《1.Introduction》
1.Introduction
Photosynthetic microorganisms such as unicellular microalgae, cyanobacteria, and plant cells are high-potential bioresources that are promising for various applications, including valuable food and animal feed production [1,2], nutraceutics [3,4], pharmaceutics [5,6], pigments and cosmetics [5,7], wastewater treatment [8,9], fine chemicals [2,10] and biofuels [11,12], carbon dioxide (CO2) biosequestration [10,13], and so forth.
The photobioreactor (PBR) process is designed to convert solar energy into desirable products. It is a highly attractive approach compared with open-air systems [14], which also use photosynthetic organisms, due to its favorable advantages such as higher photosynthetic efficiency, higher concentrations and areal productivities, low contamination, the prevention of water loss caused by evaporation, and a precisely controlled environment [13,15]. A typical PBR is a three-phase system that includes the culture medium as the liquid phase, the cells as the solid phase, and CO2-enriched air as the gas phase in autophototrophic cultivation. As a unique feature, the light in a PBR is a superimposed radiation field that is usually referred to as a fourth phase [16]. Light intensity decreases sharply due to absorption and scattering by fluids; hence, the radiation field in a PBR is highly heterogeneous. It is well known that light availability is the limiting factor for cell growth in a PBR [17,18].
Although various types of PBRs have been developed so far, including bubble column [19–22], airlift reactor [23–27], flat-plate [28], stirred-tank [29,30], tubular [31,32], conical [33,34], torus [35], and seaweed-type PBRs [36], only a few can be used for mass cultivation. In practice, PBRs are principally designed using semi-empirical methods [37–39], as is illustrated here by the example of PBRs for microalgae cultivation. Therefore, only a few species of microalgae have been cultured commercially due to poorly developed PBR technology [20]. Intensive research efforts, especially those that combine theory and practice, are still in demand to exploit the full potential of large-scale cultivation systems [40].
Although a wide variety of novel PBRs has been developed, as mentioned above, capital cost, operating cost, and the lifespan of the PBRs should be comprehensively taken into account for commercial exploitation. As pointed out by Guo et al. [41], designing an efficient PBR is still a significant challenge due to the reactor’s complexity. Important factors that must be carefully considered, such as light distribution, hydrodynamics, mass transfer, and growth kinetics, are closely interrelated, making the design more complicated. Therefore, a deep understanding of cell growth and of the theoretical foundations of the PBR in terms of light, mixing, mass transfer, and environment, is urgently needed.
This work analyzes the effects of the principal factors on the performance of commercial PBRs, and summarizes the most promising PBRs for the large-scale commercial exploitation of microalgae. It also elaborates theoretical models, which include the interplay among light, mixing, mass transfer, and cell growth, for the rational design of efficient PBRs. Further insight into the design of commercial PBRs for the mass cultivation of photosynthetic microorganisms is provided.
《2.Principal factors in PBR design》
2.Principal factors in PBR design
In the design of an efficient PBR, light penetration and distribution inside the reactor are the dominant factors. Moreover, good mixing and mass transfer as well as favorable temperature and pH can significantly improve the growth of microalgae. Lastly, capital and operating costs are crucial factors for a cost-effective PBR in the long run. Current progress in these aspects is described below.
《2.1. Light》
2.1. Light
Because solar radiation is plentiful and free, natural light is often employed for the mass cultivation of microalgae, resulting in a remarkable reduction of the cost. However, incident sunlight inevitably changes due to weather, the diurnal cycle, and the season. The photon flux density (PFD) of sunlight is over 2000 μmol·m–2·s–1 during the course of a sunny summer day in China; however, organisms that use oxygenic photosynthesis can only reach a theoretical maximum conversion efficiency of 8%–10% solar-to-biomass energy [16,32,42]. Moreover, the spectral quality of the light is an important factor in the cultivation of microalgae. Although sunlight covers a wide spectral range, only solar irradiation within the range of 400– 700 nm, which is roughly equivalent to the visible light spectrum, is photosynthetically active radiation (PAR). PAR accounts for about 50% of sunlight; the radiance falling outside of the PAR is the major reason for the temperature rise during cultivation. In addition, nonPAR light at certain frequencies (e.g., ultraviolet) is lethal for cells [13].
The light intensity distribution is crucially non-uniform inside a PBR because of absorption and scattering in the culture [43], and the attenuation of the radiation is dependent on the light wavelength, cell concentration, geometry of the PBR, and penetration distance of light. It is well known that the light intensity decreases almost exponentially with the distance away from the irradiated side of the PBR. In some cases, the light can only penetrate several millimeters when the density of the microalgae culture reaches more than 10 g·L–1 in the PBR [17]; hence, the microalgae cell growth rate significantly decreases as the PBR depth increases. If the light intensity is above a critical value and reaches the saturation level, the growth will be inhibited by the light (photoinhibition) and the light will be wasted, first as fluorescence and then as heat. On the other hand, if the light intensity is below the level that is necessary to balance the maintenance, the growth will be limited by the light (photolimitation) and the culture will collapse.
Based on the cell growth rate, the PBR can be successively divided into three distinct zones: first, the strong illumination zone, which has an inhibitory effect and extends from the illuminated wall to the point where the arriving light energy just balances the level of light energy needed for cell growth at the maximum rate; next, the weak illumination zone, which ends at the point where the light energy intake just meets the energy requirement for maintenance; and finally, the dark zone, where the cell growth rate is negative due to the limited availability of light [44,45]. This division becomes more complicated if photoinhibition is considered, because photoinhibition may bring about a decrease in growth rate near the light source [45]. Therefore, photosynthetic activity is higher than respiration when photoinhibition is not too strong in the strong illumination zone, such that net cell growth is produced; the photosynthetic rate decreases with an increase of irradiance. However, if the light intensity is higher than a critical value, a negative cell growth rate results in this zone. Therefore, the situation of light inhibition should be avoided as much as possible. Photoinhibition, which depends on light stress and on how long the microalgae are exposed to the stress, can be reversible or irreversible [13]. In the weak illumination zone, photosynthetic activity is higher than respiration, and a positive cell growth rate is observed; the cell growth rate increases with increased light absorption.
The light maintenance point (i.e., the irradiance below which photolimitation occurs) for the microalgae of Chlorella vulgaris is between 5 μE·m−2·s−1 and 10 μE·m−2·s−1, and the light saturation intensity (i.e., the irradiance above which photoinhibition occurs) is about 250 μE·m−2·s−1 [20]. It is noteworthy that the prevailing sunlight intensities are much higher than the desirable intensities for the saturation of photosynthesis under ambient conditions, leading to the dissipation and loss of excess absorbed energy [42]. However, some researchers experimentally found that weak continuous background light provided by a reflecting mirror at the back of the PBRs significantly favored the growth of microalgae [10,46]. Moreover, it was reported that a PBR aligned in a west/east orientation yielded a 1.4-fold higher biomass productivity than a PBR in a south/north orientation because the former received more solar radiation than the latter in the northern hemisphere [47–49].
To achieve high cell densities, the thickness of the PBR should be as small as possible [16]; hence, a thin optical thickness is widely applied in commercial PBRs [50]. For example, Degen et al. [20] found that a reduction in the light path from 30 mm to 15 mm increased biomass productivity by 2.5-fold in a flat-panel airlift PBR.
《2.2. Mixing》
2.2. Mixing
Mixing is a crucial feature in the cultivation of microalgae [51]; it can not only reduce the nutrients, pH, and temperature gradient in the culture broth but also prevent cell sedimentation, the emergence of dead zones, cell clumping, and cell attachment to the walls of the PBR [52]. In addition, mixing guarantees that all cells are equally exposed to the light and promotes mass transfer between phases. However, excessive mixing may produce cell damage and result in culture collapse, if the microalgae are susceptible to the shear force [53]. Posten [16] argued that a liquid velocity greater than 1 m·s–1 would produce micro eddies with diameters smaller than 50 μm, which could potentially damage the cells, and liquid velocities of 20–50 cm·s–1 were recommended.
The mixing time, tm, is a global index of mixing and is defined as the time needed for the injected tracer to obtain a prescribed uniformity that is quite close to a complete mixed state. In practice, mixing time is defined as the time required for the system to reach a state of homogeneity within ±5% deviation after the injection of the tracer [41,54]. The mixing time is affected not only by axial and radial mixing, but also by the bulk flow.
Mixing in the PBR is usually induced by aeration with CO2-enriched gas bubbles or pumping, mechanical agitation by impeller or static mixer, or a combination of these methods [13]. It is widely accepted that mixing, which brings about a favorable periodic light/dark cycle (L/D cycle, i.e., flashing-light effect) by shuttling microalgae cells between the photic zone and the dark zone, notably alters the radiation field in the reactor to promote photosynthetic conversion [55,56]. Since photosynthetic conversion is a dynamic process in microalgae cells, beneficial L/D cycles are widely thought to modify the growth rates of photosynthetic cells [2]. Therefore, mixing plays a significant role in ensuring light intensity redistribution in a PBR.
Periodic L/D cycles can be defined by the following parameters [57,58]: the flash time (tl, i.e., length of the flash), dark time (td, i.e., the dark period within an L/D cycle), cycle time (tc = tl + td, i.e., the duration of an L/D cycle), duty cycle (ϕ = tl/tc, i.e., the fraction illuminated in an L/D cycle), and flash frequency (v = 1/tc, i.e., the frequency of the cycle).
Abu-Ghosh et al. [59] observed that the flashing-light effect could enhance photosynthesis and improve the quality and quantity of the products. Iluz and Abu-Ghosh [46] found that biomass productivity increased more than 55% when the frequency of the L/ D cycles was set at 60 Hz. Degen et al. [20] discovered that the biomass yield of Chlorella vulgaris in a batch culture that was enhanced by the introduction of radial mixing was 1.7 times greater than the yield in a randomly mixed bubble column. However, it was also observed that the flashing light could decrease the production of microalgae when the L/D cycle or the frequency of the flashes was not optimized [40,58,60,61]. Kliphuis et al. [62] observed a small increase in productivity when the mixing rate was increased by 2-fold, and Zijffers et al. [63] discovered no clear improvement in cell productivity when the aeration rate increased.
The influence of the L/D cycle frequencies on cell growth is still controversial in the literature, and further research is required. For example, Huang et al. [64] proposed that a positive flashing-light effect occurred when the L/D cycle frequency was greater than 25 Hz or even 100 Hz. At a duty cycle of 0.5, Vejrazka et al. [61] observed that L/D cycles of below 10 Hz resulted in a 10% lower biomass yield on average, but L/D cycles at a frequency of 100 Hz resulted in a 35% higher biomass yield on average, in comparison with the lack of a dark zone. Posten [16] deemed that an L/D cycle frequency of mixing below 1 Hz should be avoided. However, Zhu et al. [65] found that cell growth was improved when the frequency of the L/D cycle was higher than 1 Hz, and Katsuda et al. [66] showed that 1.5-fold astaxanthin concentrations were produced in H. pluvialis at the low frequency of 1 Hz.
Moreover, the duty cycle is a vital factor in the efficient utilization of the L/D cycle. Wu and Merchuk [67] investigated the growth of Porphyridium sp. under three different PFDs (i.e., 110, 220, and 550 μmol·m−2·s−1), and studied the effect of L/D cycles by using a PBR partially covered with pieces of aluminum foil. The results indicated that dark periods were advantageous to cell growth when photoinhibition occurred and when the dark period was kept short, that is, down to a few seconds. Vejrazka et al. [58,60] and Grobbelaar [68] considered that a longer dark period could promote photosynthetic efficiency for the same light period under the condition of subsaturating or super-saturating light intensity with high L/D cycle frequencies, but not vice versa.
Why do these phenomena occur? In our opinion, cell productivity is determined by the interaction of the incident PFD, spectra of radiation, culture density, flash frequency, and duty cycle. The coupling among these factors should be investigated in more detail, and a deep knowledge of these mechanisms is desirable for the rational design and optimization of PBRs.
《2.3. Mass transfer》
2.3. Mass transfer
It is well known that the cell growth of microalgae involves three competing cellular processes: photosynthesis, photorespiration, and dark respiration [13]. The photosynthesis of microalgae makes use of light energy and CO2 to deliver oxygen (O2) as the byproduct. However, at high dissolved oxygen (DO) concentrations, ribulose-1,5-bisphosphate carboxylase oxygenase, which is the primary carboxylating enzyme that offers CO2 for the Calvin cycle, can also consume O2 to produce CO2 for photorespiration [13].
Furthermore, much of the DO that is released as the byproduct of photosynthesis is accumulated in the culture, which can lead to a high toxic level that can menace the survival of the microalgae [41,65]. Adverse conditions of high temperature and PFD combined with CO2 limitation can intensify the physiological inhibitory effects of DO [13]. It was reported that an accumulation of DO could give rise to severe inhibition of cell growth, especially in closed systems such as tubular PBRs, where the accumulated DO is removed only at the degasser through mass transfer. Since DO can not only oxidize one or more enzymes but also impact the electron transmission chain and suppress the photosynthesis process, the DO content in the culture should be limited to a certain range during the cultivation of microalgae. Chisti [69] proposed that the DO concentration should never exceed about 400% of the air saturation value in order to prevent such inhibitory effects, while Ugwu et al. [70] observed a strong decrease in cell productivity for C. sorokiniana when the DO concentration exceeded only 200% of the air saturation value (i.e., 448 μmol·L–1).
CO2 is usually used as the carbon source in the cultivation of microalgae in photoautotrophic cultures; it cannot be utilized directly, as the microalgae can only make use of it from the liquid phase. The CO2 supply can be a constraining factor if the dissolved CO2 concentration in the liquid phase is too low (e.g., when air is used as the feeding gas or when the length of a tubular PBR is too long) or if the mixing in the PBR is insufficient. Both Posten [16] and Acién Fernández et al. [53] argued that CO2 must be supplied at high enough concentrations to prevent limiting cell growth. Therefore, it has been recommended that the partial CO2 pressure should be higher than 0.2 kPa (i.e., 0.076 mol·m–3, which is equivalent to 3.3 mg·L–1). Since the partial CO2 pressure in the atmosphere is 0.04 kPa, pure air (i.e., with a CO2 content of 0.035% by volume) is insufficient for the CO2 supply, and a CO2-enriched gas mixture is required.
On the other hand, a high concentration of dissolved carbon dioxide (DCD) can lead to a low culture pH, which would also be inhibitory to some microalgae cells [13]. Therefore, maintaining the DCD level in the reasonable range is critically important, and the optimal DCD level depends on the nature of the species. CO2-air volume ratios of 1% and 4% were found to be the optimal concentrations for N. salina and Scenedesmus sp., respectively [71]. However, Chiu et al. [72] found that inhibition of Chlorella sp. growth occurred when high CO2 aeration (10%–15%) was applied. Zhu et al. [65] reported that the maximum photosynthetic efficiency was usually obtained when the CO2 concentration was in the range of 1%–5% by volume.
For the cultivation of microalgae, a dedicated space for gas exchange is usually designed in the PBR in order to maintain an optimal balance of DCD and DO. It is widely accepted that mixing is an effective method to enhance interphase mass transfer in a PBR.
《2.4. Temperature》
2.4. Temperature
The volume of a PBR is usually small because a thin optical thickness is applied for the sake of light transfer; hence, temperature rise is significant during the sunny days of summer. Temperature is a principal factor in the photocatalytic reaction [73] and has an impact on the chemical equilibrium of the species, the gas solubility, and the pH as well [4]. The optimal temperature for microalgae cultures is generally between 20 °C and 24 °C [45], and most microalgae can tolerate a water temperature between 16 °C and 35 °C [45,69,74]. Cell growth rates will decrease when the temperature is lower than 16 °C, and a temperature that is higher than 35 °C is lethal for some species [45]. A considerable variation in temperature will be experienced during commercial cultivation due to diurnal and seasonal behaviors. It has been illustrated that the temperature in a PBR may reach a level that is 10–30 °C higher than the ambient temperature in summer without a temperature controlling system [13]. Therefore, a cost-effective and reliable temperature-control system is essential in the PBR in order to keep the culture temperature within a favorable range.
In summer, popular methods of temperature control include using dark sheets for shading [75], spraying water on the illuminated surface of the PBR when the temperature of the culture exceeds a designated value [76], submerging part of the PBR or the entire culture in a water pool [13,77], overlapping tubes [39], regulating the temperature of the feed [39], or installing a heat exchanger for the PBR [78]. It should be noted that the temperature-control systems, which prevent microalgae death from an overdose of solar heating, are expensive components in the industrial system [79].
Shading the PBR is inefficient because it greatly reduces the utilization efficiency of the light. Water spraying is efficient for cooling but incurs a cost for pumping large amounts of water. As mentioned above, another commonly employed cooling method is the submersion of the PBR in a large pool of water; the reflection of the water pool has been demonstrated to promote the average light intensity in the culture [80].
《2.5. pH》
2.5. pH
Most microalgae species have a favorite pH range of 8.2–8.7, although they can also be cultivated in the pH range between 7 and 9 [81]. However, some species can tolerate adverse conditions in more acid or basic ranges [13]. It is crucial to keep the pH of the culture within the optimal value to prevent the culture from breaking down because of the destruction of cellular processes due to a detrimental pH.
In the cultivation of high-density microalgae with aeration from flue gases with a high CO2 content or from pure CO2, the CO2 is consumed by the microalgae during photosynthesis, increasing the pH of the medium [65]. As a result, the concentration of DCD may be a vital factor that influences the pH of the culture. Since CO2 solubility depends on the culture pH, the DCD is the consequence of the balance between the consumption of CO2 by the microalgae cells and the CO2 mass transfer from the gas phase to the liquid phase. Generally speaking, the solubility of CO2 in water is low: 1650 ppm at 25°C in pure water [82]. The addition of CO2 buffers the culture against pH variation as a result of the CO2 /HCO-3 /CO2-3 balance [83–85], and the influence of pH on the distribution coefficient of the components in the dissolution equilibrium of CO2 is significant.
Li [84] reported that the majority of the inorganic carbon is CO2 when the pH is below 4.5, while the proportions of the CO2 and bicarbonate (HCO3−) forms in the dissolution equilibrium of CO in fresh water are equal when the pH is about 6.5 at 25 °C. Nearly all the carbon is in the bicarbonate form when the pH is equal to 8.3. At that point, the ratio of the bicarbonate form decreases and becomes equal to that of the carbonate (CO32–) form when the pH reaches about 10.4. The bulk of the inorganic carbon is in the form of carbonate when the pH is above 12. Microalgae can quickly take full advantage of CO2 in the form of bicarbonate ions in the PBR, and some species are even able to utilize carbonate ions [82]. Therefore, CO2 availability becomes troublesome for microalgae growth at high pH levels [45,86]. In such a scenario, the pH should be brought under control during cultivation in order to intensify CO2 uptake by the microalgae [65]. For this reason, substances such as sodium bicarbonate are widely employed to control the culture pH and keep it from rising too fast.
《2.6. Capital cost and operating cost》
2.6. Capital cost and operating cost
From the economic point of view, an efficient commercial PBR should have the following features: a highly illuminated surface area/volume ratio to capture solar radiation, easy temperature control, good performance in mixing and mass transfer, low shear stress on the cells, and low capital cost and operating cost. In practice, the requirements of a high surface area/volume ratio, good performance in mixing and mass transfer, and low shear stress in the PBR can be easily satisfied; however, the temperature control and low capital cost and operating cost are difficult to ensure.
The first consideration in designing an efficient PBR with a high surface area/volume ratio is the capital cost, to which the illuminated surface area contributes substantially [16]. At present, glass, Plexiglas, polyvinylchloride (PVC), acrylic PVC, and polyethylene are the main materials applied to PBRs [13]. These materials all meet the transparency and mechanical robustness requirements for PBR fabrication. However, each material has its own pros and cons for the construction of particular types of PBRs. For example, glass is strong, transparent, and has a long life cycle; it is the perfect material for the construction of small PBRs. However, glass requires many expensive connecting parts when used for the fabrication of largescale PBRs, and leakage usually occurs when holes for aeration are present. The transparency of materials such as Plexiglas, PVC, acrylic PVC, and polyethylene decreases gradually as they oxidize in the air. It is recommended that the capital cost must fall below 40 €·m–2 for the economically feasible production of bioenergy from microalgae [16].
The second consideration is the operating cost, which includes the costs of auxiliary energy for mixing and gas transfer, temperature and pH controls, maintenance to prevent leakage, and cleaning. The mixing, mass transfer, and temperature-control systems that are required in commercial systems to prevent microalgae death due to excessive solar heating are expensive elements [79]. In addition, the mixing, mass transfer, and temperature-control systems take up most of the operating cost. Cleaning is critical for a PBR to prevent contamination [13] and cells clumping, which result in frequent reactor shut-downs for mechanical cleaning and sterilization [39]. Hence, the cost of cleaning is also high due to the rapid fouling of microalgae cells on the illuminated surfaces of the PBR.
Aeration rate is a significant parameter that affects the mixing, mass transfer, and L/D cycles inside the PBR. Gas enriched with CO2 is supplied to the culture to produce mixing (thereby reducing the pH and nutrients gradient, preventing cell sedimentation, and preventing the occurrence of dead zones, clumping, and fouling), mass transfer (thereby preventing CO2 deficiency, DO poisoning, and DCD inhibition), and an optimized L/D cycle. However, excessive aeration results in cell damage (by increasing the mechanical shear force) and a high aeration cost. The velocity of the slurry is a vital factor to prevent cell deposition and can also increase the frequency of favorable L/D cycles and the opportunity for contact with the reactant CO2. The velocity is chosen based on the settling velocity of the cells; a velocity of 0.1–0.3 m·s−1 was found to be effective in a PBR [65]. A higher velocity is also preferred but would consume too much energy. Moreover, although the L/D cycles induced by aeration are beneficial for cell growth, as mentioned earlier, no significant improvement has been observed because the intermediate frequency (0.01–1 Hz) particularly prevails in PBRs [64]. In experimental situations reported by two research groups [87,88], the maximum frequency of the L/D cycles in the PBR did not exceed 25 Hz, and the frequencies, which were below 50 Hz and 6 Hz in the plain tubes at 10 m·s−1 and 0.5 m·s−1, respectively, were also confirmed [88]. Finally, the cell movement in a PBR is chaotic [68], and the aeration amounts to about one-third of the cost for the largest scale systems [89]. Therefore, a high aeration rate is not practical for mass cultivation from an economic point of view. The optimum aeration rate by the mixture of air and CO2 for the production of microalgae in a PBR has been investigated extensively [41,65]. Air with a content of 5%–10% (v/v) CO2 at rates of 0.025–1 volume of air/medium/time (vvm) has been verified to be cost-effective for mass cultivation [45]. The conditions required for different styles of PBRs to reach the same mass transfer capacity have been compared: 2400–3200 W·m–3 for the tubular PBR, 40 W·m–3 in a bubble column, and 53 W·m–3 for the flat-plate PBR [90]. In a flat-panel airlift PBR, an optimum aeration of 8 W·m–3 has been proposed as sufficient to improve the mixing and mass transfer [41].
Although many types of PBR, including plastic light-conductive structures [13] and various types of static mixers, have been designed to increase the frequency of L/D cycles [64,91], the cleanability level is what determines the lifespan of a PBR. For example, mixers comprised of baffles or bars in the PBR have been developed to achieve rapid beneficial L/D cycles [64]. However, it is difficult to clean and periodically repair these PBRs because the baffles or bars are fabricated directly inside the reactor wall, which is usually less than 5 cm in width, for light penetration. Therefore, certain principles such as smoothing the internal surface, minimizing the amount and surface area of internals and bends, and having a large internal space, have been put forward to increase the cleanability of such PBRs [13].
To summarize, rational design of a PBR is crucial, and the capital cost, operating cost (including the cost of the auxiliary energy demand, cleaning, and maintenance), and lifespan of the PBR should be comprehensively considered regarding commercial exploitation. The best type of PBR should have a simple structure, easy operation and temperature control, low capital cost, low operating cost (including low energy consumption and convenient cleaning and maintenance), a long lifespan, and so forth.
《3.Advances in the design of PBRs》
3.Advances in the design of PBRs
To date, many new types of PBRs have been designed aside from the types mentioned earlier. However, some of these may be less robust than they appear due to low practicability resulting from cleaning and maintenance problems. Only a few types of PBRs can be used for mass cultivation, and the most promising PBRs, such as tubular PBRs, plastic bag PBRs, column airlift PBRs, and flat-panel airlift PBRs, are recommended here. Table 1 summarizes and lists the main advantages and disadvantages of these PBRs for comparison.
《Table 1》
Table 1 Characteristics of four promising PBRs.

《3.1. Tubular PBRs》
3.1. Tubular PBRs
The tubular reactor is one of the most appropriate and feasible configurations for outdoor mass cultivation because it has a relatively large available surface area for illumination. Glass or plastic tubes are usually used as the materials for construction [13,46,82]. These transparent tubes can be arranged in different patterns (e.g., straight, bent, or spiral) and orientations (e.g., horizontal, inclined, vertical, or helical) in order to maximize the sunlight capture, and the microalgae culture is circulated by a pump or by impetus resulting from aeration in the airlift systems. To improve land utilization, the tubes are usually arrayed in a horizontal fence-like structure, which increases the operation cost correspondingly [65].
The diameters of tubular PBRs are generally 10 mm to a maximum of 60 mm [16], and the length can be as long as several hundred meters. A small diameter of 10 mm or less may also be designed in some cases for the production of high cell concentrations. Liquid velocities ranging from 0.2 m·s−1 to 0.5 m·s−1 are usually adopted for mass cultivation [16].
Although tubular PBRs are popular [92], they still have many disadvantages. Photolimitation occurs very frequently in outdoor cultivations [13], especially for cell production in tubes with relatively large diameters. In such a scenario, the cells in the center part of the tube receive little light, and their growth is restricted. Furthermore, long tubular PBRs have some mass transfer problems; CO2 and O2 gradients and the pH between the medium inlet and the outlet should be carefully considered. Due to these mass transfer problems, there are usually high concentrations of DCD at the aeration zone and high concentrations of DO at the degasser zone [91]. A possible inhibiting concentration due to oxygen toxicity can occur after only 1 min in a tube without gas exchange [16]. Therefore, in theory, the length of the tubes should be kept as short as possible, depending on the potential O2 accumulation, CO2 depletion, and other factors such as convenient cleaning in practice. In addition, a high temperature rise in the culture may occur in summer because of limited volume resulting from the small diameters of tubular PBRs. Finally, cleaning the walls of tubular PBRs is an annoying problem that is closely connected to light permeability. At present, mechanical cleaning is the procedure most often used to do this washing [65].
Hence, photolimitation, O2 accumulation, CO2 depletion, high temperature, land requirement, and power consumption are the principal problems when using tubular PBRs for mass cultivation [82].
《3.2. Plastic bag PBRs》
3.2. Plastic bag PBRs
Plastic bag PBRs are attractive, and more attention has been paid to them in recent years for the commercial production of microalgae due to their low cost [13]. These bags can be installed with an aerator to promote cell yield. Fig. 1 shows an example of plastic bag PBRs. Plastic bag PBRs can be arranged in different patterns according to their respective volume. For example, a plastic bag PBR with a volume of 5 L was hung to produce C. sorokiniana microalgae on a large scale [93]. A pilot cultivation system, which included 20 bags (each bag being 20 cm in width and 2 m in length, and having 0.2 mm material thickness and a volume of 16 L) made from polyethylene and fixed on a metal stand, was shown to be a promising PBR for the cultivation of S. obliquus [94]. A flat-panel PBR that consists of a disposable 250 L plastic bag located between two iron frames has also been proposed [90]. Plastic bag PBRs with greater volumes can be immersed in a water pool to reduce cost and to control the temperature in summer. Plastic bag PBRs can even be designed to produce cells in the ocean; mixing and mass transfer are improved by the efficient utilization of ocean waves, which can reduce the cost substantially [95].
《Fig. 1》

Fig. 1. An example of plastic bag PBRs.
Although plastic bag PBRs have been favored by many researchers, they have many disadvantages. First, photolimitation commonly occurs due to distortion of the bags by gravity. Second, these PBRs can suffer from inadequate mixing, and cell growth is inhibited in some zones. Third, the bags are inherently fragile, so leakage happens more than occasionally. This circumstance can be disastrous for mass cultivation. Fourth, the lifespan of the bags is short because of the problems of cleaning and leakage, so they are not economical in the long run. Finally, the disposal of large quantities of plastic bags presents a potential problem [13].
《3.3 Column airlift PBRs》
3.3 Column airlift PBRs
Airlift PBRs have the substantial advantages of low-operational power consumption and less shear stress exerted on the cells. Furthermore, the airlift reactor has a defined circular fluid flow and relatively better gas-liquid mass transfer performance.
To obtain sufficient light, the diameter of the column of an airlift PBR should not exceed 0.2 m; otherwise, the light availability in the center of the PBR presents a severe problem [13,45]. In addition, the height of the cylinder is limited to about 4 m for structural reasons, due to the strength of the transparent materials employed and in order to reduce mutual shading in large commercial cultivations. In an airlift reactor, the column can be split with a centric tube or a flat plate in the diametrical direction. In the former case, the air can be aerated at the bottom of the annulus or in the center.
Column airlift PBRs are one of the most promising configurations for the industrial production of microalgae [96]. However, their high capital cost and cleaning problems are the main obstacles to their large-scale implementation [97].
《3.4. Flat-panel airlift PBRs》
3.4. Flat-panel airlift PBRs
A new flat-panel airlift PBR has been proposed and patented by Huang et al. [98]. We consider this PBR to be one of the most promising reactor types for mass cultivation. A schematic diagram of the reactor is illustrated in Fig. 2.
《Fig. 2》
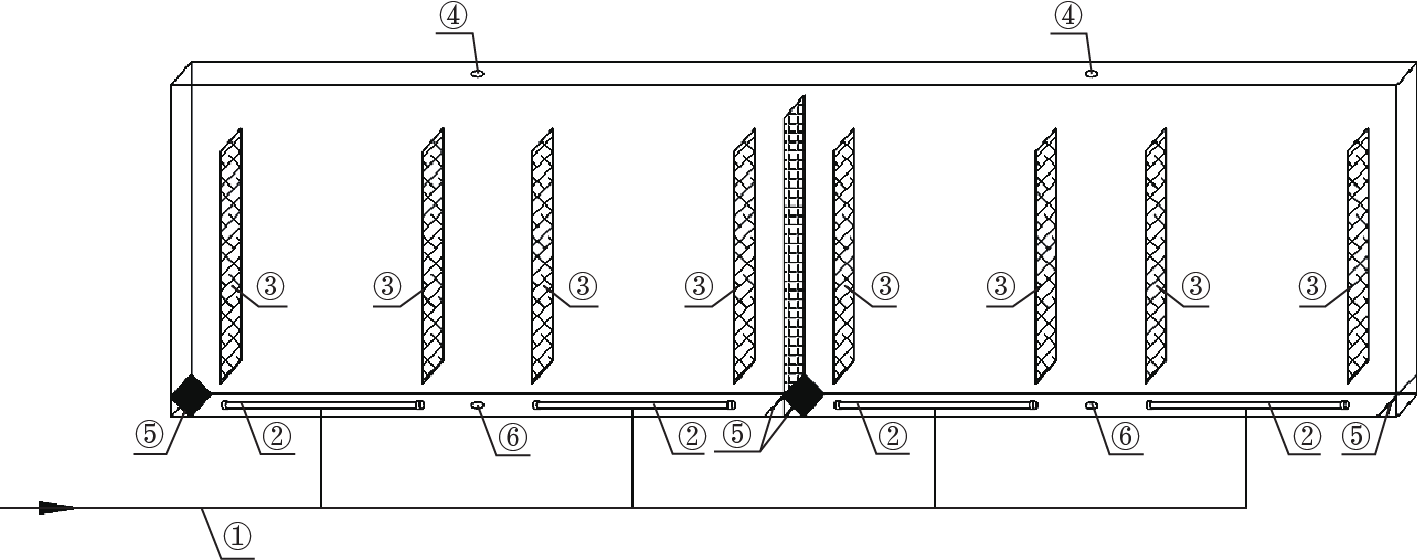
Fig. 2. Schematic diagram of a novel flat-panel airlift PBR [98]. ① Air supply tube, ② gas sparger, ③ baffle plate, ④ air hole, ⑤ dam board, and ⑥ slurry outlet.
This type of PBR combines the prominent advantages of the internal airlift loop reactor with those of the flat-panel PBR, and possesses many good features. First, from an economic point of view, it has a high ratio of illuminated surface-area-to-volume. Second, its temperature can easily be controlled by spraying water onto the irradiated surface of the PBR when the temperature of the culture exceeds a specified value [99], or by submerging the bottom of the PBR in a water pool in summer. Third, as an internal airlift loop reactor, it shows outstanding performance, including low mechanical force on the cells, low energy consumption [100], a high gas-liquid mass transfer rate, and good mixing induced by the well-defined circular flow. Fourth, this integrated PBR system contains four internal airlift loop reactors and forms two relatively independent groups in order to prevent complete culture collapse in the system when culture leakage occurs; it also has a high ratio of riser-to-downcomer surface area, with a volume as high as 200 L. Therefore, cell sedimentation, clumping, and fouling on the illuminated PBR walls can be totally avoided if the culture velocity is high enough, and the cost due to aeration is considerably reduced. Last but not least, this PBR has a long lifespan due to its cleanability and to its materials of concrete and glass; once built, it can be employed for mass cultivation over several decades. In summary, this novel type of PBR may be important for the mass cultivation of photosynthetic microorganisms in future.
It should be noted that although some novel designs have been developed for better performance [101], simple internals are highly recommended for this type of PBR due to the advantage of easy cleaning, which will result in a long lifespan.
《4.Advances in the modeling of PBRs》
4.Advances in the modeling of PBRs
Many transfer processes occur in PBRs, such as light transfer, interphase momentum, and mass and heat transfer; cell growth coupled with these physical fields further complicates the design of PBRs. Even though tremendous progress has been achieved in simulating the bubbly flow in PBRs, some researchers still consider the computational fluid dynamics (CFD) simulation of this process to be more of an “art” than a “science” because of premature models related to the turbulence and interphase momentum exchange [45,102]. However, CFD has proven to be an effective tool for predicting the inherently complex phenomena that occur inside a PBR, and is very reliable for successfully designing and developing commercial reactors.
《4.1. Light-transfer modeling》
4.1. Light-transfer modeling
It is widely accepted that radiation is the leading limiting factor governing cell growth inside a PBR, and that the light penetrating a culture medium can either be absorbed or scattered [68]. Since radiation depends on the spatial position and angular direction, the traditional light radiative transfer equation (RTE) for an absorbing and scattering medium at any position r in any direction s can be written as follows[18]:

where r, s, and s′ are the position vector, direction vector, and scattering direction vector, respectively; s, a, σs, and I are the path length, absorption coefficient, scattering coefficient, and light intensity, respectively; and Ф and Ω′ are the phase function and solid angle, respectively. In the above equation, the first term on the right-hand side accounts for the loss of photons due to light absorption, and the second term represents the loss of radiation due to light out-scattering by the bubbles and the cells. The last term indicates the profit of the radiation induced by light in-scattering, and the fraction of the radiation intensity, I (r, s′ ), in the direction s′ scattered in direction s is determined by the phase function, Ф (s·s′ ). Therefore, the total incident intensity at any point considering all directions can be computed by the following equation:
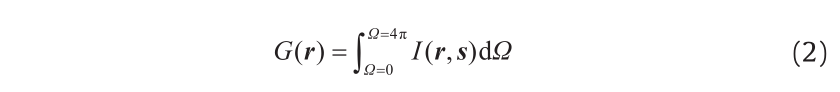
An exact analytical solution of the RTE can only be obtained for an ideal one-dimensional situation, due to its form as an integraldifferential equation [103,104]; hence, solving the RTE is not a trivial matter. Numerous analytical and empirical models have been put forward over the years to estimate the radiation distribution inside PBRs. Some researchers have ignored light scattering; hence, a simplified Beer-Lambert law has been widely applied [57,105–112]. In addition, the two-flux method that considers the absorption and scattering of one-dimensional quasi-collimated radiation was also developed [5,64,113]. During the past few decades, many numerical methods have been proposed in order to obtain a rigorous solution to the RTE, including the Monte Carlo method [114,115], the discrete ordinates method [102,116], and the finite volume method (FVM) [18,117–119]. Of these, the FVM has been popular due to favorable features such as the conservation of radiant energy and its easy incorporation into CFD simulations; this method has been developed and verified against experimental data by Huang et al. [98].
The radiative properties, such as the absorption and scattering coefficients, can be estimated using the generalized Lorenz-Mie theory with anomalous diffraction approximation [120]. Wheaton and Krishnamoorthy [102] argued that the effect of scattering by the bubbles and the cells decreased at higher cell concentrations, at which absorption was the predominant factor in the culture.
It should be noted that more attention should be paid to the specific absorption coefficient, even for the same species. Vejrazka et al. [58] reported that the average dry weight specific absorption coefficient was independent of the L/D cycles but was related to the light intensity. The absorption coefficient of Chlamydomonas reinhardtii under low radiation (100 μmol·m–2·s–1) was found to be 0.233 m2·g–1, while this value decreased to nearly half, that is, 0.127 m2·g–1, under high light illumination (500 μmol·m–2·s–1). This finding is in accordance with the results of Takache et al. [5], who reported that gradual reductions in pigmentation and corresponding reductions in the absorption coefficient were observed with the increase of the PFD for the same microalgae. Moreover, although photosynthesis works in the full range of irradiation wavelengths of the PAR, and the absorption and scattering coefficients are spectral dependent, the solution to the RTE for a PBR is currently confined to pseudo-monochromatic radiation, and averaged radiation source and radiative properties are employed in the computation in order to reduce the huge computational cost. Since the culture extinction coefficient, which is the sum of the absorption and scattering coefficients, is wavelength dependent, as shown in Fig. 3, the importance of carrying out a wavelengthdependent polychromatic radiation calculation has recently been recognized [63,102,121].
《Fig. 3》
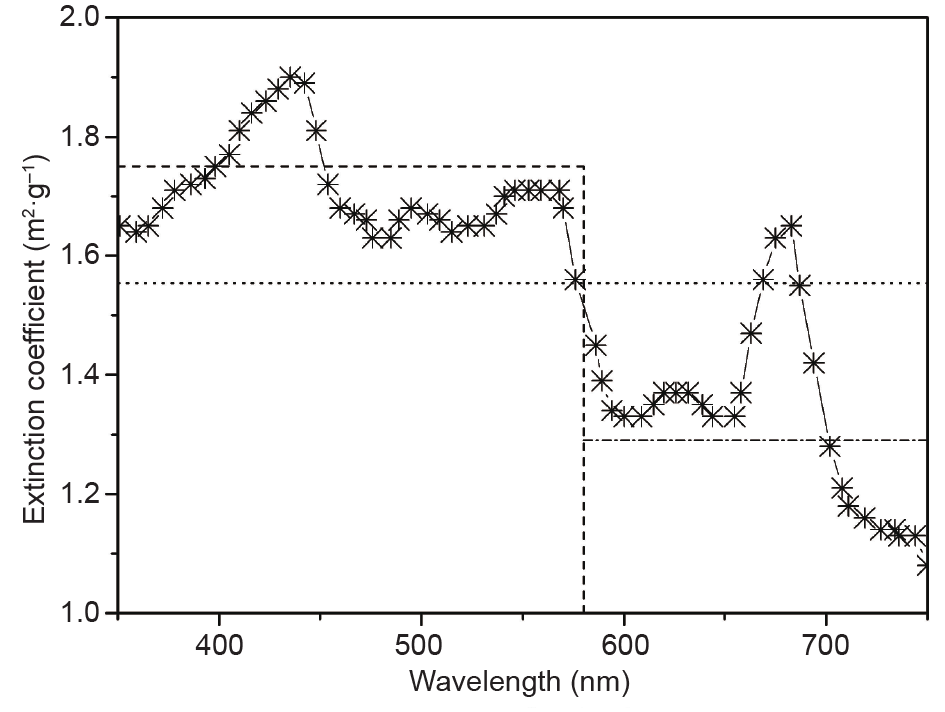
Fig. 3. The wavelength-dependent specific extinction coefficients of P. cruentum with the box model applied [121].
For polychromatic radiation, integration must be applied over the whole wavelength (frequency) range of the PAR; the total irradiance at any local point can be computed as follows [113,122]:

where λmin and λmax are the minimum and maximum PAR wavelengths of the light source, respectively; and Gλ(r) is the total incident intensity at the wavelength λ.
The accurate simulation of solar radiative transport inside a PBR is computationally challenging due to the spatial, directional, and spectral dependencies of radiation characteristics [102]. Based on the success of Berberoglu et al. [122] with one-dimensional steady light transfer, Huang et al. [121] have developed a box model to predict polychromatic radiation in a PBR, especially for a complex structure with twoor three-dimensional geometry. A detailed description of this method can be found in Ref. [121]; only the major features are presented here for the sake of brevity. In this method, the PAR is split up into several sections. The spectral quantities of the radiation are approximated by using a series of boxes [122,123], where the area under the spectrum is defined as equivalent to the quantity of area under the box [121]. Fig. 3 illustrates the extinction coefficients of P. cruentum using the box model. If only one box is applied, this is equivalent to the circumstance of monochromatic radiation. However, the number of the boxes should be determined according to both the characteristics of the source spectra and the spectral profile of the radiation characteristics. In Fig. 3, two boxes with the wavelength intervals 350–580 nm and 580–750 nm are employed. The box model is thought to be a reliable approximation of polychromatic radiation properties.
《4.2. Coupling the radiative transfer model with the Lagrangian approach》
4.2. Coupling the radiative transfer model with the Lagrangian approach
It is well known that photosynthetic conversion is a dynamic process, and the fluctuating history of cells induced by the culture flow can modify the instantaneous conversion rate and efficiency of the absorbed light [124]. In other words, the fluid flow alters the radiation spatial distribution. The distributions of the fluid flow [125–127], radiation [18,121], and cell growth [128] in a PBR can easily be obtained with the solutions of the momentum, mass transport, and radiative transfer equations along with a calculation of the photocatalysis. However, optimizing light utilization by improving the hydrodynamic conditions demands a deep knowledge of the light history that is experienced by the cells and of the global photosynthetic response to the flow history.
The coupling between radiation and cell flow history is predictable in theory. Eulerian formulation is commonly adopted for the prediction of hydrodynamics, and the history experienced by the cells can be obtained by statistical particle tracking, that is, by a Lagrangian discrete phase model. The mean intensity of the available light is defined by averaging the instantaneous irradiance that is experienced by each flowing cell in successive locations. This method, in which the cell trajectories and light absorption are directly coupled spatially and temporally, has been applied by many researchers, and great progress has been made [88,129–131]. The actual L/D cycles inside the reactor can be distinctly determined using this method. After ignoring the influence of shear stress, Pruvost et al. [124] found that although the dynamic coupling between L/D cycles and photosynthetic conversion does affect cell growth, it is not possible to promote the PBR’s performance just by considering the linear coupling between the cell trajectories’ history and radiation field. Vejrazka et al. [60] also discovered that the photosynthetic efficiency under beneficial L/D cycles with a frequency of more than 1 Hz can be portrayed by partial or full light integration, and that full light integration under lower light intensities gave rise to a higher photosynthetic efficiency. In summary, although significant information related to the L/D cycles can be provided by coupling the radiative transfer and the Lagrangian hydrodynamics model, modeling cell growth inside the PBR with this method still appears to be somewhat unrealistic.
《4.3. Coupling the radiative transfer and growth model with the Euler approach》
4.3. Coupling the radiative transfer and growth model with the Euler approach
Cell growth in a culture is related to the light intensity, mixing, shear stress, mass/heat transfer, and so forth. A deep quantitative understanding of the impact of the mixing-induced L/D cycles on biomass production is essential; however, the research in this aspect is encountering a bottleneck. Mature models of this aspect are not available; in particular, the growth model lacks a consideration of important environmental factors such as the light history the cells are exposed to, photoinhibition, photolimitation, global shear stress, temperature, pH, mass transfer, and nutrients. This lack explains why no ideal type of PBR has been developed to date, and why only a few examples dedicated to this topic and using semi-empirical models have been carried out.
Muller-Feuga et al. [132] successfully investigated microalgae production with an integrated model that combined the simple monochromatic radiation Beer-Lambert law, the mean bulk flow, and the photosynthetic growth model considering photoinhibition, photolimitation, and the L/D cycles, in a batch and continuous tubular PBR, respectively. Huang et al. [121] developed this comprehensive model by taking into account the polychromatic radiation with the aforementioned boxes model, and the complete solution for the radiation was resolved by a validated FVM. The importance of spectradependent radiation simulations was thus well illustrated in that study. The sensitivities of the box number and the L/D cycles on the global growth of the culture were discussed, and the corresponding results of the batch and the continuous cultivations are shown in Fig. 4 and Fig. 5, respectively. Their results [121] well captured the spatial and temporal evolution of the light intensity inside the PBR during cultivation, and they provided guidelines to maximize cell productivity in terms of the light transport inside the PBR, in order to maintain as much uniform distribution of light intensity as possible in space and time.
《Fig. 4》
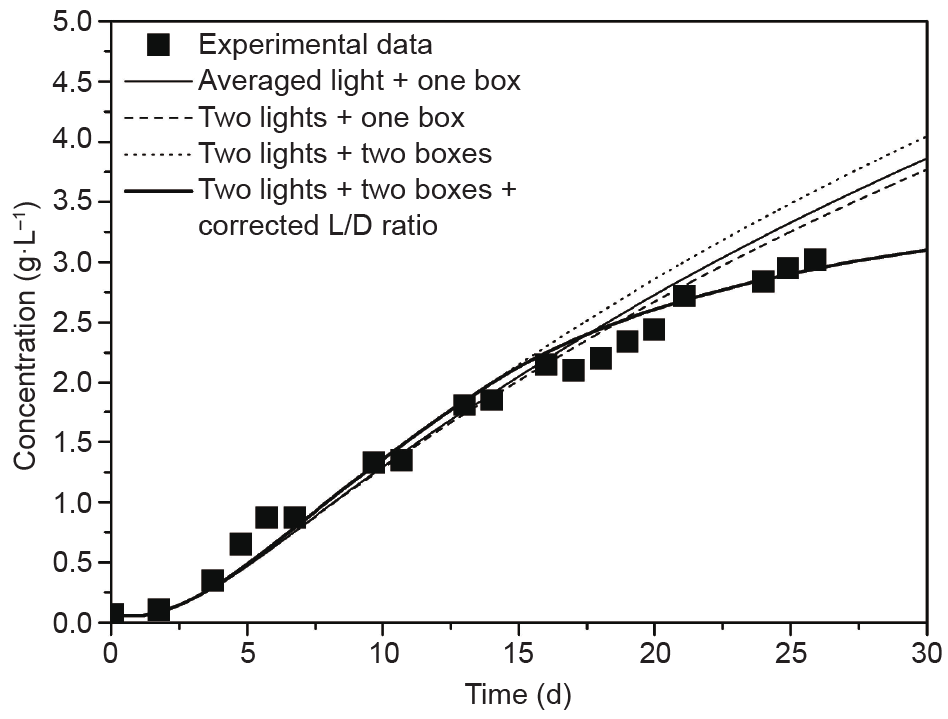
Fig. 4. Sensitivity of the radiative model on the results for a batch culture in the Euler frame [121].
《Fig. 5》
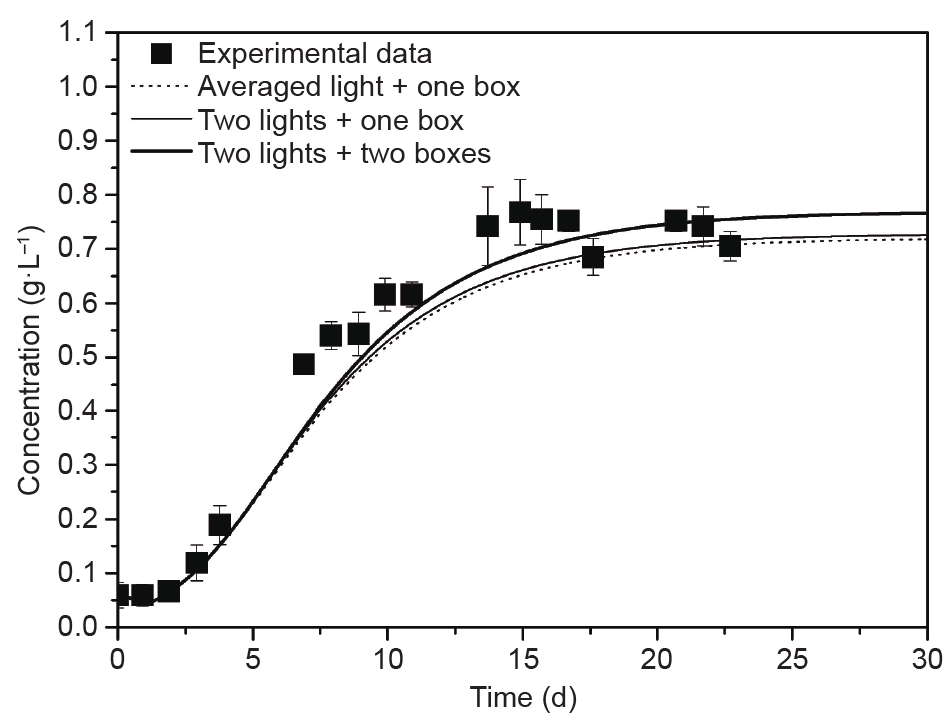
Fig. 5. Sensitivity of the radiative model on the results for a continuous culture in the Euler frame [121].
Eilers and Peeters [133] developed a dynamic model that took into account the photosaturation, photoinhibition, and global shear force inside the PBR, and used it to describe the photosynthetic response by combining the first principle of physiology with the hydrodynamics. An innovative feature of this model is that it describes photosynthesis based on the concept of photosynthetic units, which are also referred to as “photosynthetic factories” (PSFs), and which release electrons and finally produce oxygen depending on the incident PFD [4,57,133]. The rate of photosynthesis is deemed to be proportional to the light intensity received because photosynthesis is confined by the capture rate of photons at low light irradiance. When the irradiance increases to the critical value, microorganisms become “light saturated” due to their limited capability of usage. If the irradiance increases beyond the inhibitory level, the photosynthesis rate decreases sharply.
If the nutrients in the culture are excessive, light is the dominating factor affecting photosynthesis. This model regards the PSF as the sum of a light-trapping system that comprises reaction centers and associated apparatus; the system can be turned on by a certain amount of light energy to generate a particular amount of photoproduct [105]. Three states—the resting state (open, designated as x1), active state (closed, designated as x2), and inhibited state (designated as x3)—are defined in the PSF model. Fig. 6 shows the scheme of the PSF model.
《Fig. 6》
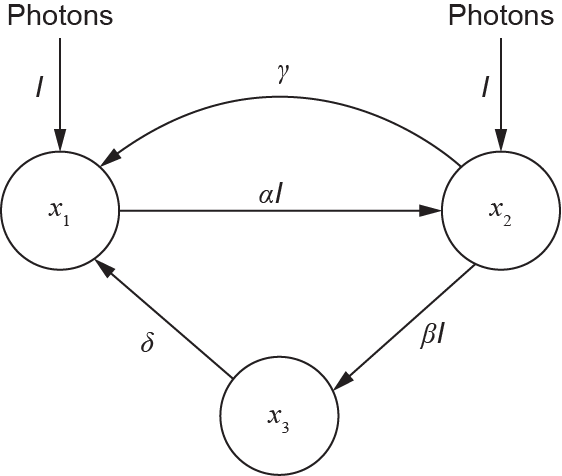
Fig. 6. The scheme of the PSF model [133].
At the start of the PSF process, a microalga is in the open state, x1,and is ready to catch a photon. The biochemical reaction starts once the photon is captured, and the microalga transits into the activated state, x2. The reaction rate is believed to be of the first order with the PFD. In the activated state, x2, the microalga can release the captured photon and go back to the open state, x1, in the dark zones, or it can catch hold of another photon and then pass on to the inhibited state, x3. The rates of these two reactions are assumed to be of zero order, and they can be determined by the rate constants of production γ and of inhibition β, respectively. Moreover, in the inhibited state, the microalga can also return to the open state, x1, with a rate constant of recovery, δ. The kinetic equations can be written as follows:

where α, β, γ, δ, and k are the rate constants; μ is the specific growth rate; τ0 is the maintenance term; and t and I denote the time and light intensity, respectively.
The mechanism-based PSF model described above takes into account the photoacclimation dynamics, the responses of overdose irradiation and dark respiration in the photolimitation zones, and the shear exerted on the cells. This model is thus a milestone in developing science-based methods for the design of commercial PBRs [57]. Great progress has been made using this model in a bench-scale bubble column PBR and in an internal loop airlift PBR [105,106]. Besides the effects of the shear stress, photoinhibition, and photolimitation, when using the PSF model, the impact of the flow history on cell growth—that is, the influence of the L/D cycles on cell production—can be well depicted by a Lagrangian approach. However, there is still a critical deficit in this model because it neglects the contribution of the culture flow to the three states when a steady Euler model is employed, and it cannot be integrated into CFD models for the prediction of global cell growth. We have recently successfully resolved this problem; the results will be elaborated in our future work.
《5.Conclusions and perspective》
5.Conclusions and perspective
PBRs are considered to be the most important devices for the large-scale production of a target product using environmentally benign photosynthetic microorganisms. As such, they play a decisive role in the green chemistry industries. Although significant achievements have been made, most PBRs are still designed semi-empirically. The growth of photosynthetic microorganisms is a complex process that is affected by many environmental factors such as light, mixing, mass transfer, temperature, and pH, if sufficient nutrients are present. However, the operating costs, which include temperature controlling, periodic cleaning, and maintenance, are commonly underestimated, and the lifespan of new PBRs is ignored by many researchers. Trade-offs among PBR efficiency, capital and operating costs, and lifespan should be carefully made. Four types of PBRs, including tubular PBRs, plastic bag PBRs, column airlift PBRs, and flat-panel airlift PBRs, are recommended here for commercial exploitation due to their unique features.
To date, no perfect type of PBR exists for the mass cultivation of microalgae, due to practical difficulties (i.e., capital cost, operating cost, and lifespan), and to a limited knowledge of the effects of light distribution on cell growth (i.e., polychromatic radiation transfer, photoinhibition and photolimitation, and beneficial L/D cycles induced by mixing) and environmental conditions (i.e., the shear force experienced by cells, nutrients, pH, temperature, deficiency in DCD, and toxic levels of DCD and DO). In addition, the strong coupling among hydrodynamics, polychromatic radiation, and cell growth further complicates PBR design, even if the cells are cultured under absolutely ideal conditions, and the solutions for modeling these issues are still acquired independently. Therefore, it is urgent to extend the mechanism-based growth kinetic model, which can easily be combined with the Euler approach model to describe the L/D cycles, for the rational design of PBRs. Regarding the innovative PSF model, building a bridge for communication between the cell growth model and the hydrodynamic model in the Euler framework is essential for the future.
It is noteworthy that a new technique called the porous substrate bioreactor (PSBR), which produces cells that are immobilized in dense biofilm culture, and which has the advantages of solving many volume-related problems, has been developed in recent years [134]. Although simple construction and low-energy demands indicate that this PSBR technology is promising, it is still being demonstrated at a small pilot scale due to problems that have been encountered, including detachment of cells from the biofilm surface, contamination, large quantities of water consumption via evaporation, and so forth. It is not possible for us to assess whether suspended cultivation in the PBR or immobilized cultivation in the PSBR are preferable, because this depends on technical progress and on the market.
《Acknowledgements》
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFB0301701); the National Natural Science Foundation of China (91434114, 21376254); the Major National Scientific Instrument Development Project (21427814); the Instrument Developing Project of the Chinese Academy of Sciences (YZ201641); the International Partnership Program for Creative Research Teams, Chinese Academy of Sciences, and the Supercomputing Center of USTC (University of Science and Technology of China).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Qingshan Huang, Fuhua Jiang, Lianzhou Wang, and Chao Yang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




