《1.Introduction》
1.Introduction
The recycling of carbon dioxide (CO2) into fuel is an attractive solution to mitigate CO2 emissions and address the growing global energy demand. However, the relative thermodynamic stability of CO2 poses a great challenge for its effective conversion without high energy consumption. Such energy consumption is often provided in the form of fossil fuel-derived heat. The need for a green and sustainable energy source is the primary motivation behind the potential utilization of sunlight for heat provision to achieve renewable catalytic recycling of CO2.
Solar heating primarily relies on increased atomic vibrational energy due to solar radiation absorption. Through such a route, high temperatures are achievable via the concentration of sunlight. Localized surface plasmon resonance (LSPR) also occurs to facilitate photothermal catalysis, which has been gaining increased attention for catalytic applications [1–5]. The resonance of oscillating free electrons on the surface of a conducting material upon interaction with impending electromagnetic wave(s) of specific wavelength(s) can result in the dissipation of intense heat energy within the vicinity of the plasmonic particle. If a plasmonic material (e.g., metal) is coupled with catalytic capabilities, the localized hotspots can also assist with the activation of a reactant molecule. The work of Wang et al. [6], which involved a zinc oxide-supported gold catalyst, shows that this application is possible for CO2 reduction. Although they do not explicitly discuss the photothermal heating aspects, Meng et al. [7] also successfully demonstrated a similar light-for-heat concept for CO2 hydrogenation, in which a catalyst bed temperature of approximately 400 °C was achieved and measured under light illumination. Another interesting finding of the work was that a reducible oxide (titania)-supported metal demonstrated enhanced CO2 conversion as compared with the insulator (aluminum oxide)-supported equivalent.
The use of reducible oxides coupled with metal catalysts to achieve activity enhancement is not unusual. In addition to the benchmark copper/zinc oxide catalyst, reducible oxides such as titania (TiO2) and ceria (CeO2) have been commonly studied for CO2 catalysis [8–10], which is attributed to the formation of an active metal oxide interface upon the utilization of such oxides. For CO2 reduction reactions, Graciani et al. [11] and Yang et al. [12] demonstrated the role of an active interface in improving CO2-to-methanol conversion for ceria-titania binary oxides coupled with copper and gold metals, respectively. The combination of cerium and titanium oxides has been shown to lead to the stabilization of reduced ceria (CeOx, x < 2) species [13–15]. These reduced species have been reported to aid in the dispersion of deposited surface metal [16,17] and surface charge redistribution within metal nanoparticles near the metal oxide interface [12], which subsequently facilitate CO2 adsorption and activation.
In our preceding study [18], the unique redox capabilities of ceriumtitanium oxide particles were utilized for the (low-temperature) steam reforming of methane. The progression in material properties across varied compositions (and synthesis pathways) was studied to examine the effect on catalytic reforming. Here, as an extension to our prior investigation, we examine ceria-titania-supported nickel (Ni) catalyst particles for CO2 methanation. In contrast to the steam-reforming work [18], and to most CO2 conversion studies in which energy is supplied in the form of heat, methanation was carried out in a photothermal reaction system in which fullspectrum irradiation was utilized to drive CO2 reduction reaction. In the earlier work, the ceria-titania-supported nickel particles were characterized according to properties that were important for defining thermal activity and catalyst selectivity (i.e., specific surface area, catalyst morphology, support crystallinity, nickel deposit size, support, and nickel reducibility). Because the current study utilizes light within the system, we assessed the optical properties of the materials via ultraviolet-visible-near-infrared (UV-Vis-NIR) spectroscopy. In addition, as the reaction being examined in this study is CO2 methanation (as opposed to steam reforming in the prior study), we used analytical techniques that were more relevant to the process (e.g., CO2 and hydrogen gas (H2) temperature-programmed desorption (TPD)) and that provided greater insight into the origins of the catalyst performance (i.e., X-ray photoelectron spectroscopy (XPS) and nickel deposit size).
《2.Experimental methods》
2.Experimental methods
《2.1. Catalyst preparation》
2.1. Catalyst preparation
2.1.1. Ceria-titania mixed-oxide synthesis
The ceria-titania mixed oxides (xCe-yTi) were prepared via a solgel route as previously described in Ref. [18]. In brief, the cerium precursor solution was prepared by dissolving the required amount of hydrated cerium nitrated salt (Aldrich) in 20 mL of ethanol, which was then acidified with 0.36 mL of concentrated nitric acid (Ajax). This solution was added dropwise to a solution containing 10 mL of titanium butoxide (Sigma-Aldrich) diluted with 60 mL of ethanol under continuous magnetic stirring at 300 r·min−1. After aging the solution for 48 h, calcination was carried out in a muffle furnace at 550 °C for 3 h, with the ramp rate set to 1 °C·min−1. The product was ground into a fine powder. These neat supports are denoted as xCeyTi_SG, where x and y indicate the relative mole ratios of cerium and titanium, respectively, and SG signifies the sol-gel synthesis method. Mixed oxides with nominal x:y ratios of 20:80, 50:50, and 80:20 were studied for this work. Pure titania (TiO2_SG) and ceria (CeO2_ SG) were also prepared according to the procedure described above.
2.1.2. Impregnation of nickel onto the xCe-yTi supports
To produce a catalyst with a nominal nickel loading of 10 wt%, hydrated nickel nitrate salt (Univar) was dissolved into 5 mL of ultrapure water (Millipore, Milli-Q 18.2 MΩ·cm) within which 1 g of the neat xCe-yTi_SG support was suspended. Continuous magnetic stirring was applied over a hot plate to evaporate excess moisture. The resulting slurry was oven-dried at 110 °C overnight. The dried particles were calcined at 400 °C (5 °C·min−1 ramp rate, held for 3 h) under a 60 mL·min-1 air flow.
To activate the catalyst, the calcined NiO/xCe-yTi_SG samples were reduced under 50 mL·min–1 10 vol% H2 /argon (H2 /Ar) flow at400 °C (5 °C·min-1 ramp rate, held for 1 h) such that Ni/xCe-yTi_SG was obtained.
《2.2. Catalyst characterization》
2.2. Catalyst characterization
UV-Vis-NIR absorption spectra were acquired using a UV-Vis-NIR spectrophotometer (Shimadzu, UV-3600). Transmission electron microscopy (TEM) images were acquired using a TEM microscope (Philips, CM200 FEG) operated at 200 kV. The samples were ultrasonically dispersed in methanol and dripped onto a carbon-coated copper grid for imaging. TPD experiments were performed using a chemisorption analyzer (Micromeritics, AutoChem 2910). For these experiments, the samples (50 mg) were first reduced in situ in 10 vol% H2/Ar flow (50 mL·min−1) at 400 °C for 1 h (5 °C·min−1) before being cooled to room temperature (under 10 vol% H2/Ar flow). For H2-TPD analyses, the samples were next exposed to the same flow of 10 vol% H2/Ar for 1 h, and then purged with Ar flow (20 mL·min−1) for 30 min before the temperature was raised at a rate of 10 °C·min−1 to 700 °C under continuous Ar flow. For CO2-TPD analyses, after reduction and cooling, the samples were first purged with helium (He) flow (20 mL·min−1) for 30 min, then exposed to CO2 flow(20 mL·min−1) for 1 h, and finally purged again with He flow for 30 min. The profiles were recorded over a temperature ramp rate of 10 °C·min−1 to 700 °C under continuous He flow. Surface elemental analysis and oxidation state evaluation were performed on the reduced catalysts using an X-ray photoelectron spectrometer (Thermo Scientific, ESCALAB 250Xi) with a mono-chromated Al-Kα X-ray source. The X-ray source was operated at 13 kV and 12 mA.
《2.3. Catalyst activity assessment》
2.3. Catalyst activity assessment
The photothermal reaction was carried out in a (glass) batch reactor with 540 cm3 volume (illustrated in Fig. 1). The reduced catalysts (0.1 g) were transferred onto a piece of glass fiber prefilter (Merck Millipore Ltd.) and spread over a circular area with a 3 cm diameter. Prior to the reaction, the reactor system was evacuated before being filled with approximately 15 kPa and 60 kPa of CO2 and H2, respectively (equivalent to 3 mmol of CO2 and 12 mmol of H2). The gas contents were constantly circulated within the reactor using a gas circulating pump. The catalyst film was irradiated from above through a quartz window using a 300 W xenon lamp (Oriel). The contents of the reaction system were periodically sampled and measured with a gas chromatograph (Shimadzu, GC-2010 Plus) equipped with a capillary column (Sigma-Aldrich, Carboxen-1010 PLOT) and a flame ionization detector.
《Fig. 1》

Fig. 1. Configuration of the batch-type photothermal reactor for CO2 methanation. The inset shows the structure within the reactor. GC: gas chromatograph.
《3.Results and discussion》
3.Results and discussion
《3.1. Material characteristics》
3.1. Material characteristics
3.1.1. Textural properties and crystallography
The textural properties and crystallography of the catalysts are discussed in detail in Ref. [18]. In addition to the previously measured specific surface areas of TiO2 _SG (< 3 m2·g−1), 20Ce-80Ti_SG (58 m2·g−1), 50Ce-50Ti_SG (35 m2·g−1), and CeO2 _SG (74 m2·g−1), the specific surface area of 80Ce-20Ti_SG was measured to be 49 m2·g−1. The crystallography of the 80Ce-20Ti_SG sample was found to be similar to that of 50Ce-50Ti_SG, in which a homogeneous cubic ceria lattice hosts the titanium ions (Fig. S1 in Supplementary Information). No detectable incorporation of nickel into the ceria-titania support was identified. This result was expected, because the heat treatment temperatures (calcination and reduction) applied after the impregnation of nickel (at 400 °C) are insufficient to induce the formation of titanate-like phases.
3.1.2. UV-Vis-NIR absorption
UV-Vis-NIR absorption was measured for the neat supports and reduced catalysts, as shown in Fig. 2. The absorptions of both of the single-phase oxides (TiO2_SG in Fig. 2(a) and CeO2_SG in Fig. 2(e)) mainly resided in the ultraviolet region. This result is also reflected by their bandgap energy values of 3.0 eV and 3.2 eV, respectively, which agree with literature-reported values [19,20]. With the exception of the 80Ce-20Ti_SG neat support, which demonstrated limited visible light absorption (bandgap energy 3.0 eV), significant red shifts were observed for the oxides in which both cerium and titanium were present. The absorption range was extended to about 500 nm for both 20Ce-80Ti_SG (Fig. 2(b)) and 50Ce-50Ti_SG (Fig. 2(c)). The corresponding bandgap energies for both these mixed oxides were calculated to be 2.6 eV. All neat supports demonstrated negligible absorption for wavelengths exceeding 600 nm.
《Fig. 2》
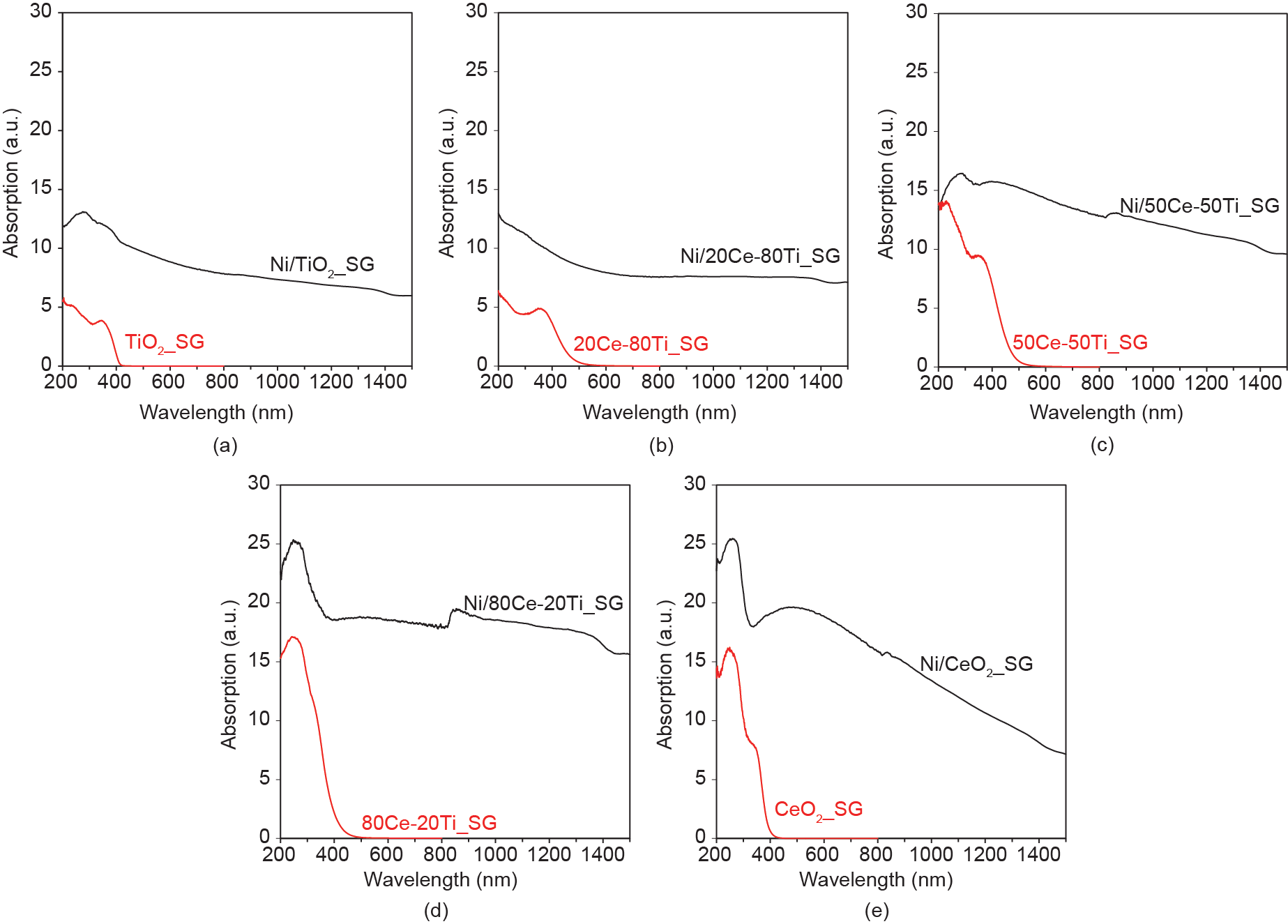
Fig. 2. UV-Vis-NIR absorption spectra (no offset) of nickel catalysts supported on sol-gel-synthesized ceria-titania mixed oxides (black curves): (a) Ni/TiO2_SG, (b) Ni/20Ce-80Ti_SG, (c) Ni/50Ce-50Ti_SG, (d) Ni/80Ce-20Ti_SG, and (e) Ni/CeO2_SG. The spectra for the corresponding neat supports are shown in red.
In the presence of reduced nickel species, the absorption intensity is observed to increase across the UV-Vis-NIR light spectrum for all catalysts, as compared with their neat counterparts (the profiles for the nickel-loaded samples have not been offset). This finding is especially noticeable in the NIR region, and is visually verified in that the reduced, nickel-loaded catalysts have a black-colored appearance in comparison with the white or pale yellow colors of the neat supports. No distinct plasmon absorption peak (which is often observed for gold or silver nanoparticles) is observed for the reduced nickel nanoparticles, aside from the increased Vis-NIR absorption. This result is consistent with other literature reports and is usually attributed to the broader absorption characteristics of metallic nickel [21]. Nevertheless, the broad Vis-NIR absorption that was demonstrated for the nickel-loaded samples is favorable for heat generation to drive catalytic conversion.
3.1.3. Nickel deposit size distribution
Nickel deposit size distributions on the different supports were obtained from 200 counts of nickel deposits for each sample (Fig. 3), measured from TEM micrographs. Ni/TiO2_SG (Fig. 3(a)) and Ni/20Ce-80Ti_SG (Fig. 3(b)) show broad distributions with metal clusters ranging in size from a few nanometers to greater than 60 nm. The dispersion of nickel species is visibly improved for supports with richer cerium content. While the Ni/50Ce-50Ti_SG and Ni/ CeO2_SG catalysts exhibited finer nickel deposit sizes, with the average sizes measured as 11 nm and 13 nm, respectively, Ni/80Ce-20Ti_ SG had the narrowest nickel distribution (< 20 nm) and smallest nickel deposit size, averaging at 6 nm. These results indicate that supports with richer cerium content are beneficial for the dispersion of nickel deposits. This finding is consistent with many literature reports in which the presence of cerium within a support is shown to facilitate higher metal dispersion [22–28].
《Fig. 3》

Fig. 3. Nickel deposit size distributions on sol-gel-synthesized ceria-titania mixed-oxide supports with different compositions: (a) Ni/TiO2_SG, (b) Ni/20Ce-80Ti_SG, (c) Ni/50Ce-50Ti_SG, (d) Ni/80Ce-20Ti_SG, and (e) Ni/CeO2_SG. The corresponding TEM images are shown as inserts. Each size distribution was obtained from 200 nickel deposit counts.
The measured distribution of nickel deposits is also in agreement with the trend demonstrated in the UV-Vis-NIR absorption spectra. Based on the nickel deposit size results, it is expected that the light absorption by nickel on the latter three supports would be stronger than that of the nickel supported by TiO2_SG and 20Ce-80Ti_SG. This is because larger metal particle sizes are known to decrease light absorption efficiency due to greater scattering effects [29]. As expected, the UV-Vis-NIR absorption spectra of the respective catalysts (Fig. 2) showed that Ni/50Ce-50Ti_SG, Ni/80Ce-20Ti_SG, and Ni/CeO2_SG had greater light absorption capacities across all three regions (UV, visible, and NIR).
3.1.4. H2-TPD
Fig. 4 shows the hydrogen desorption behaviors of the (reduced) nickel-loaded oxides. Ni/TiO2_SG demonstrates negligible hydrogen adsorption at room temperature, which is evident from its lack of distinct desorption peaks. Ni/20Ce-80Ti_SG exhibits both a low-temperature (< 350 °C) and high-temperature (350–600 °C) desorption region, whereas the desorption peaks for Ni/50Ce50Ti_SG, Ni/80Ce-20Ti_SG, and Ni/CeO2_SG primarily reside in the low-temperature (< 350 °C) region. The relative areas under the curves also reveal the greater hydrogen adsorption capacity of the ceria-rich catalysts, which is inferred from their dominant lowtemperature desorption. From the literature, it is understood that the low-temperature domain of a H2-TPD curve corresponds to the desorption from the metal surfaces and indicates the fraction of exposed nickel atoms [30–32]. Based on this interpretation, the TPD profiles in Fig. 4 reflect the degree of nickel dispersion, which falls into the following order: Ni/TiO2_SG < Ni/20Ce-80Ce_SG < Ni/50Ce-50Ti_SG ~ Ni/CeO2_SG < Ni/80Ce-20Ti_SG. The semi-quantitative nickel dispersion results from the H2-TPD agree with the results for the nickel deposit size distribution shown earlier in Fig. 3.
《Fig. 4》

Fig. 4. Amount of H2 desorbed versus temperature for nickel catalysts supported on sol-gel-synthesized ceria-titania mixed-oxide supports of different compositions. H2adsorption was conducted at room temperature for all samples.
3.1.5. CO2-TPD
The CO2-TPD technique was used to evaluate the extent of CO2 binding to the metal/metal oxide samples. In Fig. 5, it is apparent that the cerium-containing metal oxide supports are capable of significant CO2 adsorption even without the presence of metallic nickel. Negligible (room-temperature) CO2 adsorption is observed for both TiO2_SG and Ni/TiO2_SG.
As a weak Lewis acid, CO2 can adsorb onto the basic sites of a metal oxide [33]. Fig. 5 shows that the adsorption capacities of the neat metal oxides increase with increasing cerium content, indicating that the presence of cerium increases the surface Lewis basicity of the metal oxides. Since Lewis basicity is defined as the ability of a substance to donate electron pairs, the abovementioned observation implies that the presence of cerium increases the availability of electrons for improved CO2 adsorption upon the Ce-Ti oxides. One of the known properties of cerium oxide is its reducibility and defect-rich chemistry under a reducing atmosphere. Therefore, the reduction pretreatment that was intended to reduce the nickel oxide in order to active the metallic nickel simultaneously resulted in the reduction of the surface cerium oxide species. As a result, the pretreatment created electron-rich defect sites that encouraged the binding of CO2 onto these sites. Therefore, the result in Fig. 5 implies that a cerium presence is important to facilitate defect formation and thereby facilitate CO2 adsorption. Considering that the majority of the adsorbed CO2 is desorbed at relatively low temperatures (≤ 120 °C), these adsorbed species are not considered to be strongly bound to the catalyst surface when adsorbed at room temperature.
《Fig. 5》
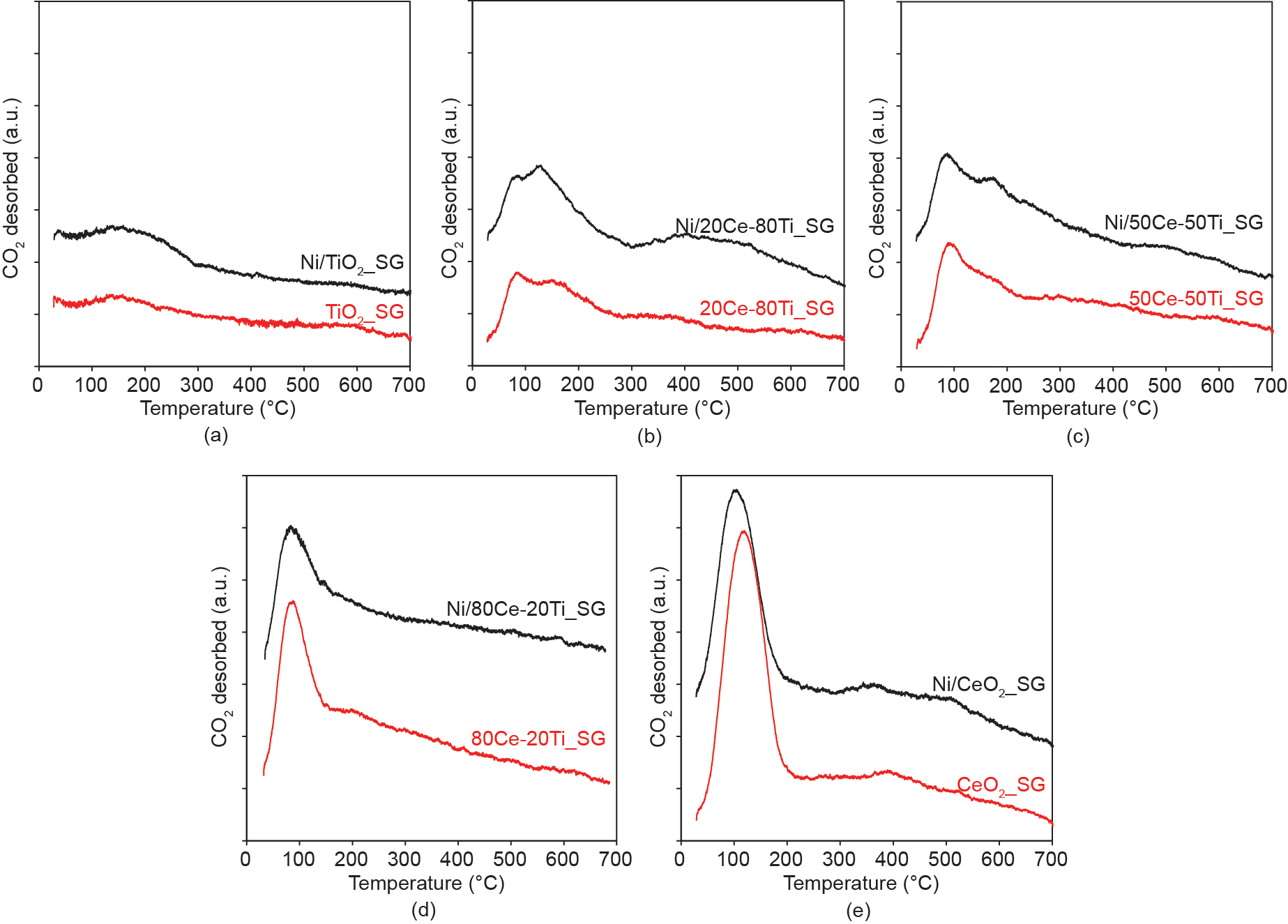
Fig. 5. Amount of CO2 desorbed with respect to temperature for nickel catalysts supported on sol-gel-synthesized ceria-titania mixed-oxide supports (black curves) with different compositions: (a) Ni/TiO2_SG, (b) Ni/20Ce-80Ti_SG, (c) Ni/50Ce-50Ti_SG, (d) Ni/80Ce-20Ti_SG, and (e) Ni/CeO2_SG. The profiles of the respective neat supports are shown in red. CO2 adsorption was conducted at room temperature for all samples.
Regarding the lack of enhanced CO2 adsorption in the presence of the reduced metallic nickel phase, it has been established that CO2 has negligible adsorption on Ni(111) sites at room temperature due to its endothermic nature [34–36]. Due to the relative thermodynamic stability of the {111} family, Ni(111) is usually present as the dominant facet of metallic nickel when metallic nickel is synthesized via the impregnation method. This was found to be the case for the catalysts used in this work, as confirmed via X-ray diffraction (XRD) (Ref. [18] and Fig. S1 in Supplementary Information). This comparison thus reveals that the ceria-rich supports primarily provide adsorption sites for CO2 activation.
3.1.6. XPS elemental analysis
The survey scans (Fig. S2 in Supplementary Information) show the elements detected on the surface of all five (pre-reduced) catalysts. Peaks corresponding to nickel (Ni2p), oxygen (O1s), and titanium (Ti2p) were found for Ni/TiO2_SG, and peaks corresponding to nickel (Ni2p), oxygen (O1s), and cerium (Ce3d) were found for Ni/ CeO2_SG. Signals belonging to carbon (C1s) were present in all samples and originate from atmospheric carbon contamination.
Although it was anticipated that both ceriumand titaniumrelated peaks would be observable for the mixed-oxide-supported catalysts, the cerium signals for Ni/20Ce-80Ti_SG were weak (Table 1), indicating its limited presence at the catalyst surface. For both Ni/20Ce-80Ti_SG and Ni/50Ce-50Ti_SG, the amount of surface cerium species was measured to be lower than their respective nominal values. Both this observation and the fact that Ni/20Ce-80Ti_SG has the lowest nominal concentration of cerium justify the lack of defined signal resolution for cerium on its surface.
Fig. 6 presents the Ni2p3/2 spectra, in which each Ni2p spectrum is deconvoluted into four peaks, indicating re-oxidation of the nickel surface into different oxidation states for all samples. In addition to a satellite peak at about 861 eV, the binding energies for Ni0, Ni2+, and Ni3+ are assigned at 852.6 eV, 854.6 eV, and 856.1 eV, respectively, for the Ni2p3/2 spectra [37]. Taking into account the relative strengths of the metallic nickel signals in their respective XPS spectra, and the fact that element detection for XPS analysis only penetrates to a depth of a few nanometers at the surface, the bulk of the nickel clusters should remain in their reduced metallic state beneath the oxidized surface. Such passivation most likely occurred due to oxygen exposure during sample transfer (since oxygen is present in the atmosphere).
《Table 1》
Table 1 Surface percentage of Ce species and atomic distribution of Ni0, Ni2+, and Ni3+ species based on their corresponding peaks at 852.6 eV, 854.6 eV, and 856.1 eV, respectively.
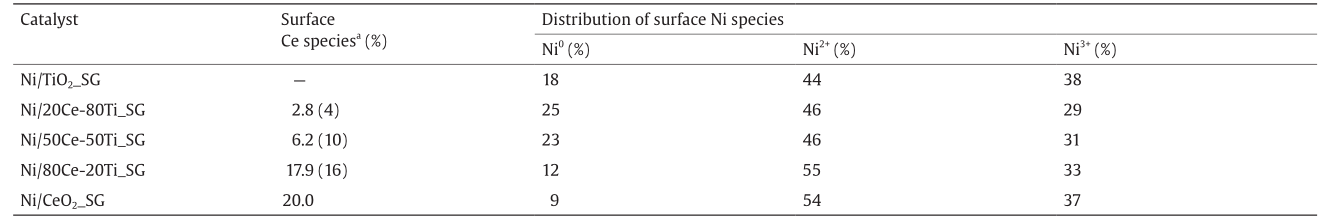
a Overall surface concentration. The expected surface concentration based on nominal Ce:Ti ratios (using the surface concentration found for Ni/CeO2 _SG as the reference) are given in the brackets.
《Fig. 6》

Fig. 6. XPS Ni2p spectra (black curves) of: (a) Ni/TiO2_SG, (b) Ni/20Ce-80Ti_SG, (c) Ni/50Ce-50Ti_SG, (d) Ni/80Ce-20Ti_SG, and (e) Ni/CeO2_SG. The deconvolution of each spectrum is shown by the red curves.
Comparing the Ce3d spectra of Ni/50Ce-50Ti_SG and Ni/80Ce-20Ti_SG with that of Ni/CeO2_SG (Fig. 7) reveals that a titanium presence within the cubic ceria lattice facilitates the preservation of the defective ceria surface after reduction treatment. A Ce3d spectrum is interpreted as follows: the u and v peaks correspond to the 3d3/2 and 3d5/2 states, respectively; the u/v and u″/v″ doublets are shakedown features arising from the transfer of electron(s) from a filled O2p orbital to an empty Ce4f orbital [38,39]; photoemissions from Ce4+ states are indicated by the u‴/v‴ doublets, and the u′/v′ doublets denote the Ce3+ (i.e., reduced cerium oxide) states; in addition to the u′/v′ doublets, the presence of u0/v0 doublets signifies the presence of oxygen vacancies [38,40]. The Ce3d spectra in Fig. 7 show that Ce4+ is present in all cerium-rich samples (Ni/50Ce-50Ti_ SG, Ni/80Ce-20Ti_SG, and Ni/CeO2_SG). Although reduced Ce3+ peaks(u′/v′ doublets) are detected for all three samples, the presence of oxygen vacancy sites is only detected for the Ni/50Ce-50Ti_SG and Ni/80Ce-20Ti_SG samples. This observation implies that the addition of titanium into the cubic lattice of ceria provides a surface that is richer in oxygen vacancy sites and thus possesses a greater electron density.
《Fig. 7》
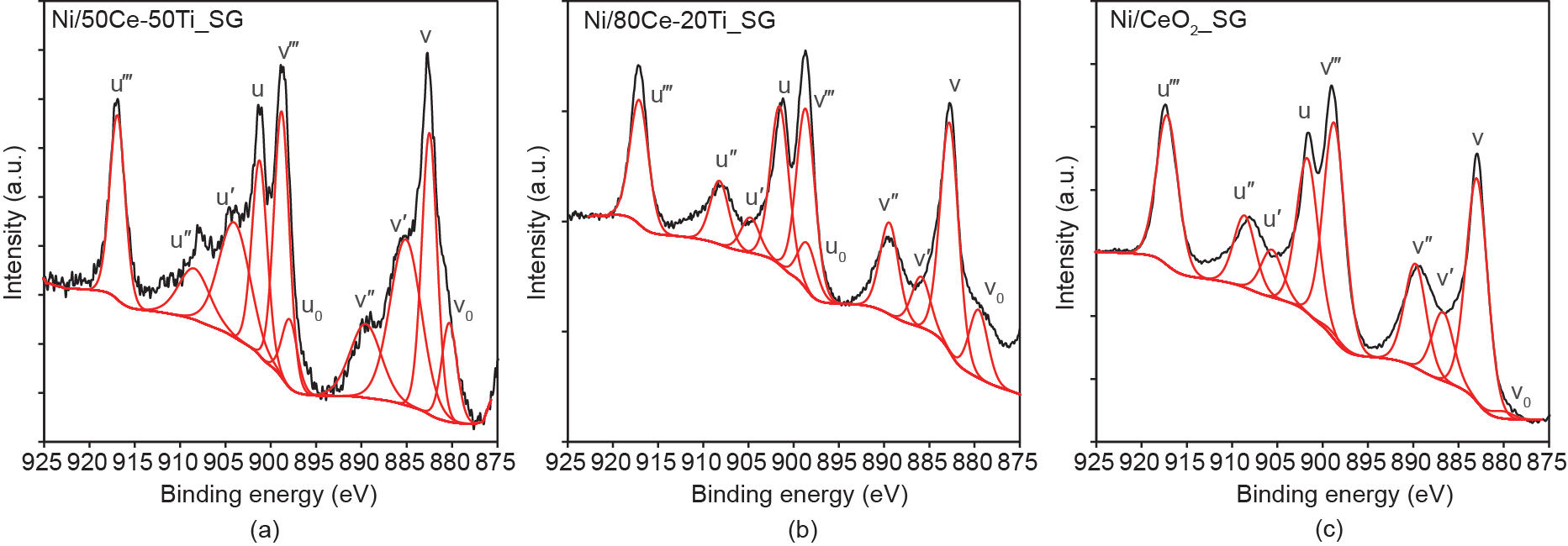
Fig. 7. Ce3d spectra (black curves) of (a) Ni/50Ce-50Ti_SG, (b) Ni/80Ce-20Ti_SG, and (c) Ni/CeO2_SG. The deconvolution of each spectrum is shown by the red curves. The Ce3d spectrum of Ni/20Ce-80Ti_SG is not shown due to the low surface Ce content, which led to poor peak resolution. The u′/v′ doublets denote Ce3+ states, the u‴/v‴ doublets denote Ce4+ states, and the u0 /v0 doublets signify the presence of oxygen vacancies.
However, there is a discrepancy between this observation and the earlier CO2-TPD findings that implied that CeO2_SG had a greater surface electron density in comparison with both 50Ce-50Ti_SG and 80Ce-20Ti_SG. This apparent conflict in the findings can be accounted for by revisiting the experimental procedure. During the CO2-TPD experiment, the catalyst was reduced in situ and was kept under an inert helium flow. Although the catalysts were also pre-reduced for the XPS analysis, the handling of the samples between catalyst preparation and analysis involved exposure (albeit limited) to non-inert conditions, thus causing the catalyst to come into contact with atmospheric oxygen. This occurrence was demonstrated earlier in the Ni2p spectra, which showed that passivation of the catalyst surface took place during sample transfer after the reduction treatment. Once this factor is taken into account, it is reasoned that although CeO2_ SG shows a higher degree of reduction after hydrogen treatment, upon exposure to air, it is more inclined to the rapid re-oxidation of its surface as compared with 50Ce-50Ti_SG. The results demonstrate the importance of having a titanium presence within a ceria lattice to preserve the stability of the oxygen vacancy sites.
The influence of a titanium presence on the surface characteristics of cerium oxide has been observed by Rodriguez and coworkers [13– 15] in their work on CeOx/TiO2 composites. Their results, both theoretical and experimental, revealed that the presence of TiO2 leads to better stability (and thus to a greater tendency for the formation) of Ce3+ species. As the observations in the current study demonstrated that the catalyst support has a preference for oxygen vacancytype species rather than for Ce3+, this comparison also highlights the influence of a mixed-oxide configuration on the electronic characteristics within the material. Whereas the active ceria-titania oxides utilized in this work comprise a ceria lattice doped with titanium ions, the materials studied in Refs. [13–15] consisted of CeOx nanoparticles deposited upon TiO2 substrates (i.e., segregated Ti and Ce oxide phases). Nevertheless, both results demonstrate the advantageous outcome of strategically combining ceria with titanium (oxides) for CO2 conversion purposes.
《3.2. Photothermal CO2 methanation activity》
3.2. Photothermal CO2 methanation activity
Fig. 8 presents the activities of the catalysts in terms of methane production. Of the five tested catalysts, Ni/50Ce-50Ti_SG and Ni/80Ce-20Ti_SG demonstrated a significantly greater methane production performance than the other catalysts, with the latter exhibiting the fastest conversion rate. Whereas Ni/50Ce-50Ti_SG and Ni/80Ce-20Ti_SG attained complete CO2 conversion within 180 min and 90 min, respectively, the other catalysts demonstrated minimal conversion within the 180-min duration. Methane-generation rates were calculated (Table 2) and the values obtained for both active catalysts were found to be two orders of magnitude greater than those for the other catalysts. The lack of methane generation for the other three samples was confirmed to be strictly due to ineffective CO2 conversion, as no other products were detected for any of the catalysts tested.
《Fig. 8》
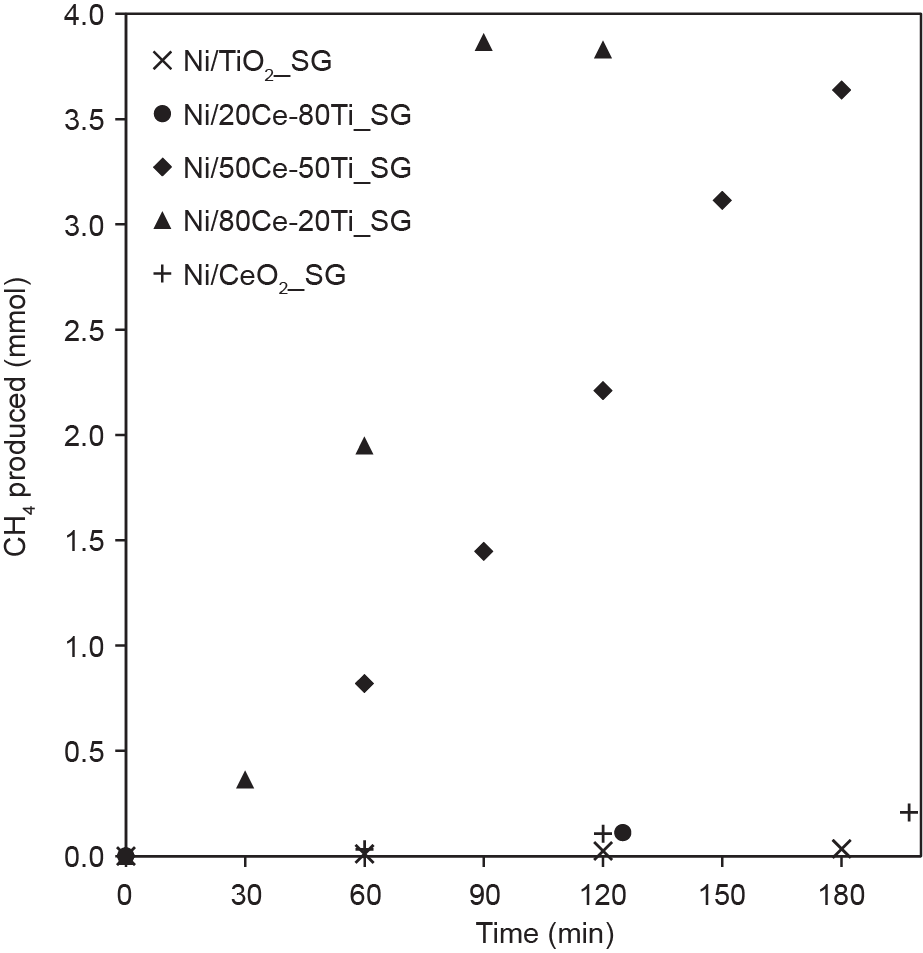
Fig. 8. Amount of CH4 produced over time by nickel catalysts supported on sol-gel-synthesized ceria-titania mixed-oxide supports with different compositions. Approximately 15 kPa of CO2 (about 3 mmol) and 60 kPa of H2 (about 12 mmol) were initially fed into the reactor system, and the catalyst bed (containing 0.1 g reduced catalyst) was illuminated with a 300 W xenon lamp.
《Table 2》
Table 2 Methane production rates by supported nickel catalysts.

Approximately 15 kPa of CO (about 3 mmol) and 60 kPa of H (about 12 mmol) were initially fed into the reactor system, and the catalyst bed (containing 0.1 g reduced catalyst) was illuminated with a 300 W xenon lamp.
Although purely photocatalytic CO2 reduction with H2 has been shown to be possible over light-active semiconductors, the conversion rates are often limited to micromole efficiencies despite long reaction periods [2,41–44]. Therefore, to achieve the conversion rates demonstrated by Ni/50Ce-50Ti_SG and Ni/80Ce-20Ti_SG, thermal energy must be involved. It is understood from the literature that temperatures greater than 200 °C are often required to drive effective methanation activity [45]. As discussed previously, smaller nickel deposit sizes lead to increased light absorption, and absorption of the NIR spectrum can commence the initial photothermal heating of the catalyst bed. Whereas the poor performance of Ni/TiO2_SG and Ni/20Ce-80Ti_SG probably stemmed from their large nickel deposit sizes, a comparison of the UV-Vis-NIR absorption spectra, deposit size distributions, and TPD results suggests that Ni/CeO2_SG should have similar CO2 methanation activity to that of Ni/50Ce-50Ti_SG, based on the abovementioned argument. Furthermore, the CO2-TPD results showed the CeO2_SG-supported catalyst to have the highest CO2 adsorption capacity out of the catalysts tested. However, as observed in the XPS results, the defective surface of Ni/CeO2_SG is prone to rapid re-oxidation upon exposure to atmospheric conditions. Due to the need to transfer activated catalysts between the reduction rig and the methanation reactor, even though the CO2-TPD indicated that Ni/CeO2_SG possessed the highest CO2 adsorption capability, the instability of the CO2 adsorption sites (i.e., oxygen vacancy sites) upon exposure to atmospheric oxygen ultimately led to the loss of this advantage. As a result, despite the effective light absorption by the small nickel particles for catalyst bed heating, the lack of active sites for CO2 adsorption led to a subsequent poor performance by Ni/CeO2_SG. In contrast, Ni/50Ce50Ti_SG and Ni/80Ce-20Ti_SG were capable of effective light absorption as well as maintaining the oxygen vacancy sites, regardless of exposure to atmospheric conditions. The superior conversion rate exhibited by Ni/80Ce-20Ti_SG over that of Ni/50Ce-50Ti_SG further emphasized the importance of nickel deposit size on the photothermal activity of a ceria-titania-supported catalyst.
《4.Conclusions》
4.Conclusions
Ceria-titania-supported nickel catalysts were tested for their CO2 reduction performance under light irradiation. When nickel was present on the supports, the catalysts were capable of greater absorption capacities within the Vis and NIR spectrum. The ceria-rich supports exhibited a greater degree of absorption in the Vis and NIR region (compared with the titania-rich supports), which was attributed to better nickel deposit dispersion invoked by the ceria presence. The increased absorption of this portion of the solar spectrum is anticipated to induce greater heating of the catalyst bed and, in turn, promote CO2 methanation activity. In addition, CO2 adsorption was facilitated by an increased presence of ceria, as it was promoted by surface defect generation within the ceria during the nickel pre-reduction stage. While titania was shown to be detrimental for nickel dispersion and CO2 adsorption, it served an important function in stabilizing the oxygen vacancy-type defects within the support, thus better maintaining the CO2 adsorption capacity during photothermal methanation. In addition to demonstrating the importance of a ceria presence for enhancing surface metal dispersion, this work shows that a titanium presence within the cubic ceria host lattice facilitates the stabilization of oxygen vacancy-type defects. Both of these characteristics are shown to be indispensable for high CO2-to-methane conversion rates driven by light irradiation, as evidenced by the distinct dominance of Ni/50Ce-50Ti_SG and Ni/80Ce20Ti_SG over the performance of the other catalysts and by the improvement in methanation rates upon a further increment in cerium concentration (i.e., from 50Ce : 50Ti to 80Ce : 20Ti). These findings demonstrate the importance of tailoring a composite metal oxide support so that the useful features of the individual components can be effectively harnessed and applied to photothermal catalytic reaction systems.
《Acknowledgements》
Acknowledgements
This work was financially supported by the Australian Research Council under the Laureate Fellowship Scheme (FL140100081). The authors would also like to acknowledge the facilities provided by the Solid State and Elemental Analysis Unit of the UNSW Mark Wainwright Analytical Center.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Ee Teng Kho, Salina Jantarang, Zhaoke Zheng, Jason Scott, and Rose Amal declare that they have no conflict of interest or financial conflicts to disclose.
Supplementary Information
http://engineering.org.cn/EN/10.1016/J.ENG.2017.03.016
Figs. S1,S2














 京公网安备 11010502051620号
京公网安备 11010502051620号




