated better on zein film than on glass from the second day after seeding, while the CCK-8 assay showed that L929 cells proliferated better on zein film than on glass on the third day after seeding and reached the peak one day earlier than on glass, although both assay methods proved that zein has a better biocompatibility. We noted that the background difference between glass and zein film was more highly significant in the MTT assay than in the CCK-8 assay, which means that the result of the CCK-8 assay was more credible.
To investigate the reason for the high background of the zein film in the MTT assay, we immersed glass slides and zein film slides either in PBS or in culture medium and performed the MTT assay. Table 4 shows the result. The background OD when immersed in culture medium was higher than that in PBS for both glass slides and zein film slides. Moreover, the background OD on the zein film slides that were immersed in either solution and incubated with MTT was much higher. For example, the background OD on the glass slides incubated with MTT was 0.045 ± 0.001 in PBS and 0.063 ± 0.002 in culture medium, while the background OD on zein film slides incubated with MTT was 0.142 ± 0.006 in PBS and 0.246 ± 0.014 in culture medium. We speculate that some components may exist in both the culture medium and the zein film, which affect the reduction reaction from MTT to formazan. In addition, zein film can absorb a considerable amount of culture medium, which is in accord with the conclusion of our previous study: that the zein film has a good swelling property in solution [19]. One such component might be cysteine, because it exists in both zein and culture medium, and has a strong reducing ability among all kinds of amino acids. It has been reported that N-acetylcysteine has the ability to reduce the MTT tetrazolium ring [13]. At the same time, we found that there was no background interference on collagen, a typical protein that contains no cysteine, in the MTT assay [20,21]—a result that supports our speculation.
《Fig. 6》
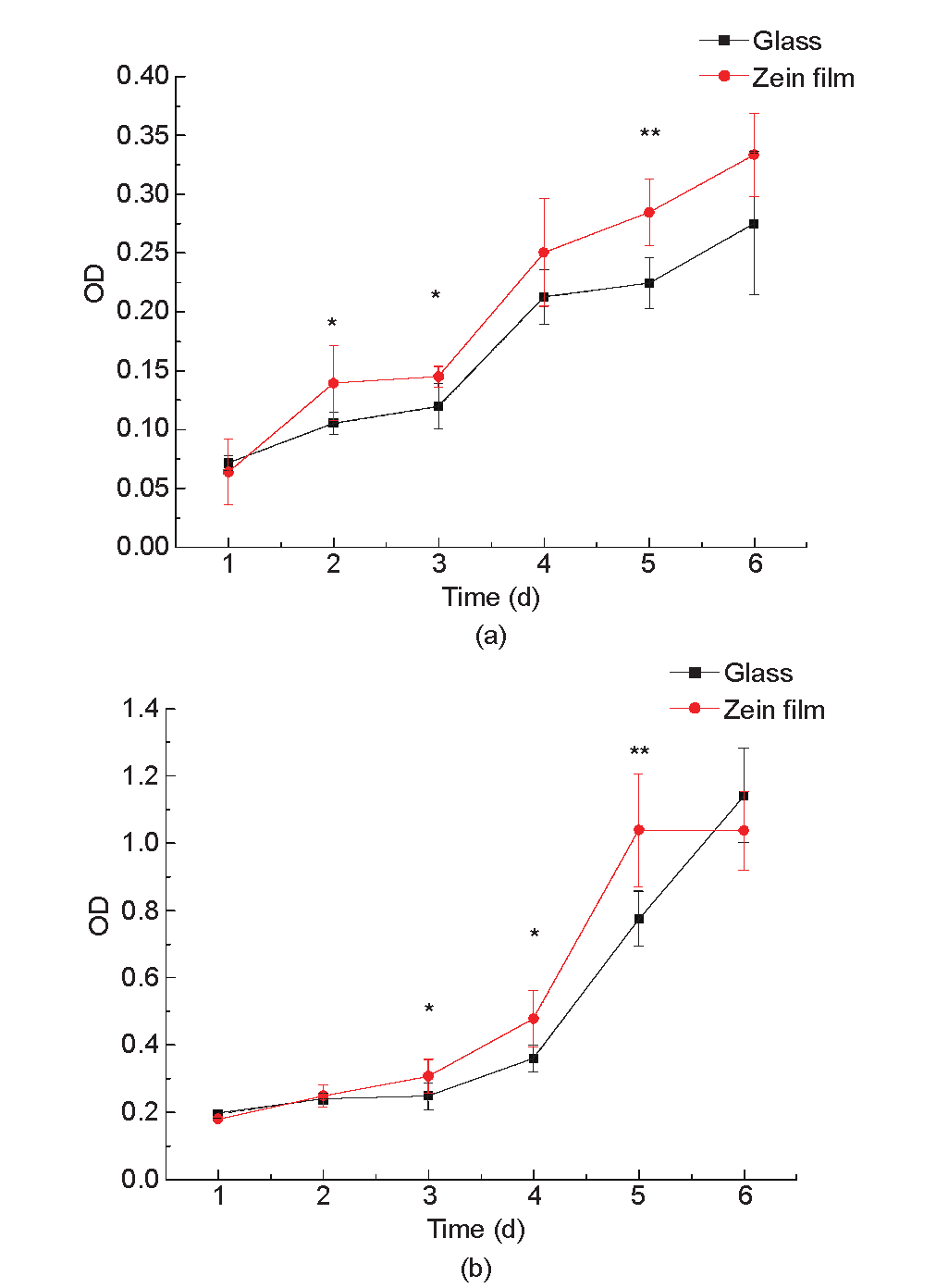
Fig. 6. Proliferation of L929 cells on glass and zein film. (a) The proliferation curve obtained from the MTT assay; (b) the proliferation curve obtained from the CCK8 assay. * indicates a significant difference (one-way ANOVA, P < 0.05, n = 6);** indicates a highly significant difference (one-way ANONA, P < 0.01, n = 6).
《Table 4》
Table 4 OD of backgrounds on glass and zein film.

《4. Conclusions》
4. Conclusions
We established an effective, biochemical method to evaluate the purity of zein from different sources, namely SDS-PAGE. We also investigated the influence of different sterilization methods on zein, and compared the MTT assay with the CCK-8 assay for the assessment of cell compatibility for this promising biomaterial. The four sources of zein that we used appeared to have no significant differences in amino acid and peptide chain composition. Neither dry-heat sterilization nor moist-heat sterilization procedures significantly affected the peptide chains of the zein. γ-ray sterilization at 25 kGy is a suitable dosage for the procedure of porous zein scaffold sterilization. When using an MTT assay, the background of zein itself affects the results of cell proliferation, while a CCK-8 assay is much more suitable to assess cell proliferation on zein films or scaffolds. From a CCK-8 assay, we obtained the conclusion that cell proliferation was better on zein film than on glass, as was previously established.
《Acknowledgements》
Acknowledgements
The authors are grateful to the financial support provided by the International S&T Cooperation Program of China (2014DFG02330 and 2015DFG32730). We also thank the Shanghai Municipal Science and Technology Commission (13JC1403400 and 15540723900) and the Medical Engineering Cooperation Program of Shanghai Jiao Tong University (YG2013MS77 and YG2014ZD03).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Yue Zhang, Wei-Ying Li, Run Lan, and Jin-Ye Wang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




