《1.Introduction》
1.Introduction
Rheumatoid arthritis (RA) is a common systemic autoimmune disease that often leads to progressive disability, long-term complications, increased mortality, and social-economic pressures [1]. RA affects 0.5%–1% of the global population [2]. Although recent advances have been made in understanding RA pathogenesis, the etiology of RA remains unknown. Both environmental and genetic factors are involved in the development of RA disease. The effects of the gut microbiota on the pathogenesis and development of RA have been an active area of research in recent years. Previous studies have shown that the gut and oral microbiomes of RA patients were different from those of healthy individuals, but that these differences were partially resolved after RA treatment [3]. The potential role of the intestinal microbiota in modulating the immune system aroused an interest in using probiotic bacteria as a preventive or supplemental therapeutic intervention.
At the present time, Bifidobacterium and Lactobacillus genera (e.g.,B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei) are widely used as probiotics. The World Health Organization (WHO) has stated that probiotics, as live microorganisms, are beneficial to host health if taken in certain amounts [4]. Probiotics can restore the microbial balance of the gut in various ways including modulation of gut immunity and competition with other gut organisms for nutrients [5]. There is increasing interest in the modulation of both human and animal immune systems by means of probiotics [6,7]. A systematic review and meta-analysis was recently done on inflammatory bowel disease; the results showed that, out of nine cohort studies, eight case studies, and one randomized controlled trial (RCT), more than 45% (54/119) achieved clinical remission after probiotic intervention [8]. Several studies were also conducted on the effects of probiotics in RA. Animal research showed that Lactobacillus significantly decreased inflammatory reactions during carrageenan-induced arthritis by down-regulating the pro-inflammatory cytokine pathway [9,10]. However, results from clinical trials on the effects of probiotics during RA treatment have not been consistent. In this paper, we perform a systematic review of RCTs that compare the effects of treating RA with or without probiotics, in an attempt to summarize existing evidence on the relationship between probiotics and RA.
《2.Methods》
2.Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines on reporting in this systematic review in order to ensure its accuracy.
《2.1. Data sources and searches》
2.1. Data sources and searches
First, we screened papers that met the inclusion criteria by going through the abstracts acquired using our search strategy. The MEDLINE (EBSCOhost), PubMed, Cochrane Library, Scopus, China National Knowledge Infrastructure (CNKI), and Wanfang databases were searched for articles published between 1995 and 2016. The following terms were used to identify relevant publications: “probiotics,” “Lactobacillus,” “Bifidobacterium,” “gut microbiota,” “microflora,” and “intestinal microbiota,” in combination with “rheumatoid arthritis (RA).” The full text of potentially relevant articles identified within these databases was then searched further.
《2.2. Inclusion criteria》
2.2. Inclusion criteria
The title and abstract of each relevant paper were examined, and then the full paper was read, if necessary. The inclusion criteria were: ① that each study focused on humans in RCTs; ② that the included participants were patients with a clear diagnosis of RA and stable treatment of disease-modifying anti-rheumatic drugs (DMARDs); ③ that the participants received additional treatment of probiotic supplementation, or else received a placebo or no additional treatment for at least eight weeks; and ④ that no restrictions were placed on sex, age, severity, or duration of RA. The diagnostic criteria for RA in the trials met the American Rheumatism Association 1987 revised criteria for the classification of RA [11]. Each article’s eligibility was determined through agreement with two reviewers.
《2.3. Outcome assessment》
2.3. Outcome assessment
The outcomes measured included clinical and biochemical assessments. The clinical data consisted of American College of Rheumatology 20% improvement criteria (ACR20) score, disease activity score in 28 joints (DAS28, referring to the number of tender and swollen joints on the basis of a 28-joint count), and health assessment questionnaire (HAQ) measurements. The biochemical data comprised measurements of the C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and tumor necrosis factor (TNF)-α, interleukine (IL)-6, IL-10, and IL-1β levels.
《2.4. Data extraction》
2.4. Data extraction
For each of the selected papers that reported sufficient followup data, details were extracted from the relevant parts of the studies regarding study design, sample size, population characteristics, probiotic source and dose, and outcomes of interest. Data were extracted from a study database according to the PRISMA 2009 statement [12]. Mean changes in the outcomes were extracted from tables, text, or graphs. Corresponding standard deviations (SDs) were extracted directly or calculated indirectly based on the following data (if available): reported standard errors with sample size, 95% confidence interval (CI), or P values.
Three authors participated in extracting data from the studies in the review. Two authors (Hudan Pan and Runze Li) extracted the relevant data from the original papers. Any disagreements were then resolved by consulting the third reviewer (Liang Liu).
《2.5. Quality assessment of the included studies》
2.5. Quality assessment of the included studies
Study quality was assessed using the Cochrane Handbook for Systematic Reviews of Interventions and the Jadad scale [13]. The assessment details were based on: ① how the random sequence was generated, ② whether allocation concealment was achieved,③ what blinding methods were adopted, ④ whether the outcomes were completed, and ⑤ whether researchers selected the reporting.
《2.6. Statistical analysis》
2.6. Statistical analysis
All statistical analyses were performed according to Review Manager (RevMan) 5.3.3 software. The difference between the change in baseline valued for the intervention and the controls was derived for each trial. Mean differences (MDs) were pooled using a fixed effects model when the study heterogeneity was not significantly different; otherwise, a random effects model was chosen. Statistical tests were two-sided (P < 0.1). Heterogeneity was tested and measured using a Q test and I2 statistics, respectively. In general, we regarded an I2 value of I2 > 50% or a P value of P < 0.1 as indicative of heterogeneity.
《3.Results》
3.Results
《3.1. Description of studies》
3.1. Description of studies
A flow diagram of this meta-analysis is shown in Fig. 1. A total of 249 articles were retrieved from a web search. After removing duplicates, we examined the titles and abstracts and read the full text if necessary. Based on the inclusion criteria, a total of six studies were selected for systematic review [14‒19]. The characteristics of the studies are summarized in Table 1 [14‒19]. All six studies were RCTs and were published between 2003 and 2016, with follow-up periods ranging from 8 weeks to 48 weeks. A total of 246 participants were included in these studies, of which 119 were in the intervention group with probiotic supplementation and 127 were in the control group without probiotic supplementation. Probiotic species and doses varied among the different studies, with five studies using capsules [14‒17,19] and one study using a caplet [18]. Most of the studies included in the systematic review were of a high quality, although the sample size of each individual study was small, resulting in varying conclusions regarding the probiotic effects.
《Fig. 1》
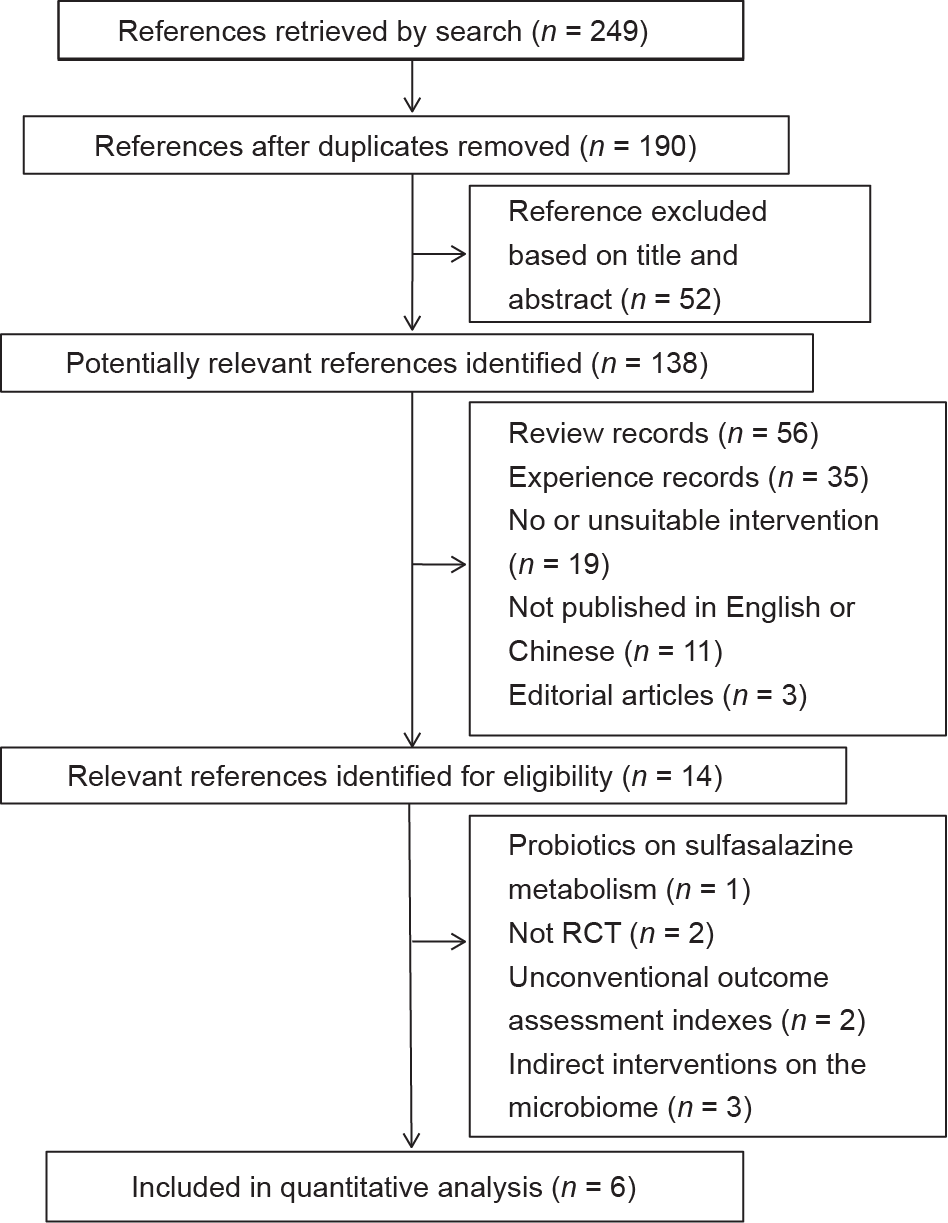
Fig. 1. Flow diagram of the selection of published articles included in the metaanalysis.
《Table 1》
Table 1 Characteristics of the studies included in the meta-analysis.

EG: experimental group; CG: control group; CFU: colony-forming unit; b.i.d.: twice a day; q.d.: once a day.
《3.2. Quality assessments》
3.2. Quality assessments
Quality assessments and risk bias measurements of the studies are shown in Fig. 2. The mean methodological quality of the included studies was 5 on the Jadad scale. All of the selected articles used a double-blind design [14‒19]. More than 66% (4/6) of the total studies had incomplete outcome data. In total, 33 patients dropped out of the studies because they had not followed the protocols. No adverse outcomes were found in these studies.
《Fig. 2》
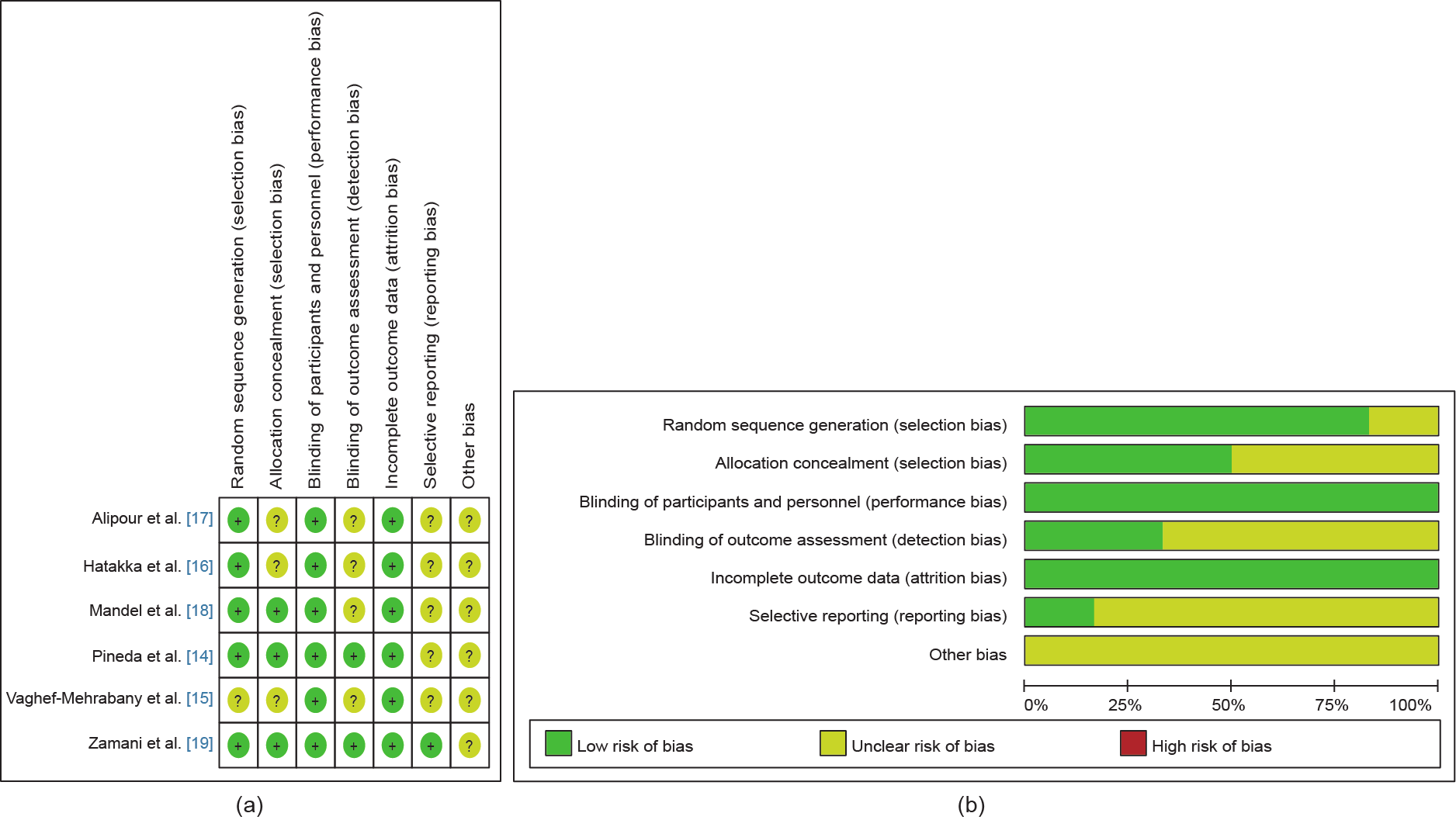
Fig. 2. (a) Risk of bias summary of the studies included in the meta-analysis, with a low risk of bias (+), high risk of bias (–), or unclear risk of bias (?) indicated; (b) bar chart comparing the percent risk of bias for each RCT included. Both parts of this figure show that the risk of bias was quite low.
《3.3. Clinical index evaluation of the probiotic effects》
3.3. Clinical index evaluation of the probiotic effects
We collected DAS28 and ACR20 data to assess the effects of probiotics on RA patients. DAS28 data were analyzed in random effects models. The data indicated that probiotics have little effect on down-regulating DAS28 (MD: –0.11, 95% CI: –0.47 to –0.24, P = 0.54; see Fig. 3) due to the limited sample size. Only two studies comprising 73 patients (37 in an intervention group and 36 in a control group) provided ACR20 count data. Eleven participants met the criteria for ACR20 after the probiotics treatments, whereas seven participants achieved ACR20 in the placebo group (Fig. 3). We also evaluated the HAQ data and the number of 28 tender/ swollen joints. However, no significant differences were found between the two groups (see Figs. S1‒S4 in Supplementary Information).
《Fig. 3》
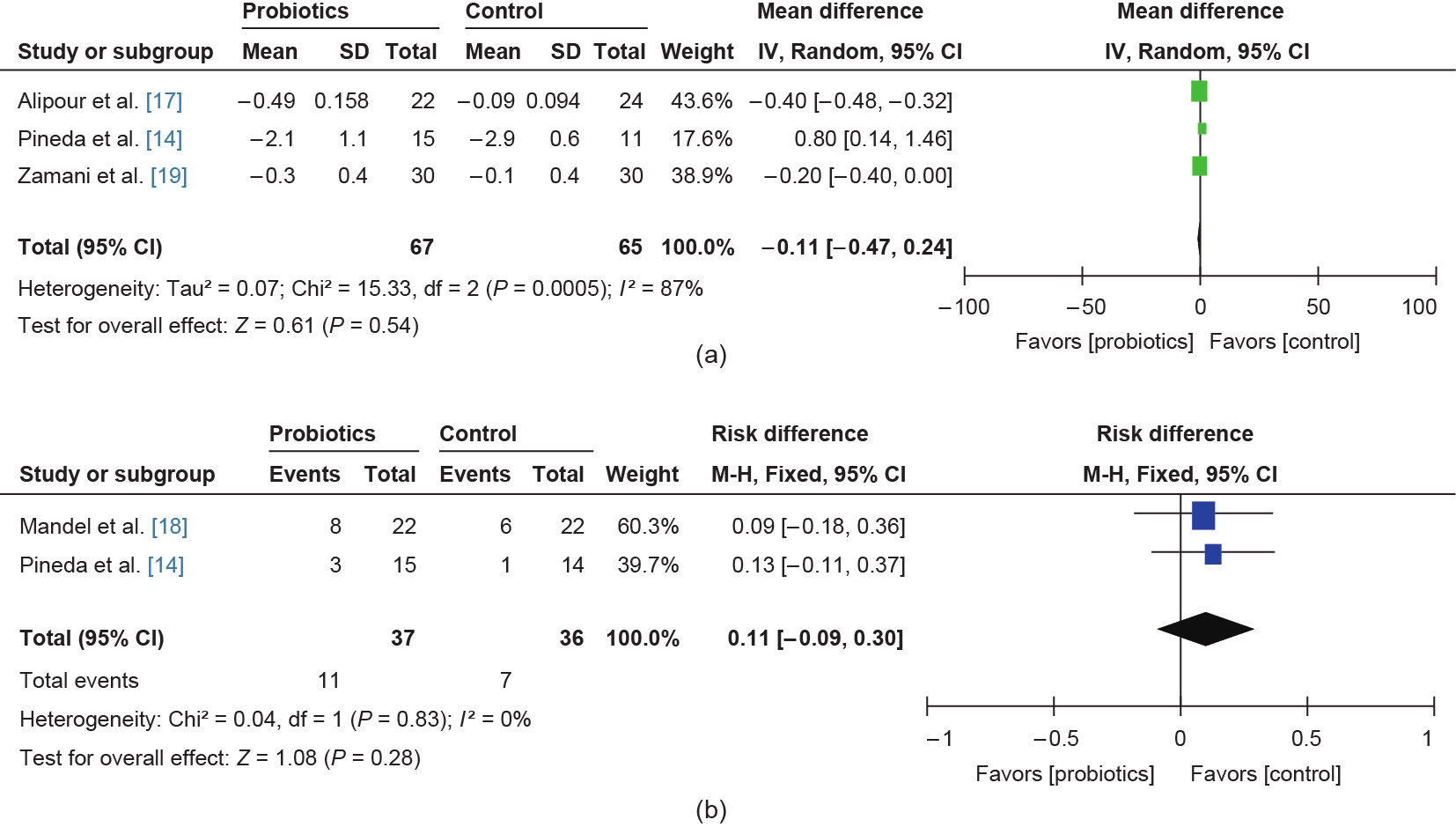
Fig. 3. Effects of probiotics on (a) DAS28 and (b) ACR20. IV: inverse variance; Random: random effects model; M-H: Mantel-Haenszel; Fixed: fixed effects model; df: degrees of freedom.
Four studies presented CRP (mg·L–1) data. A comparison of 153 participants (75 in the probiotic intervention groups and 78 in the control groups) showed a reduction of CRP expression in the intervention groups (Fig. 4). The heterogeneity test yielded a value of I2 = 75%, which was statistically significant (P = 0.007). Therefore, a random effects model was adopted for this meta-analysis.
《Fig. 4》

Fig. 4. Effects of probiotics on CRP expression.
《3.4. Effects of probiotics on inflammatory cytokine expression》
3.4. Effects of probiotics on inflammatory cytokine expression
Three studies, which used treatments of Lactobacillus, used serum TNF-α, IL-6, IL-1β, and IL-10 levels to evaluate the effect of the probiotics on inflammation (Fig. 5). This meta-analysis included 118 participants: 59 in a probiotic intervention group and 59 in a control group. The meta-analysis revealed that the expression of TNF-α and IL-1β decreased significantly in the probiotic intervention group (TNF-α: MD: –1.35, 95% CI: –1.99 to –0.71, P < 0.0001; IL-1β: MD: –6.13, 95% CI: –11.41 to –0.86, P = 0.02), while the expression of serum IL-10 significantly increased (IL-10: MD: 3.8, 95% CI: 0.4 to 7.19, P = 0.03).
《Fig. 5》

Fig. 5. Effects of probiotics on serums (a) TNF-α, (b) IL-6, (c) IL-1β, and (d) IL-10.
《4.Discussion》
4.Discussion
The condition of the gut microbiome is a crucial factor in modulating the host’s immune system responses; links have been identified between microbial dysbiosis and several autoimmune diseases such as RA [3,20], inflammatory bowel disease [8], multiple sclerosis [21], and ankylosing spondylitis [22]. The way in which commensal microbes calibrate innate and adaptive immune responses is still incompletely defined. Multiple mechanisms have been suggested, including the effects of microbial metabolites [23], pathogens [24] or certain microorganisms [25], bile acids stimulation [26], and epithelial barrier destructions [27,28]. To a great extent, microbes produce metabolites that mediate host-microbial interactions. Short-chain fatty acids (SCFAs) [29], the end products of the fermentation of dietary fibers by the anaerobic intestinal microbiota, are a classic example of how bacterial-derived molecules contribute to gut immune homeostasis. SCFAs modulate the balance of intestinal homeostasis and regulate the development of diseases [30‒32]. In contrast, certain bacteria-like segmented filamentous bacteria (SFB) can have functions in both the innate and adaptive immune systems by inducing T helper 17 cells in the small intestine [33,34].
Gut bacteria have been recognized as an autoimmune trigger for RA for decades. In 1965, Mansson and Colldahl [35] reported that levels of Clostridium perfringens type A were elevated in RA patients. However, this elevation was not specific to RA. Other chronic arthritis diseases, such as osteoarthritis, were also identified as having elevated Clostridium perfringens type A levels [36]. Later, animal studies demonstrated that the distribution of the microbiota in the gut affects the susceptibility, development, and progression of arthritis. Kohashi et al. [37] discovered that germ-free rats developed severe joint inflammation with 100% incidence of an adjuvant-induced arthritis model. Interestingly, in the K/BxN T cell receptor transgenic model, germ-free mice did not develop disease. When mono-colonized with SFB, the mice effectively stimulated an autoimmune response, resulting in arthritis. In addition, differences were found between the microbiome compositions of collagen-induced arthritis (CIA)-susceptible and CIA-resistant mice [38]. The microbiome of the former aggravated the disease condition when transplanted into germ-free mice. Recent advances in sequencing technologies have shown the enormous diversity, functional capacity, and dynamic of the human microbiome. In 2015, Zhang et al. [3] used metagenomic shotgun sequencing technology to analyze fecal, dental, and salivary samples from a large cohort of RA patients as well as from healthy controls. They detected dysbiosis in the gut and oral microbiomes of RA patients that partially resolved after RA treatment. Levels of Haemophilus spp. decreased in RA patients, whereas levels of Lactobacillus salivarius increased [3]. Symbiotic relationships between intestinal microorganisms have also been reported to be altered in RA patients [20]. The levels of strains such as Bifidobacterium, Bacteroides, and Lactopositive colibacteria were reduced, while levels of opportunistic Enterobacterium and Staphylococcus were elevated. The investigators showed that the altered gut microbiome could be used as a biomarker to identify RA patients. Thus, isolation from human gut microbial species may have therapeutic potential for inflammatory disorders.
Probiotics are living organisms that, if taken in adequate amounts, can confer a health benefit to the host [39]. Probiotics mainly exert their beneficial effects through the following three methods: antimicrobial effects, enhancement of mucosal barrier integrity, and immune modulation [40]. Several experimental and clinical studies have shown that some probiotics, particularly Lactobacillus and Bifidobacterium strains, have beneficial effects on inflammatory and autoimmune diseases. A study on the lifespan of the scurfy (SF) mouse, in which Foxp3+ Treg cells are deficient, showed that the gut microbiome was dysbiotic, and that remodeling the microbiota with Lactobacillus reuteri prolonged survival and reduced multi-organ inflammation [41]. VSL#3 probiotics were also detected to significantly down-modulate the response of Toll-like receptor (TLR)2-, TLR3-, TLR4-, and TLR9-expressing HEK293 cells, which were stimulated with Pam3CSK4, polyinosinic-polycytidylic acid, lipopolysaccharide (LPS), and ODN2006, respectively [42].
In this meta-analysis, inclusion studies used Lactobacillus or Bifidobacterium species to treat RA. The aim of this meta-analysis was to evaluate the beneficial effects of probiotic supplementation therapy for treating RA. Although several studies focused on the effect of modulating gut microorganisms in RA patients, only six studies were conducted in RCTs with specific probiotic species. Due to the limitation of the small sample sizes, the ACR20 and DAS28, which are widely used to measure changes in RA symptoms, showed no significant improvement in the group receiving probiotic supplements when compared with the control group. However, the CRP levels in plasma, which rise in response to inflammation, decreased significantly in the intervention group.
Recently, as part of the Human Functional Genomics Project (HFGP), researchers found that microbiome-host interactions could modulate inflammatory cytokine production capacity; the cytokine responses were associated with microbial taxonomic and functional features [43]. In this study, we analyzed the inflammatory cytokines expression diversity of the two groups. The results showed that the expression of TNF-α, IL-1β, and IL-6 was reduced and the expression of IL-10 was elevated in the probiotic intervention group. Oral administration of L. casei or L. acidophilus in CIA rats showed significantly down-regulated pro-inflammatory cytokines and up-regulated anti-inflammatory cytokines (P < 0.0001) [10], which were consistent with our results.
In summary, improvements in probiotic interventions have not been evident enough to demonstrate the effectiveness of treating RA patients, due to the limited studies. However, the trends of probiotics reducing inflammatory reactions imply that probiotics may have some effect on the remission of early and mild RA patients. Better-designed research needs to be conducted in order to identify the best species that relieve RA, and optimize the intake method and doses of the probiotics.
This meta-analysis has several limitations. First, we could only obtain data from published literature and from material published in English, which may have led to potential publication bias. Second, the inclusion studies had small sample sizes for each investigation group, even with different phenotypes of probiotics. Third, higher-quality research papers with multi-centered, large-sample designs are urgently required.
《5.Conclusions》
5.Conclusions
This meta-analysis supports the potential role of probiotics in relieving inflammation in RA patients; however, further evidence supported by larger samples and more rigorous RCTs is greatly needed in order to evaluate whether probiotics can significantly relieve the disease progression of RA.
《Acknowledgements》
Acknowledgements
This work was supported by the Macau Technology Development Fund (077/2014/A2).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Hudan Pan, Runze Li, Ting Li, Jun Wang, and Liang Liu declare that they have no conflict of interest or financial conflicts to disclose.
Supplementary Information
http://engineering.org.cn/EN/10.1016/J.ENG.2017.01.006
Figs. S1–S4
Refs. [1–3]














 京公网安备 11010502051620号
京公网安备 11010502051620号




