《1.Introduction》
1.Introduction
Cadaverine (1,5-diaminopentane) is a natural polyamine with multiple bioactivities that is formed through the direct decarboxylation of L-lysine catalyzed by lysine decarboxylase, and that is widely distributed in prokaryotes and eukaryotes. Cadaverine plays an important role in cell survival at acidic pH and protects cells that are starved of inorganic phosphate, Pi, under anaerobic conditions [1,2]. In plants, it is involved in regulating diverse processes such as plant growth and development, cell signaling, stress response, and insect defense [3]. Cadaverine formation is also related to animal growth [4] and tumorigenesis [5,6]. Thus, cadaverine has a wide range of potential applications in agriculture and medicine. Cadaverine has a similar structure to the synthesized petrochemical hexamethylenediamine, and thus can be used to replace hexamethylenediamine in the production of polyamides or polyurethanes [7,8]. Cadaverine is becoming an important industrial chemical because it exhibits broad prospects for various applications, and especially for the synthesis of fully bio-based polyamides by polymerization with dicarboxylic acids derived from renewable sources.
At present, cadaverine can be produced by direct microbial fermentation or whole-cell bioconversion. For direct microbial fermentation, cadaverine-producing strains are mainly engineered from the conventional L-lysine producers Corynebacterium glutamicum (C. glutamicum) [9] and Escherichia coli (E. coli) [10], because L-lysine is the direct precursor of cadaverine. For whole-cell bioconversion, the biocatalysts are dominated by E. coli overexpressed lysine decarboxylase [11,12]. In view of the above, the present review focuses on the biosynthesis, metabolism, and physiological function of cadaverine in bacteria, recent advances in cadaverine production, and applications of cadaverine in bio-based polyamide PA 5X synthesis.
《2.Cadaverine metabolism in bacteria》
2.Cadaverine metabolism in bacteria
《2.1. Anabolism of cadaverine in bacteria》
2.1. Anabolism of cadaverine in bacteria
Cadaverine is formed through the decarboxylation of L-lysine, and thus its biosynthesis depends on L-lysine. Two different pathways evolved separately for L-lysine biosynthesis: the diaminopimelic acid (DAP) route in bacteria and plants; and the α-aminoadipic acid pathway in most fungi and some archaea [13,14]. The DAP pathway has three variants for the synthesis of meso-diaminopimelate [15–17] (Fig. 1). The first, most prevalent variant, in which the intermediates are succinylated, is widely distributed in eubacteria [18], the so-called lower fungi [19], plants [20], and archaea [14,21]. The second variant involves the N-acylation of intermediates, and is only present in Bacillus species, such as Bacillus megaterium [18,22]. The third variant is the dehydrogenase pathway, in which ∆1-piperidine2,6-dicarboxylate is converted to meso-diaminopimelate by a onestep reaction; this variant has extremely limited occurrence in C. glutamicum, Bacillus sphaericus, Pseudomonas species, Brevibacterium species, and some plants such as soybean, maize, and wheat [13,18].
《Fig. 1》

Fig. 1. Schematic representation of the biosynthesis of cadaverine through the DAP pathway. TCA: tricarboxylic acid; PTS: phosphotransferase system.
The biosynthesis and metabolism of cadaverine have been extensively studied in E. coli. Two types of lysine decarboxylases are involved in cadaverine synthesis: the constitutive LdcC [23] and the inducible CadA [24]. They show about 68% and 69% identity, respectively, in DNA and amino acid sequences; however, optimum LdcC activity occurs at about pH 7.6, and optimum CadA activity occurs at around pH 5.6 [25]. Furthermore, CadA exhibits higher thermal stability and higher decarboxylation activity [25,26].
CadA is a member of the E. coli Cad system, which protects cells against acid stress by inducing protein expression under conditions of low pH with excess L-lysine [27]. The E. coli Cad system has two principal components: the cadBA operon coding for lysine decarboxylase (CadA) and lysine/cadaverine antiporter (CadB), and the regulatory protein CadC [28,29]. CadC is a multifunctional inner membrane protein, which is composed of a DNA-binding domain with a winged helix-turn-helix motif at the cytoplasmic N-terminal, a transmembrane (TM) domain, and a periplasmic C-terminal [30]. The N-terminal can bind to the Cad1 and Cad2 sites of the upstream region of cadBA to activate cadBA expression [31]. The C-terminal is a pH sensor domain that is responsible for sensing the external H+ concentration [32]. The TM domain and periplasm-exposed amino acids of CadC are important in mediating and/or stabilizing the association between CadC and LysP through the formation of salt bridges and/or disulfide bonds [30]. LysP is an L-lysine-specific permease, which perceives exogenous L-lysine and regulates CadC levels [30,33,34]. Transcription of the cadBA operon is also modulated by the histone-like DNA-binding protein H-NS, which acts to reduce the expression of CadA and CadB under non-inducing conditions [35,36].
Fig. 2 summarizes the regulatory mechanism of the cadBA operon in E. coli. Under normal growth conditions, the expression of the cadBA operon is inhibited by the binding of H-NS proteins to Cad1 and Cad2 sites, which are located at –144 bp to –112 bp and –89 bp to –59 bp of the cadBA upstream region, respectively [31,35,36]. Meanwhile, the transcriptional activator CadC is anchored in the inner membrane and cannot perform the activation function because of the interaction with membrane protein LysP. Thus, cadBA operon is not transcribed, and no cellular cadaverine is produced [37]. When acidic stress occurs (pH < 6.8) in a lysine-rich environment (> 5 mmol·L–1), the interaction between CadC and LysP is weakened due to their pH-dependent conformational transition and/or the opening of the disulfide bridge between the two proteins [32,37]. In addition, the conformation of LysP is further altered upon the binding/translocation of L-lysine, which leads to the disassociation of CadC and LysP [30,38]. The activated CadC then replaces H-NS proteins and binds the Cad1 and Cad2 sites of the cadBA upstream region to activate cadBA operon transcription and expression [36,38]. As cadaverine accumulates, the excess cadaverine (> 235 μmol·L–1 [37]) acts as a negative effector of cadBA expression by deactivation of CadC through binding to the site at the dimerization interface of CadC [28,39]. In addition, CadA activity is inhibited by ppGpp, a stringent response effector that accumulates rapidly in cells that are starved for amino acids, to prevent excessive L-lysine consumption [40].
《Fig. 2》
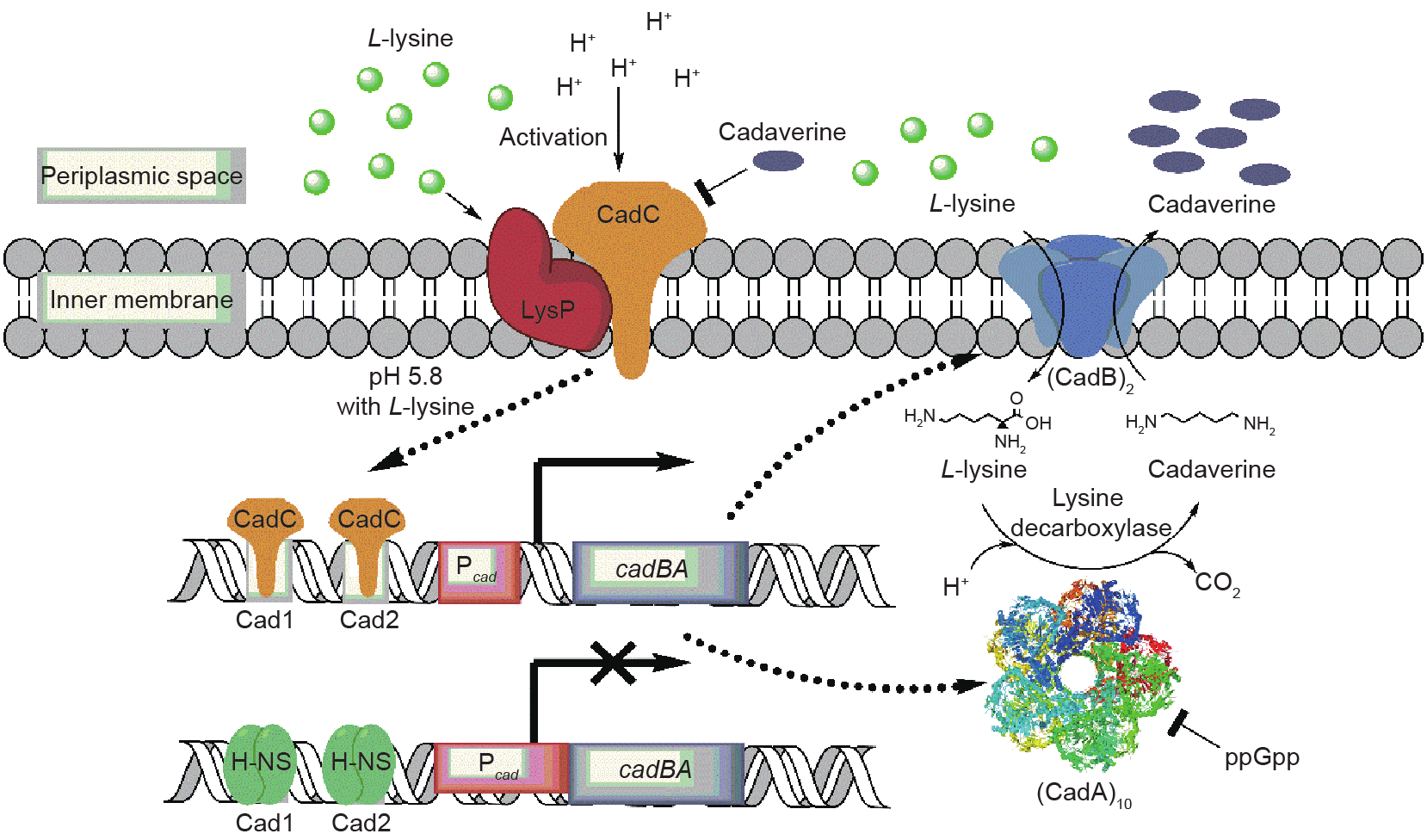
Fig. 2. Regulation of cadaverine biosynthesis in E. coli.
In addition to the Cad system of E. coli, another cadaverine synthesis system has been identified in Lactobacillus saerimneri 30a. This system consists of a specific lysine decarboxylase and a bifunctional antiporter that has an affinity for ornithine and putrescine as well as for lysine and cadaverine [41].
《2.2. Intermediary metabolism of cadaverine in bacteria》
2.2. Intermediary metabolism of cadaverine in bacteria
Although the utilization or degradation of cadaverine has not been observed for E. coli thus far [8], several bacteria species are known to use cadaverine as an intermediate. The acetylation of cadaverine has been studied in detail in C. glutamicum. In C. glutamicum, the encoding gene NCgl1469, which has been identified and functionally assigned as cadaverine acetyltransferase, is responsible for the formation of N-acetylcadaverine. Deletion of NCgl1469 led to complete elimination of cadaverine acetylation and a cadaverine yield increase of 11% [42]. Cadaverine can also be aminopropylated by agmatine/cadaverine aminopropyl transferase to form N-(3aminopropyl)cadaverine in Pyrococcus furiosus [43]. Another cadaverine catabolic pathway has been confirmed in members of Pseudomonas, in which cadaverine is metabolized by transamination to α-piperidine and oxidized to δ-aminovaleric acid [44].
Cadaverine has been proven to be an essential constituent of the cell wall, and plays an important role in the structural linkage between the outer membrane and peptidoglycan in some strictly anaerobic bacteria [45]. This role was first discovered in a peptidoglycan of Selenomonas ruminantium by Kamio et al. [46,47]; a similar structure was found in Veillonella alcalescens [48], Veillonella parvula [49], and Anaerovibrio lipolytica [50]. The proposed primary structure of the cadaverine-containing peptidoglycan is shown in Fig. 3 [45,50].
《Fig. 3》

Fig. 3. Chemical structure of the cadaverine-containing peptidoglycan. MurNAc and GlcNAc refer to N-acetylmuramic acid and N-acetylglucosamine, respectively.
Cadaverine is also involved in the biosynthesis of siderophores, which are exported into the local extracellular milieu where they bind Fe3+ with high avidity [51,52]. The currently known cadaverinebased siderophores (Fig. 4) include arthrobactin [51,53], bisucaberin B [54], bisucaberin [55], desferrioxamine B [56–58], desferrioxamine E [57,59], and desferrioxamine G1 [60].
《Fig. 4》
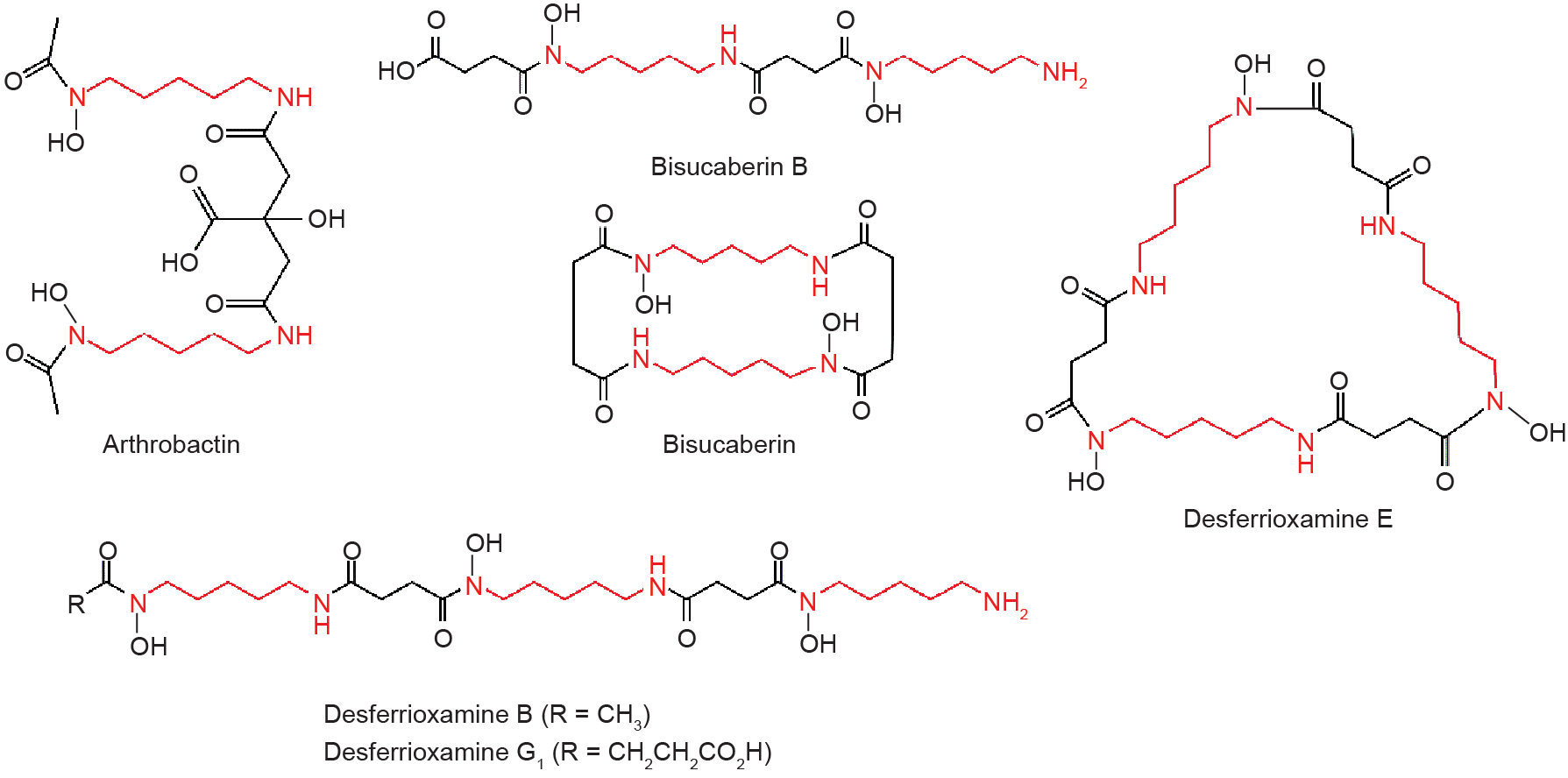
Fig. 4. Cadaverine-based siderophores.
《3.Bio-based production of cadaverine》
3.Bio-based production of cadaverine
Cadaverine is synthesized through the direct decarboxylation of L-lysine, which is catalyzed by lysine decarboxylase in living cells. However, little research is available on the production of cadaverine by direct enzyme catalysis, due to product inhibition in lysine decarboxylase, which loses 50% activity at 3 g·L–1 cadaverine [61]. In one recent exception, an attempt was made to catalyze L-lysine decarboxylation using glutaraldehyde-cross-linked lysine decarboxylase; however, the immobilization yield and activity retention were only 30.5% and 8.04%, respectively [62]. Thus, it is still impractical to produce cadaverine by direct enzyme catalysis, whether using a free or an immobilized enzyme. On the other hand, great progress has been achieved in cadaverine production by direct fermentation of metabolic engineering strains or by whole-cell bioconversion from L-lysine.
《3.1. Metabolic engineering of cadaverine production》
3.1. Metabolic engineering of cadaverine production
In fermentative cadaverine production, the most commonly used producer strain is engineered from the conventional L-lysine producer C. glutamicum, which was first isolated by Kinoshita et al. in 1957 [63], and which has been continuously optimized for L-lysine production for up to 50 years [7]. Especially in recent years, metabolic engineering has strongly contributed to the performance of C. glutamicum [64–69], which is able to produce 120 g·L–1 of L-lysine with a productivity of 4 g·(L·h)–1 and a yield of 0.55 g(lysine)·g(glucose)–1 in fed-batch culture within 30 h [7,70]. The annual global production of L-lysine is currently approximately 2 × 106 t, using a mutant strain of C. glutamicum [71].
Because of its remarkable performance in L-lysine production, C. glutamicum was one of the first species engineered for fermentative cadaverine production. In a first proof of principle, Mimitsuka et al. [72] constructed an L-homoserine auxotrophic C. glutamicum, in which the L-homoserine dehydrogenase gene (hom) was replaced by the lysine decarboxylase gene (cadA) of E. coli; the resulting strain produced 2.6 g·L–1 of cadaverine after 18 h fermentation. However, a concentration of 2.3 g·L–1 L-lysine remained in the fermentation broth, which was presumed to be caused by the cadaverine excretion deficiency in C. glutamicum. Hence, lysine decarboxylase activity was inhibited by high concentrations of intracellular cadaverine. This hypothesis was confirmed by Li et al. [73], who constructed an engineered C. glutamicum by co-expression of the E. coli cadaverine/ lysine antiporter CadB and the Hafnia alvei (H. alvei) lysine decarboxylase. Results showed that the cadaverine secretion rate increased by 22% and the yield of total cadaverine and extracellular cadaverine increased by 30% and 73%, respectively [73]. A similar study on modulation of cell permeability was carried out by Matsushima et al. [74] through the addition of Tween 40 at the mid-exponential phase, causing a 1.5-fold increase in cadaverine yield compared with cultivation without Tween 40 addition.
The real breakthrough in cadaverine production using C. glutamicum was accomplished by Kind et al. [42]. Using transcriptome analysis, they identified a major facilitator superfamily permease, cg2893, which is a cadaverine exporter in C. glutamicum, and subsequently examined the effect of cg2893 overexpression on cadaverine production. Their results showed a 20% increase in product yield, with 40% enhancement in specific production rate [61].
The cadaverine producer C. glutamicum DAP-16 was then engineered from the L-lysine hyper-producer C. glutamicum LYS-12 [70] using the following process: genome-based expression of a codonoptimized variant of E. coli ldcC; deletion of N-acetyltransferase NCgl1469 and lysine exporter (lysE); and overexpression of permease cg2893 by replacement of the native promoter by the sod promoter. Fed-batch fermentation of DAP-16 resulted in the production of 88 g·L–1 of cadaverine in 50 h, with a productivity of 2.2 g·(L·h)–1 and a yield of 0.29 g(cadaverine)·g(glucose)–1 [75]. Buschke et al. [76] also contributed to improvements in cadaverine production. They targeted the genes that limited cadaverine production performance from five-carbon sugars using a systems-wide comparison of the fluxome and transcriptome on glucose and xylose as the sole carbon sources in the cadaverine producer C. glutamicum DAPXyl1. Next, they constructed the cadaverine producer C. glutamicum DAP-Xyl2 with the following characteristics: Fructose bisphosphatase (fbp) and the entire tkt operon were overexpressed; isocitrate dehydrogenase (icd) was attenuated; L-lysine exporter (lysE) and N-acetyl transferase (cg1722) were knocked out; and the xylose isomerase (xylA) and xylulokinase (xylB) from E. coli were introduced. As a result, C. glutamicum DAP-Xyl2 achieved 103 g·L–1 of cadaverine from xylose with a product yield of 32%, in a fed-batch process [76].
In addition to C. glutamicum, E. coli is a competitive L-lysine producer and has been engineered for cadaverine production. E. coli and C. glutamicum have a similar biosynthesis pathway and metabolic regulation of L-lysine, so E. coli has been systematically engineered and fermented accordingly to produce L-lysine [77–80], resulting in the production of 136 g·L–1 of L-lysine with a productivity of 2.8 g·(L·h)–1 and a yield of 0.56 g(lysine)·g(glucose)–1 [81]. However, the use of E. coli for cadaverine production has not been studied extensively; the highest titer of cadaverine produced by metabolic engineered E. coli is only 9.61 g·L–1 thus far, with a productivity of 0.32 g·(L·h)–1 and a yield of 0.12 g(lysine)·g(glucose)–1 [10]. There are two possible reasons for the low bioconversion rate of L-lysine to cadaverine in E. coli. The first is that E. coli exhibits low tolerance to cadaverine, which inhibits cell growth and causes cell lysis after 8 h exposure to 0.3–0.5 mol·L–1 cadaverine [10]; the other is that cadaverine regulates membrane permeability of E. coli. Cadaverine has been shown to interact with the bacterial porins OmpC and OmpF, inducing porin closure in a concentration-dependent manner [82,83], and the extent of this effect is greater from the periplasmic side than from the extracellular side [84]. As a result, the uptake of nutrients and the efflux of deleterious metabolites are decreased, which halts the bioconversion of L-lysine to cadaverine. Since E. coli possesses considerable capability to produce high amounts of L-lysine and has a competitive advantage in L-lysine decarboxylase activity [12], it is the most promising species for fermentative cadaverine production and is not being used to its full potential.
Several studies have been carried out to extend the substrate range for cadaverine production. Tateno et al. [85] developed an approach to produce cadaverine from soluble starch using engineered C. glutamicum overexpressing α-amylase from Streptococcus bovis 148 and CadA from E. coli, and the cadaverine titer reached 2.24 g·L–1 after 21 h fermentation. The feasibility of using methanol as a carbon source for cadaverine fermentation was also evaluated in Bacillus methanolicus overexpressing E. coli CadA, which led to a volume-corrected concentration of 11.3 g·L–1 of cadaverine by highcell density fed-batch fermentation [86].
《3.2. Cadaverine production by whole-cell bioconversion》
3.2. Cadaverine production by whole-cell bioconversion
Whole-cell bioconversion is a promising method due to its convenience and high efficiency, as well as its high product concentration and purity, which reduce the cost of product separation. In addition, L-lysine is economically viable as a raw material for cadaverine production, because there is a thriving, million-ton industry for its production [8].
In comparison with fermentative cadaverine, which is mainly produced by C. glutamicum, whole-cell bioprocesses are dominated by E. coli. The use of whole-cell bioconversion for cadaverine production was initially reported in a patent by Nishi et al. [87]. Nishi et al. [87] reported obtaining 69 g·L–1 cadaverine after 6 h whole-cell bioconversion using the E. coli overexpressed CadA, with a productivity of 11.5 g·(L·h)–1.
In whole-cell bioprocesses, limited permeability is a widespread problem: The natural barrier functions of the cell wall and cell membrane limit the transport of starting material into the cell, as well as the release of products accumulated in the cell [88,89]. Thus, an efficient substrate and product transport system is important in whole-cell bioconversions. There are several ways to reduce the permeability barrier, including chemical (e.g., by adding detergents or solvents) or physical (e.g., temperature shock) treatment [90]. However, these treatments may damage the biocatalysts and pose further difficulty for downstream processes [88]. In comparison, modulating cell permeability through genetic approaches is more promising. For cadaverine transportation in E. coli, there is a lysine/ cadaverine antiporter (CadB) that facilitates cadaverine excretion and coordinates cadaverine excretion with lysine uptake, which is driven solely by concentration gradients without other energy inputs [29,91]. The effect of CadB overexpression on biocatalyst activity was first investigated by Ma et al. [11]. Their results showed that co-overexpression of CadA and CadB with N-terminal fused pelB signal peptide from the pectate lyases of Erwinia carotovora was effective in reducing the biocatalyst permeability barrier. The resulting strain, BL-DAB, produced cadaverine with a titer of 221 ± 6 g·L–1 and a molar yield of 92% [11].
Pyridoxal 5′-phosphate (PLP), the cofactor of lysine decarboxylase, is critical for whole-cell activity. The activity of lysine decarboxylase is positively correlated to the amount of PLP, and the maximum activity occurs at a ratio of 1.0–1.2 mol(PLP)·mol(protein)–1 [24]. Kim et al. [92] reported that when no PLP was added in wholecell bioconversion, only 20% of the 1 mol·L–1 L-lysine was decarboxylated to cadaverine, whereas the conversion rate reached 80% with the addition of 0.025 mmol·L–1 PLP. PLP can also be self-supplied by enhancing intracellular PLP synthesis in biocatalysis. For example, Ma et al. [93] constructed the whole-cell biocatalyst E. coli AST3, in which PLP synthesis was enhanced by introducing the de novo PLP synthesis pathway (R5P pathway) of Bacillus subtilis into a CadA/CadB overexpressing strain, BL-DAB. This E. coli biocatalyst was capable of accumulating intracellular PLP at a concentration of 1051 nmol·g(DCW)–1 (DCW: dry cell weight) after optimizing the co-expression of proteins and culture conditions. As a result, cadaverine productivity reached 28 g·[g(DCW)·h]–1 without PLP addition, which was 1.2-fold and 2.9-fold higher than that of BL-DAB with and without PLP supplementation, respectively [93].
The effect of substrate L-lysine concentration on biocatalyst activity was also investigated. Oh et al. [12] reported that in a bioconversion system with cells concentrated to OD600 of 10, an increase in the initial L-lysine concentration of up to 150 g·L–1 resulted in significant decreases in the activity of whole-cell biocatalysts. Fortunately, substrate inhibition can be relieved by optimizing L-lysine feeding strategies [11].
Another research focus is to maintain biocatalyst activity, which is significantly reduced with the increase of cadaverine concentration during the whole-cell bioconversion process. Oh et al. [12] reported that an initial rate of L-lysine consumption of 40.42 mmol·(L·h)–1 could be reached, whereas the average reaction rate was only 10 mmol·(L·h)–1, indicating a significant decrease in biocatalyst activity. Cell lysis may be one of the reasons for this decrease; therefore, the application of immobilized cells for cadaverine production has been investigated recently. Bhatia et al. [94] examined the deactivation energy (Ed) of free and immobilized cells during cadaverine production. Their results showed that the Ed of immobilized cells was almost 10% higher than that of free cells: 66.7 kJ·mol–1 and56.9 kJ·mol–1, respectively. The immobilized cells retained 56% activity after 18 reaction cycles, whereas the free cells lost catalytic activity completely after 10 reaction cycles [94]. These results indicate that the application of immobilization techniques was effective for maintaining biocatalyst activity.
In addition to inducible lysine decarboxylase CadA from E. coli, the use of constitutive lysine decarboxylase LdcC in the construction of whole-cell biocatalysts was reported. The resulting strain produced 133.7 g·L–1 cadaverine in 120 h from a fermentation broth containing 192.6 g·L–1 L-lysine, with a yield of 99.9%; however, the productivity was only 4.1 g·(L·h)–1 [12]. Li et al. [95] investigated the biotransformation of cadaverine using a codon-optimized lysine decarboxylase from Klebsiella oxytoca, which was introduced into E. coli MG1655 and overexpressed under the control of a Ptac promoter. The resulting strain, LN24, converted L-lysine to cadaverine at a conversion rate of 0.133% (min·g)–1 with a yield of 92% [95]. Moreover, the lysine decarboxylase from H. alvei AS1.1009 was mutated by error-prone polymerase chain reaction and DNA shuffling, and the mutant LDCE583G (which showed 1.48-fold improved activity in comparison with the wild type) was introduced into E. coli JM109 in order to construct a whole-cell biocatalyst for cadaverine production. The resulting biocatalyst produced 63.9 g·L–1 cadaverine with a conversion yield of 93.4% during 5 h whole-cell bioconversion [96].
《3.3. Separation and purification of cadaverine》
3.3. Separation and purification of cadaverine
Cadaverine separation and purification methods are developed based on experience with other diamines. Solvent extraction is the most popular method due to low energy consumption, high selectivity, and separation efficiency, as well as simple equipment and operation.
To identify a suitable solvent for extracting cadaverine from fermentation broth, the distribution coefficients for cadaverine in n-butanol, 2-butanol, 2-octanol, and cyclohexanol were compared, with the results showing that n-butanol was better for cadaverine extraction [75]. Meanwhile, the cadaverine distribution coefficients in 4-nonylphenol, di-2-ethylhexyl phosphoric acid, Versatic acid 1019, di-nonyl-naphthalene-sulfonic acid, and 4-octylbenzaldehyde were also investigated. The results showed that 4-nonylphenol is a suitable extractant, and that over 90% of cadaverine can be extracted from fermentation broth after a one-step extraction and backextraction [97].
After phase separation, cadaverine can be recovered from the cadaverine-containing phase by chromatography, distillation, or precipitation as a salt with suitable acids [9,98]. Of these measures, distillation is a promising method for cadaverine purification on an industrial scale; however, the specific conditions for distillation are dependent on practical factors such as the concentration of cadaverine in the extract phase and the properties of the extractant.
To avoid cadaverine decomposition during distillation at high heating temperatures, a purification process using high boiling point solvents was proposed in a recent patent application [99]. This method involves adding high boiling point solvents, such as C14 alkanes or a C11/C14 alkane mixture, to the distillation system in order to improve fluidity during reduced-pressure distillation. As a result, nearly 100% of cadaverine can be recovered at the lower heating temperature of 130 °C, which is 50 °C lower than the boiling point of cadaverine.
Although many advances have been made in cadaverine separation and purification, successful commercial application has not yet been achieved. Purity higher than 99.5% is a basic prerequisite for monomers to polymerize effectively; thus, developing the separation and purification technology of bio-based cadaverine is crucial in order to meet the needs of industrial production.
《4.Application of cadaverine in bio-based polyamides》
4.Application of cadaverine in bio-based polyamides
《4.1. Research progress of bio-based polyamides》
4.1. Research progress of bio-based polyamides
Research on bio-based polyamides (nylon) began in the 1940s and has become an important focus area in recent years due to economic and environmental factors. According to statistics from the European Bioplastics e.V., the global production of bio-based plastics was about 1.7 × 106 t in 2014 and will reach 7.8 × 106 t by 2019 [100].
At present, the commercialized bio-based polyamides include completely bio-based polyamides (e.g., PA 11 and PA 1010) and partially bio-based polyamides (e.g., PA 610, PA 1012, PA 410, PA 10T, etc.); the classification and producers of each of these are listed in Table 1 [101–105]. The main bio-based monomers used are sebacic acid, decan-1,10-diamine, and ω-amino-undecanoic acid, which are all prepared from castor oil as shown in Fig. 5 [106–108].
《Table 1》
Table 1 Classification and producers of commercialized bio-based polyamides.

《Fig. 5》
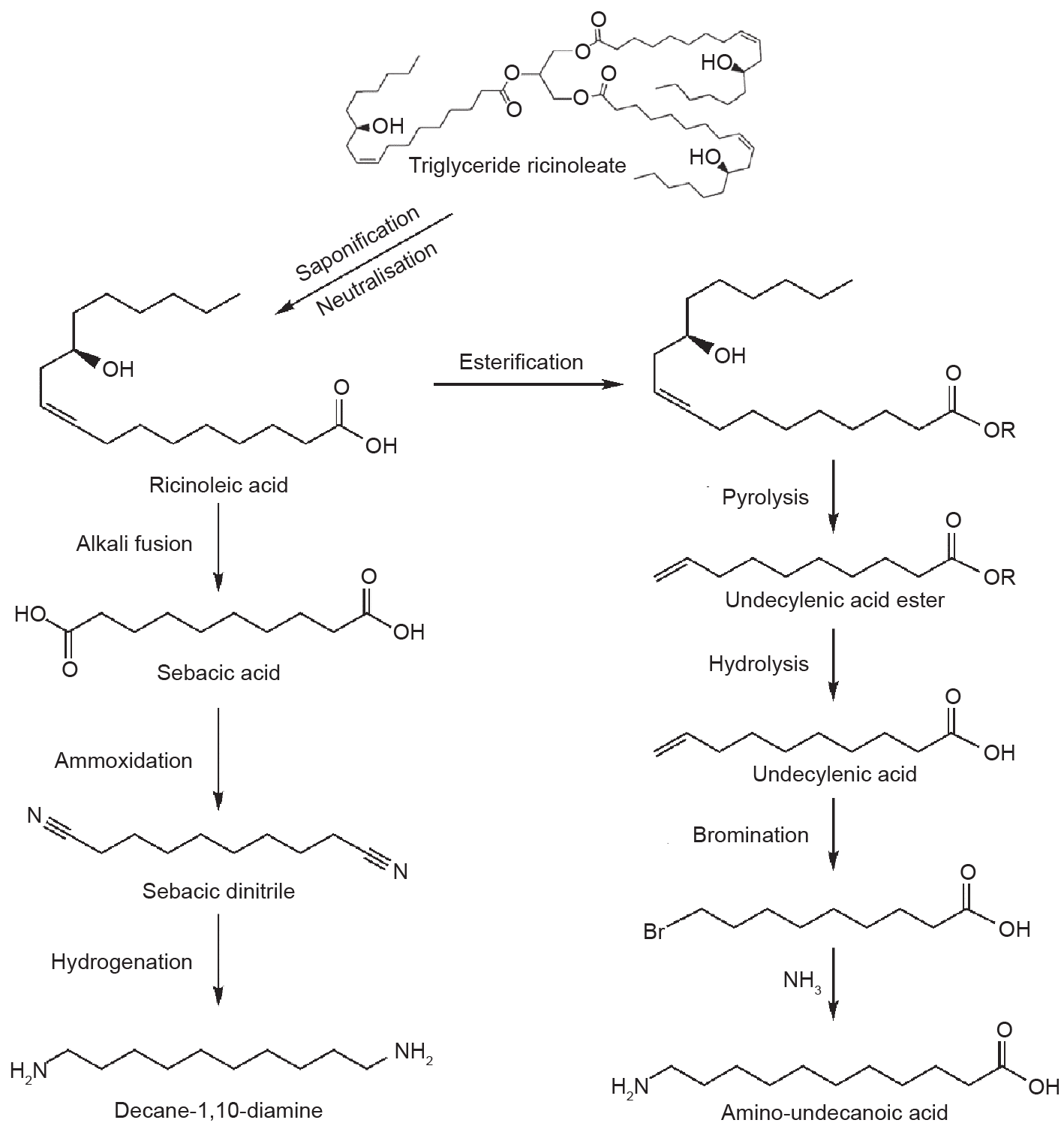
Fig. 5. Transformation of castor oil to sebacic acid, decan-1,10-diamine, and ω-amino-undecanoic acid.
Apart from the commercialized bio-based polyamides discussed above, some new types of fully bio-based polyamides are being extensively researched, such as bio-based PA 46, PA 6, and PA 5X. The fully bio-based PA 46 is polymerized from bio-based adipic acid and putrescine. Several synthesis methods have been developed using different renewable materials to produce bio-based adipic acid. For example, Draths et al. [109] and van Duuren et al. [110] developed a fermentation approach and an enzymatic catalytic approach, respectively, to synthesize muconic acid from D-glucose, followed by the hydrogenation of muconic acid to adipic acid. Lange et al. [111] reported a method to synthesize levulinic acid from cellulose by acid hydrolyzation; the levulinic acid is then hydrogenated and dehydrated to generate γ-valerolactone, followed by ester exchange, an addition reaction, and a hydrolysis reaction to obtain adipic acid. Boussie et al. [112] invented a method to produce adipic acid via the hydrogenation of glucaric acid, which can be obtained by the catalytic oxidation of glucose. Research on putrescine biosynthesis is also in progress. Schneider et al. [113] have studied putrescine production by the fermentation of glucose using genetically engineered C. glutamicum, which achieved a putrescine titer of 19 g·L–1 at a volumetric productivity of 0.55 g·(L·h)–1 and a yield of 0.16 g(putrescine)·g(glucose)–1. Qian et al. [114] reported a titer of 24.2 g·L–1 putrescine with a volumetric productivity of 0.75 g·(L·h)–1, which was obtained by fermentation using engineered E. coli.
Research on production of the PA 6 monomer caprolactam has also made some progress. Caprolactam can be obtained from L-lysine by catalytic conversion [115] or from γ-valerolactone, which is a component of cellulosic hydrolysate, by acid catalysis [116]. In addition, the production of caprolactam from adipic acid, adiponitrile, 1,3-butadiene, 6-aminocaproic acid, adipamide, and muconic acid has been reported [117].
《4.2. Research status of bio-based polyamide 5X》
4.2. Research status of bio-based polyamide 5X
In recent years, many studies have been carried out to investigate polyamide synthesis from bio-based cadaverine, instead of from petroleum-based hexanediamine. Using cadaverine polymerization with appropriate bio-blocks, such as succinate, adipic acid, or sebacic acid, results in the completely bio-based polyamide family PA 5X, such as PA 54, PA 56, and PA 510, respectively. Compared with PA 66, which is obtained from non-renewable fossil fuels, cadaverinebased polyamides also exhibit excellent tensile strength, high melting points, and resistance to organic solvents; thus, they can be used to replace PA 66 in many applications. Table 2 compares several material properties of PA 6, PA 66, PA 56, and PA 510 [75,105,118].
《Table 2》
Table 2 Material properties of PA 6, PA 66, PA 56, and PA 510.
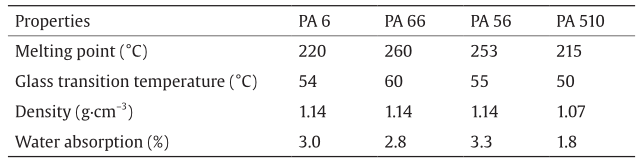
PA 56 has a competitive advantage in the preparation of textile fibers due to its high water absorption and a low glass transition temperature. The high water absorption of PA 56 endows fibers with excellent moisture-wicking performance, which keeps the people wearing the textiles comfortable and prevents static electricity. A low glass transition temperature is conducive to material performance of low temperature resistance, so clothes made from these textiles will not be brittle in high-altitude areas. Moreover, PA 56 has outstanding strength and toughness along with excellent resistance to abrasion, which makes clothes made from such textiles more durable [118].
PA 510, which is synthesized from cadaverine and sebacic acid, possesses the properties of low water absorption, outstanding impact strength at low temperatures, and dimensional stability [75]; thus, it can be applied in fields where conventional polyamides are not suitable. PA 510 has wide application prospects in machinery, electronic appliances, radio technology, and other industrial areas and daily necessities, and can be used to manufacture industrial parts, friction-reducing materials, and electrical insulation. In addition, PA 510 is highly suitable for automobiles, aircraft, and other vehicles because of its low density, which can reduce vehicle weight, resulting in energy saving and environmental protection.
However, no high-purity cadaverine is produced on an industrial scale as yet, because high-performance PA 5X has not yet been commercialized. Studies of other completely bio-based nylons based on cadaverine are still in the early stages [119], but cadaverine-based PA 5X has broad application prospects due to its exceptional performance and environment-friendly characteristics.
《5.Outlook and suggestions for future development》
5.Outlook and suggestions for future development
Studies on the biosynthesis of cadaverine are still in the initial stages, and many factors restrict its large-scale industrial production. The toxic effect of cadaverine on bacterial strains is one of the factors restricting industrialization. Further studies on the mechanisms of cellular damage are needed to provide a basis for the specific engineering of production strains and the optimization of fermentation conditions. For whole-cell catalysis, substance transport in cells and the mechanisms of the catalytic reaction require further study. In addition, from the perspective of fermentation economics, the release of a molecule of carbon dioxide (CO2) during the process of L-lysine decarboxylation imbalances the carbon cycle and consequently leads to an increase in production costs. Therefore, CO2 recycling is a key issue in reducing the cost of cadaverine production by whole-cell bioconversion.
The production cost of cadaverine can also be reduced by screening and engineering lysine decarboxylase. At present, the commonly used lysine decarboxylase is the protein CadA from E. coli. Although this enzyme has higher decarboxylase activity than other lysine decarboxylases, it suffers from serious substrate and product inhibition. In addition, CadA can only maintain catalytic activity under mild acid conditions (pH 5–6.8), which restricts the production of high concentrations of alkaline cadaverine. To meet the requirement of cadaverine separation by solvent extraction, alkalinity (pH > 12) of the fermentation broth (or transformation solution) is needed, while a mild acid condition is needed during production. This apparent contradiction results in excessive consumption of acids and bases during the whole process, which not only increases production cost, but also restricts cleaner production. Therefore, screening novel lysine decarboxylases with alkali resistance or engineering lysine decarboxylases using molecular and synthetic biology approaches, such as site-directed mutagenesis and directed evolution, can be done to reduce substrate and product inhibition and enhance acid/ base stability. These steps could greatly enhance cadaverine yield and productivity, and reduce acid-base consumption and production costs.
《Acknowledgements》
Acknowledgements
This work was supported by the National Key Research and Development Program (2016YFA0204300); the National Natural Science Foundation of China (21390200, 31440024); and the Technology Support Program of Gansu Provincial S&T Department, China (1304FKCE106).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Weichao Ma, Kequan Chen, Yan Li, Ning Hao, Xin Wang, and Pingkai Ouyang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




