《1.Introduction》
1.Introduction
Carbon dioxide (CO2) flooding is one of the most widely used enhanced oil recovery(EOR)methods. In the United States, CO2 EOR has recovered over 1.5 billion barrels of oil, and estimates of the amount of oil that is recoverable by CO2 EOR range from 47 billion to 137 billion barrels[1–3]. Even though CO2 flooding is an attractive EOR method by itself, it can be enhanced using additives that improve the ability of the CO2 to displace trapped oil. Under the conditions of a typical oil reservoir, the viscosity of CO2 can be 10–50 times lower than that of the oil, which makes CO2 likely to channel through the oil and preferentially flow through more permeable rock sections[4]. This problem can be alleviated by adding surfactants to generate in situ CO2 foams[2–10]. These foams have higher viscosity than each of their components, and their viscosity increases with increasing pore diameter, making them less likely to preferentially flow through more permeable rock sections. The result is a more uniform front of foam that pushes oil rather than fingering through the reservoir’s most open geological formations.
Ideally,using CO2 foams for EOR would be a nontoxic method of carbon sequestration that would reduce industrial contributions to global warming. This process meets the criteria established by the US Environmental Protection Agency (EPA) for a process to be considered "green chemistry”[11]. The use of CO2 foams would decrease our impact on global climate change by trapping carbon in the depleting reservoir, thus reducing greenhouse gas emissions. Incorporating CO2 EOR into carbon capture and sequestration systems results in lower environmental impact and higher thermodynamic efficiency compared with schemes that do not use it[12]. Furthermore,experiments have shown that CO2 foams are most effective at low pressures[13], thus reducing compression costs and improving the thermodynamic efficiency of the process.
Given the appeal of CO2 EOR as an economically feasible method of carbon sequestration, there have been numerous recent studies on the physics and economics of the process[14,15] as well as research on novel foaming agents such as CO2 soluble surfactants, polymer-coated nanoparticles, and surfactant/ nanoparticle blends[15,16]. Although much progress has been made in improving the stability and performance of CO2 foams for EOR, the rapid development of foaming agents raises the question of their environmental safety. Most studies on the potential environmental impacts of carbon sequestration via CO2 EOR focus on CO2 leakage; far fewer studies consider the potential impact of the leakage of foaming agents. Considering the large amounts of surfactant injected into the ground in commercial CO2 EOR operations typically hundreds to thousands of tons of surfactant[17] this impact could be substantial.In this paper, we explain why the environmental impact of foaming agents warrants concern and highlight the recent advances in developing stable CO2 foams for EOR, with an emphasis on their potential environmental effects. We will show how new technology has enabled future research to revisit the necessity of using harsh anionic surfactants.
The rest of this work is organized as follows: Sections2 and 3 outline the most recent developments in surfactant and surfactant/ nanoparticle-based CO2 foam systems. Section 4 discusses recent studies on the potential environmental impacts of CO2 leakage,and Section 5 considers the potential impact of leakage of the foaming agents.Finally, we offer some closing remarks,including suggestions for future research.
《2.Advances in surfactant CO2 foam systems》
2.Advances in surfactant CO2 foam systems
There are several CO2 flooding processes that rely on surfactants to produce foams[2,4–10]. Surfactant/ polymer (SP) flooding involves in situ foam creation as the surfactant interacts with the brine and oil present in the reservoir, while the added polymer improves the mobility of the displacing phase. Foam flooding involves injecting a foamed gas into the reservoir to displace the oil and to improve the mobility of the oil as the gas dissolves in it. Water alternating gas (WAG) flooding involves the alternate injection of CO2 with slugs of soapy brine containing ionic surfactants, leading to in situ foam generation. The majority of studies and field tests for surfactant-enhanced CO2 EOR have used the WAG process.
Several improvements to the WAG process have been proposed, including the foam-assisted WAG(FAWAG) process, which integrates foam flooding and WAG flooding[10], and the chemically augmented WAG process, which combines WAG with the injection of alkali/ surfactant/ polymer(ASP) mixtures[18]. Research on these improved methods has found success in utilizing amine-functionalized CO2 switchable chemicals such as Nerucamidopropyl-N, N-dimethylamine[18,19] and N, N, N',N'-tetra methyl-1, 3-propanediamine[20]. Upon contact with CO2, these chemicals react with the dissolved carbonic acid, leading to the formation of foams and gel-like structures with enhanced sweeping efficiency. N-erucamidopropyl-N,N-dimethylamine has been shown to provide good mobility control and foam performance under harsh conditions[18].
More recently,there has been increased interest in the development of CO2-philic surfactants,whether by means of functional groups or by modifying the tail group topology.Using CO2-soluble surfactants has several advantages[2,4,21]. First, it ensures that the surfactant is available for foam generation right where the CO2 flows. Second, it eliminates the need to inject water (brine is usually present in the reservoir from prior water flooding). Third, it makes the surfactant less likely to be lost due to adsorption on rock surfaces or trapping in "thief zones,”thus reducing the amount of surfactant required.
In a recent study, Sagir et al.[21] examined the performance of a synthetic CO2-philic surfactant, nonylphenol ethoxylate sulfonate(NPES), using betaine as a foam booster. The study found that NPES lowers the surface tension of the CO2/ brine interface from 30 to 5.2 mN·m-1 and reduces the mobility of the CO2 by a factor of three, making it a very promising surfactant for CO2 EOR. However, the study did not consider the potential environmental impact of the surfactant. Another recent study by Talebian et al.[22] tested blends of three newly developed surfactants,FomaxII, FomaxVII, and UTP-Foam, which also contain CO2-philic groups, as foaming agents in surfactant alternating gas(SAG)flooding. The study found that surfactants containing bulky, branched tail groups resulted in more stable foams, and that CO2-philic surfactants with higher activity at the gas/water interface resulted in improved stability in the presence of oil. The results of these studies support prior findings that surfactants with bulkier, branched tail groups result in improved stability of the CO2/brine interface[8]. More recent molecular simulation studies have also found that branching increases the effectiveness of the surfactant, although there is a complex relationship between the tail group architecture and the surfactant performance.
Alkalis are often used as additives in WAG flooding. Recent work by Farzaneh and Sohrabi[23] studied the stability of CO2 foams in the presence of crude oil using eight different surfactants and three different alkaline additives(sodium hydroxide, sodium carbonate, and sodium borate). The study included one nonionic alcohol ethoxylate surfactant (Neodol 25-7TM) and seven anionic surfactants:four from the sodium olefin sulfonate family (Pet-rostep C1TM,C2TM,and S2TM,and Bioterge AS-40TM), two ammonium alkyl ether sulfate surfactants (Rhodapex CD-128TM and Alpha Foa-mer®), and a proprietary surfactant (XP-0010TM). All the anionic surfactants resulted in more stable foams than the nonionic surfactant, with XP-0010TM resulting in the most stable and oil-resistant foam.Of the alkalis, sodium hydroxide was found to decrease foam stability. Sodium carbonate and borate resulted in more stable foams, with the borate outperforming the carbonate. In all cases, the study observed that there is an optimal concentration of alkali and surfactant that produces the most stable foams.
Polymer additives are another method of improving foaming properties. A recent study found that combining foam flooding and SP flooding significantly increases the oil recovery in CO2 EOR, particularly at higher reservoir pressures[24]. The study used a mixture of sodium alpha olefin sulfate(AOS), the foaming agent N70K-T, and the thickener AVS (consisting of a novel terpolymer of acrylamide, 2-acrylamido-2-methylpropane sulfonic acid (AMPS), and an additional (variable) monomer[25]). Other recent studies have tested mixtures of sodium dodecyl benzene sulfonate (SDBS) with partially hydrolyzed polyacrylamide (HPAM)[26], and of sodium dodecyl sulfate (SDS) with a hydrophobic modified water-soluble polymer, alkyl acrylate cross polymer(HMPAA)[27]. The latter system led to ultra-stable foams due to the forma-ion of an HMPAA hydrophobic network on the CO2/water interface.
Several recent studies have also considered foams prepared with the nonionic surfactant Triton X-100TM (TX-100), an octylphenol ethoxylate with 9–10 ethylene oxide units[25,28–30], as well as nonylphenol polyethoxylates(NPs)[31]. One flooding study sought to improve sweep efficiency by blending surfactant concentrations at reservoir conditions[28]. The study tested three surfactants: pure AOS, a blend of equal parts of AOS and lauramidopropyl amine oxide (LMDO), and a blend of equal parts of AOS and TX-100. The study found significant enhancements in oil recovery in all cases,with the AOS/TX-100 blend resulting in the highest recovery.
A study by AttarHamed and Zoveidavianpoor[29] in 2014 also tested mixtures of AOS and TX-100 at different concentrations, and found that a 4:1 mixture produced better oamability and stability than other mixtures or the surfactants alone. A more recent study by Xu et al.[25] tested several mixtures of surfactants, including TX-100 , alkyl polyglycoside (APG), SDS ,and AOS with the additives N70K-T, triethanolamine(TEA),AVS, and HPAM. The study found that the AOS/AVS/N70K-T mixture showed superior performance in terms of foamability, foam stability, relative modification ability, and amount of oil recovered.
From the studies discussed so far, it is clear that anionic surfactants are generally superior to nonionic options and more stable in brine for CO2 EOR, although adding the nonionic surfactant TX-100 to an anionic formulation can result in improved foam properties. The results also suggest that modifying the chemistry and architecture of the surfactant tail can be an effective way to improve foam-ing performance. However, these studies mostly focus on the properties of the resulting foams and not on the potential toxicity or environmental impact of the formulations (see Section 5). The use of biosurfactants would be a greener alternative[32]. In addition to discovering that the foaming performance and thermal stability of nonylphenol polyethoxylate surfactants increased with the number of ethylene oxide units, recent work by Wang et al.[31] found that APG-1214, a biodegradable polyglycoside nonionic surfactant, is a very effective CO2 foaming agent.In their study, the addition of APG-1214 produced a foam that outperformed all the individual NP and anionic surfactant systems alone. The authors attributed this result to the presence of negative charges on the APG micelles, due to excess adsorption of OH-at the interface. This results in a higher effective hydrophilicity of the surfactant, leading to higher foaming capability. Another recent study found that poly-oxyethylene sorbitan monooleate (Tween 80), an inexpensive non-toxic emulsifying agent commonly used in food and cosmetics, can be used in combination with small amounts of SDS (10:1 weight ratio of Tween 80 to SDS) to produce stable CO2 foams suitable for injection in hydrate reservoirs[33]. These studies suggest that synthetic and caustic additives could be replaced by greener alternatives in CO2 EOR operations in order to reduce the impact of such operations on the environment.
《3.Advances in surfactant/nanoparticle CO2 foam systems》
3.Advances in surfactant/nanoparticle CO2 foam systems
Most of the recent research in the field of CO2 EOR has focused on the use of nanoparticles as foaming additives. Emulsions, including solid nanoparticles, often exhibit higher long-term stability, and can be designed to be biocompatible and environmentally friendly[34,35]. In this section, we discuss studies from the last five years on the use of surfactant/nanoparticle foams for CO2 EOR, with their environmental impact in mind.A recent general review of the uses of nanoparticles in various EOR applications has been published by Sun et al.[36].
A recent study by Yekeen et al.[37] showed that the synergy of SDS with either alumina (Al2O3) or silica(SiO2) nanoparticles resulted in smaller bubbles, a longer half-life,and greater viscosity. The authors attributed these properties to the accumulation of nanoparticles in the foam lamellae and plateau borders,which led to increased film thickness and elasticity. The positioning of the nanoparticles prevents liquid drainage, film thinning,and bubble coalescence. This analysis was supported by similar recent studies on foams that were stabilized by polyelectrolyte complex nanoparticles with the nonylphenol ethoxylate surfactant Surfonic N120TM[38], and by studies that combined SiO2 nanoparticles with bis (2-ethylhexyl) sulfosuccinate (AOT) [39], SDS[40], AOS[41,42], cetyl trimethylammonium bromide (CTAB)[43], and ethyl hexadecyl dimethylammonium bromide[44]. A separate study confirmed the improvement in the properties of foams formulated with AOS and mixtures of AOS with guar gum and viscoelastic surfactants (VESs) when nanoparticles are added[45].
Silica nanoparticles are the most commonly studied nanoparticles in the recent literature. Silica is an abundant natural material;for this reason, it is generally assumed that silica nanoparticles are an environmentally friendly additive, even though it is unclear whether this is true (see Section 5). Furthermore, silica nanoparticles often outperform other additives. A few recent studies have compared silica with other materials. Emrani and Nasr-El-Din[46] recently compared the foaming properties of AOS/guar gum solutions containing SiO2 and iron(III) oxide(Fe2O3) nanoparticles, and found that silica nanoparticles resulted in higher stability. They attributed the poorer performance of Fe2O3 nanoparticles to their tendency to aggregate due to their high surface energy. Another study by Manan et al.[47] compared the performance of SiO2, Al2O3, copper (II) oxide (CuO), and titanium dioxide (TiO2) nanoparticles as additives to AOS. Their study found that alumina nanoparticles resulted in more stable foams that recovered the highest amount of oil after water flooding,with silica nanoparticles being a close second.
One disadvantage of silica nanoparticles is that they have a negatively charged surface,which precludes the tuning of their hydrophobicity [48–50]. In order to control hydrophobicity,a chosen surfactant should have a charge that is opposite to that of the nanoparticle. The negative charge of the silica nanoparticles means that cationic surfactants should be used for this purpose; however, these surfactants are easily trapped in the negatively charged rock surfaces [49]. For this reason, other materials such as boehmite (AlOOH) have been studied. Yang et al.[49] tested SDS foams stabilized with AlOOH nanoparticles, and found that the resulting foam exhibited much better stability at high temperature and in the presence of oil than SDS foams. In a separate study, Yang et al.[51] used foams stabilized by AlOOH nanoparticles combined with sodium cumenesulfonate (SC), and found that the foams were highly stable over a wide electrolyte concentration range, and resulted in improved oil displacement.
Recent studies have also tested other nanoparticle materials for CO2 EOR.Guo and Aryana[52] studied foams stabilized by different mixtures of SDS,AOS, and lauramidopropyl betaine(LAPB)in combination with nanoclays and silica nanoparticles. Their study found that the combination of a mixture of AOS and LAPB with silica nanoparticles resulted in better foamability and higher stability. However, an AOS/LAPB mixture with nanoclay particles produced a better oil-recovery performance. As another alternative material, Lee et al.[53] explored the use of nanoparticles made by grinding coal fly ash, a waste product of coal power plants.Their study found that due to their negative charge, the fly ash particles could not stabilize CO2 foams by themselves. However, adding dimethyl trimethylammonium bromide (DTAB), a cationic surfactant, to low-carbon fly ash resulted in stable foams.Nevertheless, a cationic surfactant would typically be adsorbed by the reservoir rocks, making this process inefficient. On the other hand,the study also found that highly carbonaceous fly ash particles combined with turpentine oil did produce stable foams, suggesting that these particles might produce a foam in situ due to interactions with the oil in the reservoir.
Several recent studies have tested nanoparticle/ surfactant systems with other additives, particularly alkalis, alcohols, and polymers. Adding polymers to nanoparticle/ surfactant foams at optimal concentration enhances the foam stability due to steric repulsion between bubbles. However,too much polymer can break the foam due to settling.Alcohols and alkalis also exhibit optimal concentrations for foaming performance[54]. A recent study by Wang et al.[55] used mixtures of the "green”nonionic surfactant APG in combination with SiO2 nanoparticles and the gemini surfactant C12C3C12Br to form a stable foam. Although anionic surfactants are known to outperform nonionic surfactants in brine[56], their results suggest a possible line of research in developing"green”foaming blends.
《4.Environmental impacts of CO2 foam leakage》
4.Environmental impacts of CO2 foam leakage
Liu et al.[57] have compiled a thorough analysis of the environmental impacts and risks of CO2 EOR due to short-term leakage, large-scale leakage,and long-term diffusion or seepage of stored CO2. Examples of these phenomena are illustrated inFig.1[57]. These processes have direct and indirect impacts on human health and the environment, at both local and global levels. At the local level,the increased CO2 levels in the vicinity of the leaking reservoir cause changes in the groundwater and soil chemistry, leading to impaired plant health and diminished crop yields[58]. These high CO2 concentrations can also have a direct impact on human health,with levels above 5%–10% potentially resulting in loss of consciousness and death. At the global level,the release of CO2 results in increased greenhouse gas levels, thereby diminishing or eliminating the benefits of geological storage[57].
《Fig.1》
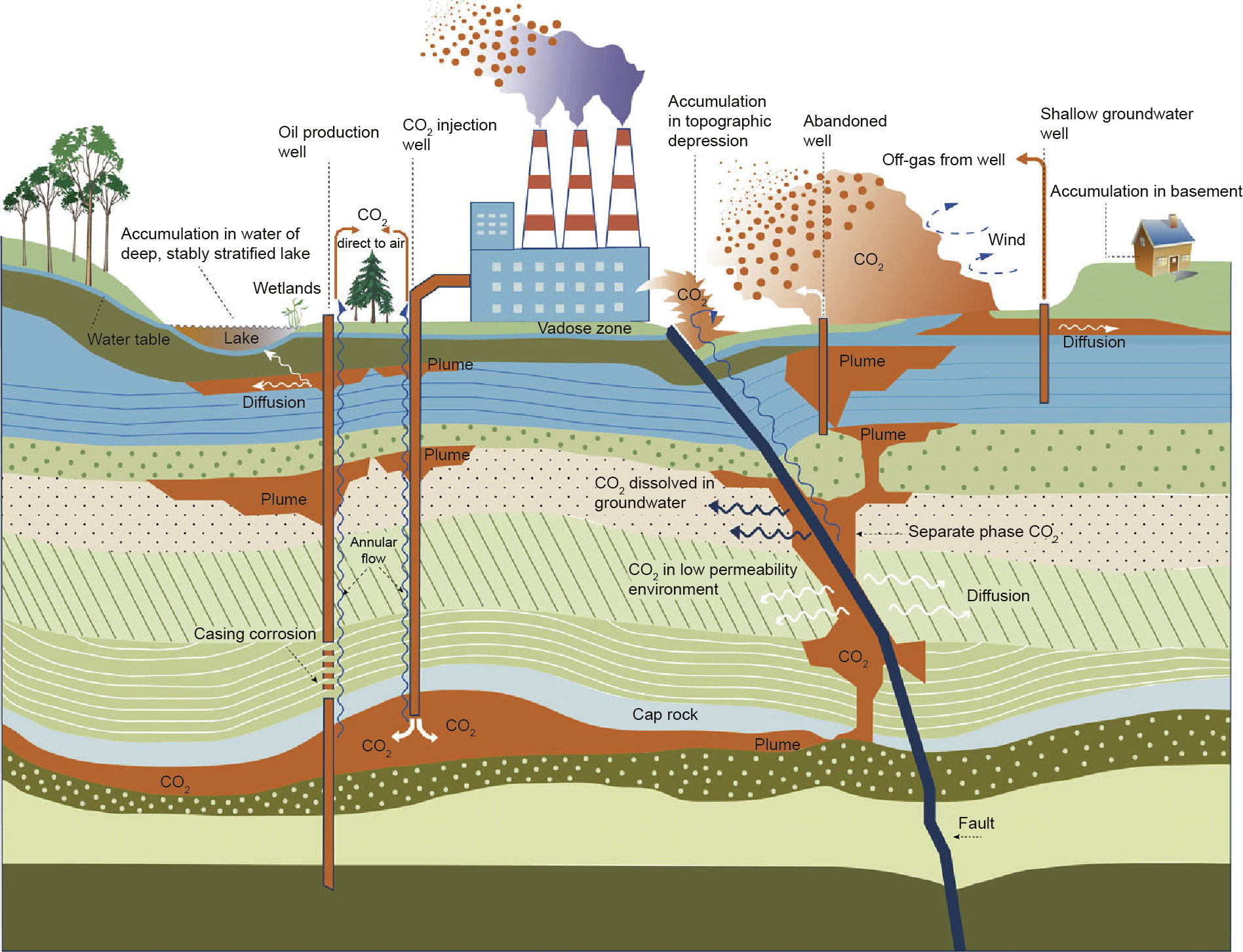
Fig.1.Mechanisms and impacts of CO2 leakage from geological sequestration sites[57]. (Copyright 2016 Springer,reproduced with permission)
Given the different possible failure scenarios for CO2 storage and the lack of an empirical knowledge base for evaluating the related risks, it is very difficult to carry out a quantitative risk analysis with high confidence [59]. Developing models for fluids within oil reservoirs is also challenging, as the fluids are complex and their properties within a rock formation may be very different from those of a bulk fluid[60–62]. Several recent case studies have considered potential risks due to gas leakage in individual CO2 reservoirs, as well as monitoring and remediation strategies. The Zama Lake site in Alberta, Canada, has been the subject of a few such studies [63–65]. This site contains tens of thousands of metric tons of a mixture of carbon dioxide and hydrogen sulfide that has been used for EOR. These sites are typically sealed after the injec-tion process has completed in order to keep the toxic gas deep underground. However, the cement and steel used to seal the well-bore will degrade over time, and this toxic mixture will inevitably leak into the environment, as it is predicted that the mixture will remain unreactive and thus free-flowing[63]. Another area that has recently been studied extensively is the Jingbian Gas Field in Shaanxi Province, China,which houses a major CO2 EOR demonstration project that has been running since 2012 [66]. The Ordos Basin, where the Jingbian Gas Field is located, is the second-largest sedimentary basin in China, and has a very large potential capacity for CO2 storage [67,68]. However,even though the Jing-bian Gas Field is located in the most geologically stable area of the Ordos Basin,a number of potential risks have been identified:The region has a history of earthquakes and also contains collapsed mine shafts and abandoned boreholes, which could result in CO2 leakage[67,69]. Therefore,a comprehensive geological and environmental monitoring strategy is essential for the success of the project.
Besides the environmental risk due to CO2 leakage,there is a potential risk due to the additives used to stabilize the CO2 foams. Given the recent advances in both surfactant-based and surfactant/ nanoparticle-based foaming additives, there is a need for a comprehensive study of the potential environmental impact of these additives. These impacts are discussed in the next section.
《5.Environmental impacts of surfactant and nanoparticle leakage》
5.Environmental impacts of surfactant and nanoparticle leakage
In contrast to the large number of studies on the potential risks of CO2 leakage, few studies have been performed on the potential risks of foaming agent leakage in the context of CO2 EOR. When the risks of chemical exposure involve adverse environmental or human health effects (whether acute or chronic), the US EPA classifies that chemical as toxic [70]. In this section,we outline some of the risks associated with the components used in the previously described studies, in the hope that it will be helpful to guide future studies of CO2 EOR as a"green”process.
The research reviewed in the present paper examines an assortment of anionic surfactants, as well as a much narrower set of nonionic surfactants (since cationic surfactants are unattractive due to their propensity to adsorb in rock formations, we do not consider them in this discussion). The anionic and nonionic surfactants used in the most recent studies (Sections 2 and 3) are summarized inTable 1. All of the anionic and most of the nonionic surfactants listed have potential toxic effects on the environment, depending on the amount released. For example, alkylphenol ethoxylates, which include some of the anionic and most of the nonionic surfactants discussed in this work, are well known for being endocrine disruptors and for having major deleterious impacts on aquatic organisms [71–73]. Releasing large quantities of these surfactants near bodies of water is very likely to catastrophically impact the aquatic wildlife in those environments.
《Table 1》
Table 1 Summary of environmental impacts of surfactants used in recent CO2 foam studies.
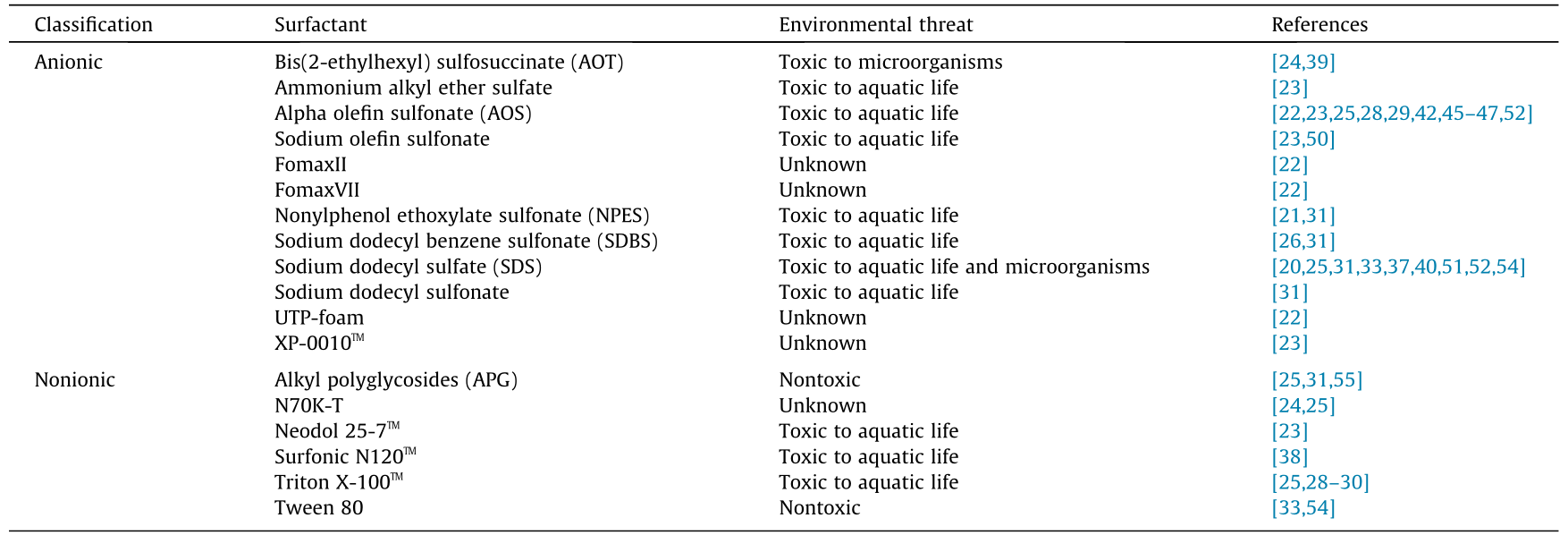
Anionic surfactants deserve the most attention here, as they are the most effective for CO2 foams for EOR and are also a cause for great concern in terms of ecological impact . Among the anionic surfactants described in Sections2 and 3, SDS and AOS are by far the most common. In high concentrations, even common household surfactants such as SDS can have major, widespread environmental impacts [74]. The hazardous effects of SDS on the environment have motivated studies on how to rapidly degrade SDS for wastewater treatment[75,76]. Of the remaining surfactants considered, many are olefin sulfonates,which provoke that the same environmental concerns as AOS. Olefin sulfonates show greater toxicity to aquatic life than alkyl sulfates such as SDS[77]; however,unlike SDS, olefin sulfonates have not been shown to have toxic effects on microorganisms [78]. Similarly, alkyl ben-zene sulfonates negatively affect aquatic life[79,80]. On the other hand,sulfosuccinates, such as AOT ,have been found to be toxic to aquatic microorganisms and harmless to crustaceans [81].
When addressing the degradation of surfactants, however, it is important to indicate whether aerobic or anaerobic conditions are involved. Most reports refer to aerobic conditions;however, in the case of EOR,we are concerned with anaerobic degradation,which is not as well understood.Surfactant biodegradation is dependent on multiple factors[82]: Microorganisms either cause a structural change that eliminates their function as surfactants,or are completely broken down. Because degradation is carried out by microorganisms, the presence of organisms capable of metabolizing a surfactant, as well as appropriate pH, temperature, nutrients, and water content, is necessary. Studies under anaerobic conditions have shown that alkyl and olefin sulfonates will not degrade, while sulfosuccinates are digested[78] and alkyl ester sulfonates and alkyl benzene sulfonates are slowly digested[82]. The difference in biodegradation is thought to be due to the presence of functional groups (i.e.,ester ,ether, and aromatic groups) that allow anaerobic microorganisms to cleave the sulfonate from the tail group. Although there is some information on the degradation of surfactants under ideal anaerobic conditions[83], our understanding of how various environmental conditions relate to the degradation rate is still limited.Most oil reservoirs are unlikely to exhibit ideal degradation conditions; thus, the storage of these compounds in a reservoir with a risk of leakage or seepage can be seen as a significant environmental risk.
Nanoparticle additives also pose environmental risks. Silica nanoparticles have traditionally been regarded as environmentally friendly due to the common natural occurrence of silica. However, the toxicity of silica nanoparticles is poorly understood.Recent studies have found that silica nanoparticles exhibit significant cytotoxicity depending on the particle size and level of exposure[84,85], and in vivo experiments have shown that silica nanoparticles can lead to liver [86] and kidney injury[87], among other harmful effects [85]. More studies are needed to understand the potential environmental effects of injecting large quantities of silica nanoparticles underground.
Other nanoparticle systems are equally uncertain regarding their environmental impact. As discussed in Section 4, AlOOH nanoparticles have also shown enhanced performance as foaming additives. However, AlOOH nanoparticles are known to be toxic,depending on the size of their agglomerates [88]. As in the case of silica nanoparticles, a study on the potential impact of releasing large quantities of AlOOH nanoparticles into the environment is necessary in order to outline an effective monitoring and mitiga-tion strategy.
《6.Closing remarks》
6.Closing remarks
In recent years, there has been an increasing focus on novel technologies for CO2 EOR,given its special status as an economically feasible process for carbon sequestration. Recent studies have resulted in highly effective foaming additives to enhance stability, resilience, and oil recovery. These additives include nonionic surfactants, nanoparticles, and other additives such as polymers,alco-hols,and alkalis. The continued preferential use of anionic surfactants that are known to be toxic may no longer be necessary, given recent advances in using additives,although studies regarding their toxicity are needed.
Studies from recent years have resulted in a large knowledge base on the use of different types of additives to improve foams for CO2 EOR. Given this knowledge,and considering the potential environmental risk of a major leak from a sealed CO2 reservoir, we believe that it is imperative to focus on characterizing and developing less toxic, biodegradable anionic surfactants and foaming additives, considering the particular challenges of biodegradation under reservoir conditions.
《Acknowledgements》
Acknowledgements
The authors acknowledge support from the American Chemical Society Petroleum Research Fund(ACS-PRF)(55801-DNI6).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jennifer A.Clark and Erik E.Santiso declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




